Abstract
Background
The purpose of this study was to compare the efficacy and safety of dexmedetomidine versus dexmedetomidine and ketamine for sedation during awake fibreoptic intubation (FOI) in patients posted for elective surgeries. Ninety-eight American Society of Anesthesiologists Physical Status (ASA-PS) I–II patients with difficult airway and scheduled for elective surgeries were enrolled in this study after institutional ethics committee approval. Patients were randomly allocated into 2 groups, i.e. 49 patients in each group. Group D patients received 1 μg/kg dexmedetomidine IV over 10 min in 100-mL normal saline followed by a continuous infusion at 0.5 μg/kg/h till FOI and 5-mL normal saline followed by saline infusion. Group DK patients received 1 μg/kg dexmedetomidine IV over 10 min in 100-mL normal saline. Further, they received IV ketamine 15 mg as a bolus of 5 mL, followed by continuous infusion of ketamine at 20 mg/h until the end of intubation. The primary objective was to compare the efficacy of the combination of IV dexmedetomidine and ketamine with IV dexmedetomidine alone as sedation for FOI. Vocal cord movement, sedation, coughing, facial grimace score, recall of procedure, and haemodynamics were also compared in both groups.
Results
Demographic data, vocal cord movement, cough score, facial grimace score, total drugs used, hoarseness, sore throat and level of recall were comparable in both groups. Haemodynamics were significantly better in group DK at 2, 3, 4 and 5 min compared to group D.
Conclusions
Addition of ketamine to dexmedetomidine did not improve intubating conditions, reduce cough or improve recall of FOI. However, patients remain sedated when ketamine was used with dexmedetomidine. The study was not registered prospectively in any clinical trial registry.
Similar content being viewed by others
Background
Airway management is an important skill set learnt during training, and tracheal intubation is frequently required to ensure adequate airway management, while providing optimal operating conditions. However, in the presence of certain anatomical variants or airway pathology, visualization of the glottis by direct laryngoscopy can be difficult or impossible (Cook & MacDougall-Davis, 2012). Problems with tracheal intubation could be failure, difficulty, delay and cannot intubate cannot ventilate situation leading to aspiration and hypoxia which is undesirable. The principal adverse outcomes associated with the difficult airway include but are not limited to death, brain injury, cardiopulmonary arrest, unnecessary tracheostomy, airway trauma and damage to teeth (Cook et al., 2011a; Cook et al., 2011b). A fibreoptic intubation (FOI) plays a special role in securing the airway when tracheal intubation is difficult, when the airway is compromised, when the neck extension is to be avoided and in the presence of lower airway pathology (Wong et al., 2019; Collins & Blank, 2014). Nasal FOI is easier for securing the airway rather than an oral technique for anatomical reasons. Oral and facial surgeries can only be performed under nasotracheal intubation. However, it involves the risk of nasal bleeding (Hall & Shutt, 2003).
Currently, benzodiazepines, opioids and propofol are used alone or in combination for this purpose. Midazolam produces amnesia and makes the patient comfortable. Propofol has rapid onset and offset of action with profound amnesia. Opioids such as fentanyl suppresses respiratory centre and produces chest wall rigidity, and there is a risk of hypoxia and desaturation (Johnston & Rai, 2013; Liu et al., 2016).
Dexmedetomidine which is an α2-adrenoreceptor agonist is a valuable drug of use during fibreoptic intubation as it induces sedation and analgesia without depressing respiratory function (Chopra et al., 2016). In addition, xerostomia is commonly reported by patients. These two effects make dexmedetomidine highly desirable for awake, nasotracheal FOI. Unlike patients sedated with propofol, patients receiving dexmedetomidine are easily aroused to cooperate with medical procedures without expressing irritation. The relative sympatholysis achieved during dexmedetomidine infusions is an additional benefit in a procedure that may lead to elevations of heart rate and blood pressure (Cabrini et al., 2019). Ketamine, an NMDA antagonist, has been studied extensively as an adjunctive analgesic in the perioperative setting. At sub-anaesthetic doses, ketamine is well tolerated and has a low incidence of mild psychomimetic symptoms, nystagmus and double vision (Porter, 2019). While bradycardia and hypotension have been reported with dexmedetomidine, this is not observed in patients receiving a concurrent ketamine bolus injection. The undesirable feature of increasing airway secretions with ketamine administration is attenuated by the xerostomia induced by dexmedetomidine. Ketamine is selected to take advantage of its minimal impact on ventilator drive and analgesic properties. In addition, dexmedetomidine attenuates ketamine-induced cardiostimulatory effects and drug-induced delirium. Ketamine combined with dexmedetomidine provided excellent conditions for awake FOI including satisfactory sedation, patient cooperation and dry airway in randomized-controlled trials (Kumar et al., 2019; Sinha et al., 2014).
We hypothesized that sedation with dexmedetomidine plus ketamine combination for FOI could produce better intubating conditions when compared to using dexmedetomidine alone. The primary objective was to compare and study the efficacy of the combination of intravenous dexmedetomidine and ketamine with intravenous dexmedetomidine alone as sedation agents to facilitate awake, fibreoptic-guided nasal intubation with respect to intubation conditions (sedation score, vocal cord movement, cough score). Secondary outcomes were to compare the haemodynamic responses to intubation, incidence of desaturation, time taken for intubation, total dose of lignocaine/dexmedetomidine used, patient satisfaction score, level of recall and adverse events like sore throat and hoarseness.
Methods
This was a prospective, interventional, randomized controlled, double-blinded study which included patients between 18 and 60 years of either gender posted for elective surgeries (laparoscopic surgeries like appendicectomy, cholecystectomy, umbilical and epigastric hernioplasty, laparoscopic inguinal hernioplasty), belonging to ASA-PS I/II and with anticipated difficult intubation (Modified Mallampatti score III/IV). The study was approved by the Institutional Ethics Committee. Pregnant ladies, known alcoholics or drug abusers and those who have an allergy to the drugs involved in the study, bradycardia (baseline HR <40 beats/min), atrioventricular block, heart failure, significant neurological, hepatic, renal and pulmonary disease, emergency surgeries and any contraindication for nasal intubation like thrombocytopenia or coagulopathies were excluded from this study.
All the patients underwent a thorough pre-anaesthetic evaluation including relevant investigations like complete blood picture, bleeding time, clotting time, blood urea, serum creatinine and blood sugar. All systems were examined including the airway, and the procedure to be carried out was explained to the patients. After confirming 6 h nil by mouth, patients were shifted to OR and appropriately sized intravenous (IV) access was secured. After obtaining written informed consent, patients satisfying the inclusion criteria were randomized into 2 groups using a computer-generated random number list. All patients were premeditated with IV pantoprazole 40 mg, IV ramosetron 0.3 mg and IV glycopyrrolate 0.2 mg as an institutional protocol for awake FOI. Standard monitoring using Philips Intellivue MP20 (pulse oximetry, non-invasive blood pressure monitors on the upper limb, respiratory rate, electrocardiography with lead 2, 3) was used for all patients. Oxygen at 2 L/min was administered through a nasal cannula.
Group D (dexmedetomidine) patients received a bolus dose of dexmedetomidine at 1 μg/kg over 10 min in 100-mL normal saline followed by a continuous infusion at 0.5 μg/kg/h using an infusion pump (Smiths Medical, Graseby™ 2000 syringe pump). Further, they received 5-mL normal saline, followed by plain normal saline infusion until the end of intubation. Group DK (dexmedetomidine plus ketamine) patients received a bolus dose of dexmedetomidine at 1 μg/kg over 10 min in 100-mL normal saline followed by a continuous infusion of dexmedetomidine at 0.5 μg/kg/h using an infusion pump. Further, they received IV ketamine 15 mg as a bolus of 5 mL, followed by a continuous infusion of ketamine at 20 mg/h until the end of intubation.
Any decrease in heart rate below 50/min was considered as bradycardia and was treated with 0.6 mg of atropine injection. Similarly, any fall of the mean blood pressure of more than 20% of baseline was considered as hypotension and was treated with fluid boluses and 6 mg of mephentermine injection boluses 6 mg IV. Any drop in saturation of oxygen below 90% was considered desaturation and was treated by stopping of study drug infusions and intubating the patient. Both groups had two infusion pumps.
Following the bolus doses, sedation score was assessed by an anaesthesiologist unaware of the regime used by modified observer assessment of alertness sedation (5 = respond readily to name spoken in a normal tone, 4 = lethargic response to name spoken in a normal tone, 3 = respond only after name spoken loudly or repeatedly, 2 = respond after mild prodding or shaking, 1 = does not respond to mild prodding or shaking). Xylometazoline nasal drops 0.1% (2 drops in each nostril), followed by 2 mL of 4% of lignocaine, were administered intranasally. Two puffs of 10% lignocaine spray were instilled in the same nostril immediately before inserting a nasal fiberscope. An endotracheal tube (ETT) of appropriate size (softened in warm water) was mounted over the fiberscope (Olympus BF Type PE2/TE2) and introduced through the selected nostril after 10 min of the start of study drugs. After visualization of the glottis and vocal cords, 2 mL of 4% lignocaine was injected through the epidural catheter passed through the working channel. Further aliquots were given if the vocal cords moved vigorously. A lubricated ETT was passed over it into the trachea and positioned 2–3 cm above the carina. The cuff was inflated, and the bronchoscope was withdrawn. General anaesthesia was administered using 2–2.5 mg propofol IV, and neuromuscular blockage was achieved using 0.5-mg/kg atracurium followed by discontinuation of study drugs.
The primary outcome was the comparison of intubation scores as assessed by vocal cord movement (1 = open, 2 = moving, 3 = closing, 4 = closed). Secondary outcomes were comparison of modified observer assessment of alertness/sedation (5 = respond readily to name spoken in a normal tone, 4 = lethargic response to name spoken in a normal tone, 3 = respond only after name is spoken loudly or repeatedly, 2 = respond after mild prodding or shaking, 1 = does not respond to mild prodding or shaking), coughing (1 = none, 2 = one gag or cough only, 3 ≥ 1 gag or cough, acceptable conditions, 4 = unacceptable conditions) and patient tolerance as assessed by facial grimace score (1 = no grimace, 2 = minimal grimace, 3 = mild grimace, 4 = moderate grimace, 5 = severe grimace, 6 = very severe grimace). Haemodynamics and oxygen saturation were monitored and noted at regular intervals (T1 = baseline, T2 = 2 min after sedation, T3 = at the beginning of fiberscope as it passes through the nostril, T4 = after advancing the ETT through the nasopharynx, T5 = 2 min after endotracheal intubation).
Other parameters compared were the time taken for intubation (time from the start of FOI to the correct placement of endotracheal tube), total dose of lignocaine and dexmedetomidine used, satisfaction score (1 = excellent, 2 = good, 3 = fair, and 4 = poor), level of recall (memory of pre-anaesthetic preparations, topical anaesthesia, endoscopy and intubation) and adverse events (sore throat, hoarseness). To achieve blinding, three anesthesiologists were required to conduct the study. One anesthesiologist prepared and controlled the drug infusions (who was not concerned with the patient management and data collection), the second one performed FOI and assessed the intubating conditions, and the third anesthesiologist documented the data and made postoperative visits the next day.
Statistical analysis
Descriptive and inferential statistical analysis has been carried out in the present study. Results on continuous measurements were presented as mean ± standard deviation, and results on categorical measurements are presented as number (%). Significance was assessed at 5% level of significance. The Student t test was used to find the significance of the study parameters on a continuous scale between the two groups. The chi-square test was used to find the significance of the study parameters on a categorical scale between the two groups. Statistical analysis of the data collected was done using suitable statistical methods using GraphPad Prism 8. We referred to the study by Sinha et al. (Sinha et al., 2014) for sample size where authors enrolled 60 patients, i.e. 30 patients in each group. However, we included 49 patients in each group to compensate for possible exclusion due to adverse events on non-cooperation. Therefore, we decided to perform a post hoc analysis of power subsequently.
Results
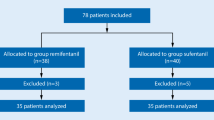
A total of 98 patients completed the study, 49 in group D and 49 in group DK. Figure 1 shows the CONSORT flow diagram. Demographic data (age, gender, weight, ASA-PS) were comparable (Table 1). Sedation score and time for intubation (in minutes) was more in group D compared to group DK (p = 0.003 and p = 0.010, respectively) which was statistically significant (Table 1). Other variables like vocal cord movement, cough score, facial grimace score, total drugs used, hoarseness, sore throat and level of recall were comparable in both groups (Tables 2 and 3). The mean heart rate and MAP (at T2, T3, T4 and T5) were statistically significant in group DK than in group D (p value < 0.05) [Table 4]. Oxygen saturation was comparable between both groups at T1, T2, T3, T4 and T5 (Table 4). We performed a post hoc analysis of power mean ± standard deviation of primary outcomes and a type 1 error as 0.05 and found it to be 88%.
Discussion
Based on the analysis of our results, we found that although the addition of ketamine to dexmedetomidine did not improve intubating conditions, reduce cough or improve haemodynamic fluctuations, patients were more sedated compared to patients receiving dexmedetomidine alone. However, patients in the DK group were more satisfied.
Awake FOI is a stressful procedure, and thus, sedoanalgesia may be necessary to relieve anxiety, ease discomfort and reduce pain. A patient who undergoes FOI frequently experiences pain, cough and sensation of asphyxiation and might remember the procedure as a noxious experience. With the intention of alleviating apprehension before the procedure, providing amnesia and patient comfort, sedation is frequently used (Knudsen et al., 2016; Ramkumar, 2011). Propofol, ketamine, benzodiazepines, opiates and dexmedetomidine are the most commonly used, alone or in combination for sedation (Zhou et al., 2016; Yousuf et al., 2017). But deeply sedated patient may be unable to follow commands, like protrusion of the tongue and swallowing. Even worse, excessively sedated patient might aspirate and might even compromise airway and ventilation (Gonzalez et al., 2003).
The primary goals for sedation during awake FOI are maintenance of spontaneous respiration, adequate oxygenation, and sufficient ventilation. The patient’s ability to inspire deeply on command facilitates ventilation and oxygenation (Kar Kurt et al., 2015). Secondary goals also include amnesia and the ability to cooperate with instructions to take deep breaths or protrude the tongue. Amnesia of potentially uncomfortable procedures is desirable for the patient’s psychologic outlook towards repeat awake fibreoptic intubation and other medical care (Wahidi et al., 2011). No single agent provides amnesia, anxiolysis and analgesia, so a combination of drugs is necessary (Johnston & Rai, 2013). The sedation regimen should be simple and the degree of sedation kept to the minimum required for patient comfort.
Sedation induced by dexmedetomidine has unique properties, which is well documented in intubated and ventilated ICU patients (Sharma et al., 2017; Dhasmana, 2014; Hoy & Keating, 2011). It produces an unusually cooperative form of sedation in which the patient is calm and easily roused from sleep to wakefulness to allow task performance and excellent communication and cooperation while intubated and ventilated and then quickly back to sleep when not stimulated. The primary site of action of α2-adrenoceptor agonists is the locus ceruleus (nucleus in the pons) and not the cerebral cortex, as would be the case with GABA-mimetic drugs (Reel & Maani, 2020). This should be the reason why this class of drugs produces a different type of sedation when compared to propofol and benzodiazepines.
While bradycardia and hypotension have been reported with dexmedetomidine, this is not observed in patients receiving a concurrent ketamine bolus injection. It has been suggested that low-dose ketamine infusion (4 μg/kg/min) effectively lowers postoperative narcotic requirements without significant effect on mood, perception and cognition (Kumar et al., 2019; El Sharkawy, 2019). The undesirable feature of increasing airway secretions with ketamine administration was attenuated by the xerostomia induced by dexmedetomidine. In addition, dexmedetomidine attenuated ketamine-induced cardio stimulatory effects and drug-induced delirium. The combination of dexmedetomidine and ketamine may have resulted in higher sedation level in group DK patients, which was statistically significant (p < 0.003 ). The sedative effects of the combination of ketamine and dexmedetomidine were found to be additive at the endpoints of hypnosis and anaesthesia. Synergism has been found between agents with known functional links in the central nervous system.
Sedation produced by dexmedetomidine is unique when compared to propofol and other narcotics because of its mechanism of action in the locus ceruleus (nucleus in the pons) which is involved in the physiological response to stress and anxiety. The fact that patients maintain spontaneous breathing with dexmedetomidine while attempts are made to secure their airway, makes it an ideal agent for use in elective and critical intubation (Gao et al., 2017; Hassan & Mahran, 2017; He et al., 2014). Our study showed similar results to Sinha et al. providing similar sedation and patient satisfaction (Sinha et al., 2014). Dexmedetomidine blocks the sympathetic supply of the upper airway and produces xerostomia, while ketamine attenuated the xerostomia by producing increased airway secretions. In addition, the use of glycopyrrolate in both groups effectively decreased airway secretions. We used the ‘spray as you go’ technique which involves instillation of a topical agent through the advancing bronchoscope (Xue et al., 2009). Lidocaine jelly was used to lubricate the flexible FOI prior to nasal insertion.
Our study was similar to that performed by Sinha et al. in terms of methodology. However, our sample size was more (60 patients in the previous study versus 98 patients in our study). We also performed a post hoc analysis of power which was 88%.
One of the limitations of the study was the small sample size. We suggest further randomized controlled trials before extrapolating the results of this study in clinical practice.
Conclusion
To conclude, the addition of ketamine to dexmedetomidine does not improve intubating conditions, reduce cough or improve recall of FOI. Rather, patients are more likely to be sedated compared to patients receiving dexmedetomidine alone.
Availability of data and materials
Not applicable
Abbreviations
- FOI:
-
Fibreoptic intubation
- ASA-PS:
-
American Society of Anesthesiologists Physical Status
- D:
-
Dexmedetomidine
- DK:
-
Dexmedetomidine ketamine
- GABA:
-
Gamma amino butyric acid
- ETT:
-
Endotracheal tube
- IV:
-
Intravenous
References
Cabrini L, Baiardo Redaelli M, Ball L, Filippini M, Fominskiy E, Pintaudi M et al (2019) Awake fiberoptic intubation protocols in the operating room for anticipated difficult airway: a systematic review and meta-analysis of randomized controlled trials. Anesth Analg. 128:971–980
Chopra P, Dixit MB, Dang A, Gupta V (2016) Dexmedetomidine provides optimum conditions during awake fiberoptic intubation in simulated cervical spine injury patients. J Anaesthesiol Clin Pharmacol. 32:54–58
Collins SR, Blank RS (2014) Fiberoptic intubation: an overview and update. Respir Care. 59:865–880
Cook TM, MacDougall-Davis SR (2012) Complications and failure of airway management. Br J Anaesth. 109(Suppl 1):i68–i85
Cook TM, Woodall N, Frerk C (2011a) Fourth National Audit Project. Major complications of airway management in the UK: results of the Fourth National Audit Project of the Royal College of Anaesthetists and the Difficult Airway Society. Part 1: anaesthesia. Br J Anaesth. 106:617–631
Cook TM, Woodall N, Harper J, Benger J (2011b) Fourth National Audit Project. Major complications of airway management in the UK: results of the Fourth National Audit Project of the Royal College of Anaesthetists and the Difficult Airway Society. Part 2: intensive care and emergency departments. Br J Anaesth. 106:632–642
Dhasmana SC (2014) Nasotracheal fiberoptic intubation: patient comfort, intubating conditions and hemodynamic stability during conscious sedation with different doses of dexmedetomidine [published correction appears in J Maxillofac Oral Surg. 2015 Jun;14(2):519]. J Maxillofac Oral Surg. 13:53–58
El Sharkawy RA (2019) Efficacy of adding low-dose ketamine to dexmedetomidine versus low-dose ketamine and propofol for conscious sedation in patients undergoing awake fiber-optic intubation. Anesth Essays Res. 13:73–78
Gao Y, Kang K, Liu H et al (2017) Effect of dexmedetomidine and midazolam for flexible fiberoptic bronchoscopy in intensive care unit patients: a retrospective study. Medicine (Baltimore) 96:e7090
Gonzalez R, De-La-Rosa-Ramirez I, Maldonado-Hernandez A, Dominguez-Cherit G (2003) Should patients undergoing a bronchoscopy be sedated? Acta Anaesthesiol Scand. 47:411–415
Hall CE, Shutt LE (2003) Nasotracheal intubation for head and neck surgery. Anaesthesia. 58:249–256
Hassan ME, Mahran E (2017) Evaluation of different doses of dexmedetomidine alone versus the combination of dexmedetomidine and fentanyl in sedation during awake fiberoptic intubation in oral cancer surgery patients: a prospective, randomized, double-blind clinical trial. Saudi J Anaesth. 11:196–202
He XY, Cao JP, He Q, Shi XY (2014) Dexmedetomidine for the management of awake fibreoptic intubation. Cochrane Database Syst Rev:CD009798
Hoy SM, Keating GM (2011) Dexmedetomidine: a review of its use for sedation in mechanically ventilated patients in an intensive care setting and for procedural sedation. Drugs. 71:1481–1501
Johnston KD, Rai MR (2013) Conscious sedation for awake fibreoptic intubation: a review of the literature. Can J Anaesth. 60:584–599
Kar Kurt Ö, Talay F, Karğı A, Yaşar Z, Tuğ T (2015) Fiberoptik bronkoskopide sedasyon: Literatürün gözden geçirilmesi [Sedation for fiberoptic bronchoscopy: review of the literature]. Tuberk Toraks. 63:42–47
Knudsen K, Nilsson U, Högman M, Pöder U (2016) Awake intubation creates feelings of being in a vulnerable situation but cared for in safe hands: a qualitative study. BMC Anesthesiol. 16:71
Kumar A, Verma S, Tiwari T, Dhasmana S, Singh V, Singh GP (2019) A comparison of two doses of ketamine with dexmedetomidine for fiberoptic nasotracheal intubation. Natl J Maxillofac Surg. 10:212–216
Liu GP, Xue FS, Sun C, Yang GZ (2016) Comparing sedation regimens for awake fiberoptic intubation. Chin Med J (Engl) 129:502–503
Porter SB (2019) Perioperative ketamine for acute analgesia and beyond. Rom J Anaesth Intensive Care. 26:67–73
Ramkumar V (2011) Preparation of the patient and the airway for awake intubation. Indian J Anaesth. 55:442–447
Reel B, Maani CV. Dexmedetomidine. [Updated 2020 May 21]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2020 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK513303/[last accessed on 17 Sept 2020].
Sharma J, Purohit S, Bhatia S, Kalra P, Sharma M, Meena R (2017) Awake orotracheal fibre-optic intubation: comparison of two different doses of dexmedetomidine on intubation conditions in patients undergoing cervical spine surgery. Indian J Anaesth. 61:811–817
Sinha SK, Joshiraj B, Chaudhary L, Hayaran N, Kaur M, Jain A (2014) A comparison of dexmedetomidine plus ketamine combination with dexmedetomidine alone for awake fiberoptic nasotracheal intubation: a randomized controlled study. J Anaesthesiol Clin Pharmacol. 30:514–519
Wahidi MM, Jain P, Jantz M, Lee P, Mackensen GB, Barbour SY et al (2011) American College of Chest Physicians consensus statement on the use of topical anesthesia, analgesia, and sedation during flexible bronchoscopy in adult patients. Chest. 140:1342–1350
Wong J, Lee JSE, Wong TGL, Iqbal R, Wong P (2019) Fibreoptic intubation in airway management: a review article. Singapore Med J. 60:110–118
Xue FS, Liu HP, He N, Xu YC, Yang QY, Liao X et al (2009) Spray-as-you-go airway topical anesthesia in patients with a difficult airway: a randomized, double-blind comparison of 2% and 4% lidocaine. Anesth Analg. 108:536–543
Yousuf A, Ahad B, Mir AH, Mir AW, Wani JG, Hussain SQ (2017) Evaluation of effectiveness of dexmedetomidine and fentanyl-midazolam combination on sedation and safety during awake fiberoptic intubation: a randomized comparative study. Anesth Essays Res. 11:998–1003
Zhou LJ, Fang XZ, Gao J, Zhangm Y, Tao LJ (2016) Safety and efficacy of dexmedetomidine as a sedative agent for performing awake intubation: a meta-analysis. Am J Ther. 23:e1788–e1800
Acknowledgements
Not applicable
Ethical approval and consent to participate
The study was approved by the Ethics Committee of Yashoda Hospitals, Secunderabad, Telangana State, India. Written informed consent was obtained from all patients. Reference number not available.
Funding
None.
Author information
Authors and Affiliations
Contributions
DJ: literature review, manuscript preparation, and followed the patients. GF: manuscript review, data analysis, and followed the patients. SD: concepts and design. BV: concepts and design. AN: literature review, manuscript editing, final draft, and data analysis. All authors read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Jamgade, D., Fultambkar, G., Dara, S. et al. A prospective randomized controlled study comparing intravenous dexmedetomidine plus ketamine combination with intravenous dexmedetomidine alone for awake fibreoptic nasotracheal intubation. Ain-Shams J Anesthesiol 13, 16 (2021). https://doi.org/10.1186/s42077-021-00133-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s42077-021-00133-x