Abstract
Background
Epidermal growth factor receptor (EGFR) is a cellular oncoprotein which is overexpressed in many human cancers including a subset of endometrial cancers. Immunohistochemical (IHC) expression of EGFR has been investigated in previous studies; Role of EGFR in endometrial carcinoma as a prognostic biomarker has not been studied in our population; therefore we aimed to evaluate the expression of EGFR in cases of endometrial carcinoma in loco-regional population and its association with histologic variables.
Methods
Total 89 cases of endometrial carcinoma were selected from records of pathology department archives. All patients underwent surgeries at Liaquat National hospital, Karachi from January 2012 till December 2017 over a period of 6 years. Slides of all cases were retrieved and reviewed by two senior histopathologists and pathologic characteristics were evaluated. Moreover, representative tissue blocks of all 89 cases were selected for EGFR immunohistochemistry.
Results
73% (65 cases) showed no EGFR expression, while 21.3% (19 cases) showed low EGFR expression and 5.6% (5 cases) revealed high EGFR expression. Significant association of EGFR expression was noted with histologic type. Serous carcinoma and carcinosarcoma showed high expression of EGFR. On the other hand, no significant association of EGFR with other histopathologic parameters was found.
Conclusion
Overall, we found a low EGFR expression in endometrial carcinoma in our population without any significant pathological association except for its high expression in serous carcinoma and carcinosarcoma; however, more large scale studies are warranted to validate these findings.
Similar content being viewed by others
Introduction
Epidermal growth factor receptor (EGFR) is a cellular oncoprotein overexpressed in many human cancers including a subset of endometrial cancers (Battaglia et al., 1989; Bauknecht et al., 1989; Miyazawa, 1992). EGFR is also used as a prognostic biomarker and therapeutic target in many cancers including head and neck, breast, bladder and lung cancers (Ali Hashmi et al., 2018; Hashmi et al., 2018)
Endometrial carcinoma is one of the most common gynaecological malignancies in women. There are two major types of endometrial carcinoma (type I and II) on the basis of genetic alteration and phenotypic appearance. Type I endometrial carcinoma is hormone driven and include endometroid subtype. On the other hand, type II endometrial cancers are caused by p53 gene mutations. Major prognostic parameters of endometrial cancers include histologic subtype, grade, depth of myometrial invasion and extrauterine spread (Ambros & Kurman, 1992; Kurman & Norris, 1987) Immunohistochemical (IHC) expression of EGFR has been investigated in previous studies; however its prognostic significance has not been validated yet in Pakistan. Role of EGFR in endometrial carcinoma as a prognostic biomarker has not been studied in our population; therefore we aimed to evaluate the expression of EGFR in cases of endometrial carcinoma in loco-regional population and its association with histologic variables.
Methods
Case selection
Total 89 cases of endometrial carcinoma were selected from the files of pathology department archives. All patients had surgeries at Liaquat National hospital, Karachi from January 2012 till December 2017 over a period of 6 years. The study was approved by research and ethical review committee of Liaquat National Hospital. Informed written consent was taken antecedent to surgery. Hematoxylin and eosin stained slides and paraffin blocks were retrieved. Slides of all cases were re-evaluated by two senior histopathologists and pathologic characteristics were recorded. Representative tissue blocks of all cases were selected for EGFR immunohistochemistry.
Immunohistochemistry
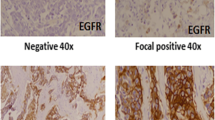
EGFR immunohistochemistry was performed using DAKO EnVision method using DAKO Monoclonal Mouse Anti-human Epidermal growth factor Receptor (EGFR), clone H11 according to manufacturers protocol. Both membranous and cytoplasmic staining for EGFR were evaluated. Intensity of staining was categorized into no staining (0), weak (1+), intermediate (2+), strong (3+) while percentage of positively stained cells were scored as continuous variable (Fig. 1). Intensity and percentage cores were multiplied to generate an H-score ranging from 0 to 300. A cut off score of 10 i.e. at-least weak expression (intensity score 1+) of EGFR in 10% of tumor cells was taken as positive EGFR expression. On the other hand, cut-off value of 200 was used to categorize positive EGFR expression into low and high. Cases above 200 H-score was considered high EGFR expression. H-scoring system in evaluating biomarker testing is widely used in different cancers (McCarty Jr. et al., 1985).
Statistical analysis
Statistical package for social sciences (SPSS 21) was used for data compilation and analysis. P-value ≤0.05 was taken as significant. Mean and standard deviation were calculated for quantitative variables. Frequency and percentage were calculated for qualitative variables. Fisher exact test was applied to determine association.
Results
Demographic patient characteristics
Mean age of the patients involved in the study was 55.76 + 9.17. Endometroid carcinoma was the most common subtype (86.5%), followed by serous (7.9%) and carcinosarcoma (4.5%). Most of the cases were of either grade I or II (39.3 and 42.7% respectively). Fifty-two cases (58.4%) showed more than half of myometrial invasion. Cervical invasion, adnexal involvement and nodal metastasis were seen in 25.8, 10.1 and 5.6% cases respectively. 12.4% cases were found to be at high T stage (T3/T4). Similarly, high FIGO stage (III/IV) was noted in 12.4% cases (Table 1).
EGFR expression in endometrial carcinoma
73% (65 cases) showed no EGFR expression, while 21.3% (19 cases) showed low EGFR expression and 5.6% (5 cases) revealed high EGFR expression. Significant association of EGFR expression was noted with histologic type. Serous carcinoma and carcinosarcoma showed high expression of EGFR. Median IHC score for endometroid carcinoma was 0 with standard error 4.5; median IHC score for serous carcinoma was 8.0 with standard error of 7.2 while median IHC score for carcinosarcoma was 80.0 with standard error 34.7. Non parametric Kruskal-Wallis Test was used to compare mean difference. We found significant mean difference between EFGR IHC scores of various histologic subtypes of EC (p = 0.013).On the other hand, no significant association of EGFR with other histopathologic parameters was found (Table 2).
Discussion
In the present study, we found a low overall expression (26.9%) of EGFR in endometrial carcinoma. Moreover, no significant association of EGFR expression was noted with tumor grade and other histologic parameters.
Comparison of our results with reported literature revealed that most of the authors found a relatively high expression of EGFR in endometrial carcinoma. Niikura H et al., found 67.1% expression of EGFR in a study involving 140 patients of endometrial carcinoma. They found significant association of EGFR expression with grade and age, however no significant association was noted with other prognostic parameters like depth of myometrial invasion and tumor stage (Niikura et al., 1995). On the other hand, in another study involving 96 and 40 cases of endometrial carcinoma, revealed 74 and 67.5% EGFR expression with no significant association with tumor grade and depth of invasion (Berchuck et al., 1989a; Nyholm et al., 1993). Similarly, Berchuck A et al., didn’t find any significant association of EGFR expression with histologic grade and depth of myometrial invasion (Berchuck et al., 1989b). Khalifa MA et al., found EGFR expression in 49% cases of endometrial carcinoma and found that EGFR expression as a significant predictor of survival (Khalifa et al., 1994).
Cai S et al., in a study involving 152 cases of endometrial carcinoma concluded that, EGFR along with COX-2 and VEGF-C expression can help in predicting FIGO stage, degree of differentiation, and depth of myometrial invasion in endometrial carcinoma (Cai et al., 2017). Ramalingam P et al., investigated expression of various biomarkers in undifferentiated and basal like EC. They noted EGFR expression in 22% cases of undifferentiated EC; on the other hand, basal like EC lack EGFR expression.
In this era of personalized medicine and changing trends of cancer management, more and more molecular targets are being identified. Apart from WHO classified histologic subtypes of EC, two distinct molecular pathways of EC are well known with type II EC frequently having p53 and EGFR mutations. Therefore, there is need for a molecular based classification of EC that can help in personalizing targeted therapy in EC. Although, molecular classifications of EC have been proposed in the past, however they have not been implemented yet. Jones NL et al., correlated histologic types with gene mutations and described EGFR mutations in EC including mucinous subtype (Jones et al., 2017). Thoury A et al., investigated gene expression and molecular targets in low and high grade EC and proposed role of anti-EGFR agents and rapamycin derivatives (anti-mTOR) for low grade and anti c-MET/ligand complex in high grade EC (Thoury et al., 2014). On the other hand, Jones NL et al., suggested hormonal receptors, as well as genes implicated in cell proliferation, DNA repair, and cell cycle pathways as possible therapeutic targets in EC (Jones et al., 2015)
In addition to the prognostic significance of EGFR, predictive role of EGFR in anti-EGFR targeted therapy was also suggested in a few studies. Nishimura T et al., proposed possible role of Erlotinib in EGFR over-expressed EC (Nishimura et al., 2015)
The main limitation of the study was that long term follow-up of the patients was not available to evaluate association of EGFR expression with recurrence and disease free survival. In addition, the number of cases of non-endometroid cancers was low. Therefore, we recommend large scale studies to evaluate EGFR expression in endometrial carcinoma in our population.
Conclusion
Overall, we found a low EGFR expression in endometrial carcinoma in our population without any significant pathological association except for its high expression in serous carcinoma and carcinosarcoma, however, more large scale studies are warranted to validate these findings.
Abbreviations
- EGFR:
-
Epidermal growth factor receptor
- IHC:
-
Immunohistochemistry
References
Ali Hashmi A, Hussain ZF, Aijaz S, Irfan M, Khan EY, Naz S, Faridi N, Khan A, Edhi MM (2018) Immunohistochemical expression of epidermal growth factor receptor (EGFR) in South Asian head and neck squamous cell carcinoma: association with various risk factors and clinico-pathologic and prognostic parameters. World J Surg Oncol. 16(1):118.
Ambros RA, Kurman R (1992) Combined assessment of vascular and myometrial invasion as a model to predict prognosis in Stage I endometrial adenocarcinoma of the uterine corpus. Cancer 69:1424–1423 1.
Battaglia F, Scambia G, Panici PB, Baiocchi G, Perrone L, Iacobelli S et al (1989) Epidermal growth factor receptor expression in gynecological malignancies. Gynecol Obstet Invest. 27(1):2742–2744.
Bauknecht T, Kohler M, Janz I, Pfleiderer A (1989) The occurrence of epidermal growth factor receptors and the characterization of EGF-like factors in human ovarian, endometrial, cervical and breast cancer. Cancer Res Clin Oncol 115:193–199.
Berchuck A, Soisson AP, Olt GJ (1989a) et a1: Epidermal growth factor receptor expression in normal and malignant endometrium. Am J Obstet Gynecol 161:1247–1252.
Berchuck A, Soisson AP, Olt GJ, Soper JT, Clarke-Pearson DL, Bast RC Jr, McCarty KS Jr (1989b) Epidermal growth factor receptor expression in normal and malignant endometrium. Am J Obstet Gynecol. 161(5):1247–1252.
Cai S, Zhang YX, Han K, Ding YQ (2017) Expressions and clinical significance of COX-2, VEGF-C and EFGR in endometrial carcinoma. Arch Gynecol Obstet. 296(1):93–98.
Hashmi AA, Hussain ZF, Irfan M, Khan EY, Faridi N, Naqvi H, Khan A, Edhi MM (2018) Prognostic significance of epidermal growth factor receptor (EGFR) over expression in urothelial carcinoma of urinary bladder. BMC Urol. 18(1):59.
Jones NL, Xiu J, Chatterjee-Paer S, Buckley de Meritens A, Burke WM, Tergas AI, Wright JD, Hou JY (2017) Distinct molecular landscapes between endometrioid and nonendometrioid uterine carcinomas. Int J Cancer. 140(6):1396–1404.
Jones NL, Xiu J, Reddy SK, Burke WM, Tergas AI, Wright JD, Hou JY (2015) Identification of potential therapeutic targets by molecular profiling of 628 cases of uterine serous carcinoma. Gynecol Oncol. 138(3):620–626.
Khalifa MA, Abdoh AA, Mannel RS, Haraway SD, Walker JL, Min KW (1994) Prognostic utility of epidermal growth factor receptor overexpression in endometrial adenocarcinoma. Cancer. 73(2):370–376.
Kurman RJ, Norris HJ (1987) Endometrial carcinoma. In: Kurman RJ (ed) Blaustein’s pathology of the female genital tract, 3rd edn. Springer-Verlag, New York, pp 338–372.
McCarty KS Jr, Miller LS, Cox EB et al (1985) Estrogen receptor analyses: correlation of biochemical and immunohistochemical methods using monoclonal antireceptor antibodies. Arch Pathol Lab Med 109(8):716–721.
Miyazawa K (1992) Role of epidermal growth factor in obstetrics and gynecology. Obstet Gynecol 79:1032–1040.
Niikura H, Sasano H, Matsunaga G, Watanabe K, Ito K, Sato S, Yajima A (1995) Prognostic value of epidermal growth factor receptor expression in endometrioid endometrial carcinoma. Hum Pathol. 26(8):892–896.
Nishimura T, Nakamura K, Yamashita S, Ikeda S, Kigure K, Minegishi T (2015) Effect of the molecular targeted drug, erlotinib, against endometrial cancer expressing high levels of epidermal growth factor receptor. BMC Cancer. 15:957.
Nyholm HU, Nielsen AL, Ottesen B (1993) Expression of epider- mal growth factor receptors in human endometrial carcinoma. Int J Gynecol Pathol 12:241–245.
Thoury A, Descatoire V, Kotelevets L, Kannengiesser C, Bertrand G, Theou-Anton N, Frey C, Genestie C, Raymond E, Chastre E, Lehy T, Walker F (2014) Evidence for different expression profiles for c-Met, EGFR, PTEN and the mTOR pathway in low and high grade endometrial carcinomas in a cohort of consecutive women. Occurrence of PIK3CA and K-Ras mutations and microsatellite instability. Histol Histopathol. 29(11):1455–1466.
Acknowledgments
We gratefully acknowledge all staff members of Pathology, Liaquat National Hospital, Karachi, Pakistan for their help and cooperation.
Funding
No Funding was provided.
Availability of data and materials
Please contact author for data requests.
Author information
Authors and Affiliations
Contributions
AAH, ZFH and MI: main author of manuscript, have made substantial contributions to conception and design of study. MN, SKH, HA, SB and NF: been involved in drafting the manuscript, revising it critically for important intellectual content. MN, SKH, HA, SB and NF have been involved in analysis of the data and gave final approval and revision of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Ethics committee of Liaquat National Hospital, Karachi, Pakistan approved the study. Written informed consent was obtained from the patients for the participation.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Hashmi, A.A., Hussain, Z.F., Irfan, M. et al. Epidermal growth factor receptor (EGFR) overexpression in endometrial carcinoma: association with histopathologic parameters. Surg Exp Pathol 2, 8 (2019). https://doi.org/10.1186/s42047-018-0028-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s42047-018-0028-1