Abstract
Background
Epidural hematomas have been treated with urgent surgical evacuation to prevent catastrophic neurological sequelae and death. Brain Trauma Foundation recommends EDH volume greater than 30 cm3 and warrants surgical evacuation irrespective of GCS. However, due to increase in number of patients undergoing brain CTs following head injuries, more patients have been detected with EDH causing minimal symptoms.
Aims and objectives
To study factors influencing patients being treated conservatively for head injury with supratentorial epidural hematomas.
Material and methods
Our study is a retrospective analysis of supratentorial epidural hematoma treated conservatively from august 2018 to July 2020 at Sher-i-Kashmir institute of medical sciences, Srinagar, Jammu and Kashmir.
Results
A total of 19 patients with EDH were treated conservatively and fulfilled the inclusion criteria.(GCS of 13–15 with no neurological deficit, mild signs of elevated ICP, EDH thickness < 1.5 cm on CT, EDH volume on CT < 30 ml, midline shift on CT less than or equal to 5 mm with no significant intradural pathology)Age ranged from 2 months to 70 years (average 27.15 yrs)males (89.47%) predominated females (10.53%). Motor vehicular accidents were the most common mode of injury (42.1%). EDH was localised 13 times on right side, 5 times on left side and bilateral in one, supratentorially. A midline shift of 5 mm was found in 3 of 19 patients; GCS was > 13 on admission. 8 patients were hospitalised for a week, whilst 2 patients stayed in the hospital for 20 and 25 days, respectively, due to problems not related to EDH. One patient in whom conservative treatment had to be changed to surgical evacuation after 6 days of observation because of worsening headache, impaired alertness repeated imaging showed slight increase in EDH.
Conclusions
EDH can be managed conservatively in carefully selected patients of minor head injury with radiological surveillance and close neurological monitoring. Patients with GCS on admission more than 13, midline shift of less than 5 mm, location and volume of EDH less than 30 ml. Thus, leading to optimal utilisation of hospital resources. So, we conclude that even a dreaded entity like extradural haemorrhage can be managed conservatively in selected cases with strict clinical and radiological surveillance. We have called them ‘’Benign extradural haemorrhages’’.
Similar content being viewed by others
Background
Epidural hematomas (EDH’s) frequently present with dramatic neurological deterioration that requires urgent surgical evacuation to prevent severe neurological sequelae or death [1]. It occurs in about 2% of all cases of head injury but 5–15% of cases of fatal head injury [2]Males outnumber females by 4:1 [3]. It is rare in small children because of the plasticity of the skull and is less common over the age of 60 because the dura is tightly adherent [4]. Bilateral extradural haematomas are rare (< 5% of all cases) and are caused by an extended linear fracture around the superior sagittal sinus [5]. Contralateral extradural haematoma often develops following surgical decompressive craniectomy for subdural haematoma [6]. Almost all the patients with an EDH volume greater than 30 cm3 should have a surgical evacuation regardless of GCS [7]. Furthermore, the epidural hematoma will start the ossification process 2 weeks after the injury. Without any treatment, this collection will become a mass that appears hyperdense on a CT scan [8]. Extradural haematoma can result from injury to the middle meningeal artery, the middle meningeal vein, the diploic veins or the venous sinuses [9]. Historically bleeding from the middle meningeal artery has been considered the main source for EDH [10]. It is uncommon in infants and is associated with skull fracture [11]. Most typical clinical feature is the presence of lucid interval, during which headache, restlessness, nausea, vomiting, vertigo, confusion, poor reactivity or convulsion are observe [12]. A head injury patient had only temporary loss of consciousness and was left asleep may sometimes be found dead in the bed next morning because of slowly developing extradural haematoma [13]. The earliest neurosurgical abnormality other than disturbance of consciousness is pupillary abnormality, which amounts to 90.0% for mydriasis on the side of haematoma [9].
Recently due to pronounced increase in the number of patients who undergo brain CT scans following minor head injuries, we noticed greater proportion of EDH’s being detected in patients who are neurologically intact or with minimal symptoms [14, 15].
The aim of this study is to report the outcome after nonsurgical management in those patients with EDHs who remain in good clinical condition.
Methods
In this retrospective study, 19 patients with radiologically significant EDH (volume < 30 ml) were identified from August 2018 to July 2020 for conservative management. We reviewed the charts of all patients who were intended to follow a conservative treatment for their EDH if they were compatible with our study inclusion criteria, focusing on accident mechanism, initial neurological symptoms, time between accident and diagnosis, characteristics of CT findings and neurological symptomatology. The authors have clinically followed all patients during their hospital stay; neurological examination was performed by not less than a senior resident. All the data were tabulated in a Microsoft excel spreadsheet and was assessed with regards to age at presentation, gender, mode of injury, clinical presentation, GCS on presentation, radiological and imaging profile.
Inclusion criteria
-
1.
Glasgow Coma Scale (GCS) of 13-15 and no neurological deficits when admitted to the hospital.
-
2.
Clinically none or only mild signs of elevated intracranial pressure.
-
3.
EDH thickness of < 1.5 cm on initial CT.
-
4.
Volume < 30 ml
-
5.
Shift ≤ 5 mm
-
6.
Not associated with significant intradural pathology
Exclusion Criteria
-
1.
GCS < 13, Neurological deficit with associated injuries and hemodynamic instability.
-
2.
Haematoma volume > 30 ml
-
3.
Midline Shift > 5 mm
-
4.
Posterior fossa EDH
Patients were under close clinical observation in our emergency surgical ward for their period of stay. A 24-h access to emergency surgery in case of deterioration was provided during the whole hospital stay. Regular follow-up included visits in the outpatient clinic after 1, 3, 6 months and 1 year. CT was repeated until the EDH resolved.
Results
A total of 19 patients with radiologically significant EDH were conservatively treated and fulfilled the study inclusion criteria. Age at the time of the accident ranged from 2 months to 70 years (average age 27.15 years). Injury mechanism in all cases included trauma to the head caused by falls, hit by stones, log of wood or road traffic accidents. All patients had CT scan done in up to 72 h as ours is the only tertiary care centre for management of neurosurgical patients in Kashmir valley.
The EDH was localised thirteen times on the right side, five times on left and bilateral in one. All of them were located supratentorially. A midline shift of 5 mm was found in 3 of 19 patients.
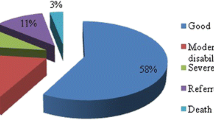
Eight patients were hospitalised for 7 days. There were two patients who stayed in the hospital for 20 and 25 days because of problem not related to EDH (patient number 9 and 16; both had multiple bony fractures in chest and leg). There was only one patient (patient number 18) in whom the conservative treatment had to be changed to surgical evacuation of right temporal EDH after 6 days of observation. During observation, he complained of worsening headache, mildly impaired alertness and repeated CT revealed a slightly increasing size of EDH. Although his GCS was 15 and had no neurological deficit, we decided to operate because of worsening of symptoms. The patient recovered uneventfully. Average follow-up was 1.3 years. All patients were clinically stable and had no disability. None of them needed regular pain medication. No episodes of seizures were reported (Figs. 1, 2, 3). All patients returned to their premorbid socio-intellectual state. Up to 12 months all patients followed up but at 1.5 year, only 15 patients followed up. Those who did not follow-up cited reasons such as “no problems” with the patients, just belonging to far flung regions or busy in work (Table 1, 2).
Discussion
McLauren and Towbin [16], mentioned: ‘the definitive treatment of EDH should always be surgical removal, and delay of such treatment is unacceptable. On the other hand, some of the studies have clearly shown that old fear of extradural haemorrhages is sometimes exaggerated and that intervention is not always necessary [17, 18]. Patient selection and hospital set up are the key factors for successful treatment; normal neurological examination and close monitoring are mandatory for choosing this method of treatment. It is also important to have a set up where you can perform immediate craniotomy and evacuation at any time. In marginal cases, the authors maintained a low threshold for craniotomy [19].
Pang et al [20] reported the nonsurgical management of subacute and chronic EDHs in 11 children, two of whom required operation later. These authors concluded that criteria for conservative treatment included were that patient should be awake, oriented, free of neurological deficit and without evidence of associated intradural lesions. Most of their patients had CT examination 48 h after injury in the subacute phase, when the deterioration is generally less dramatic. The conservative management of chronic EDHs is based upon the belief that they result from venous haemorrhage. That is slow and therefore, allows the brain time to accommodate to the enlarging mass.
In our institution, we have followed the criteria described by Bullock et al [21], for the surgical treatment of patients with EDH: hematoma volume larger than 30 ml, midline shift over 5 mm and clot thickness over 15 mm on cerebral CT scan. Patients who did not fit into this criteria were managed conservatively. Also, deleterious effects of EDH are dependent on various factors—size, location and configuration of the clot, the rapidity of accumulation and age of the patient.
Focal EDHs seem to produce more brain shift than do hematomas spread over large surface of dura mater [20]. The shape of the clot seems to be an important parameter in determining the clinical course. Thus, in children in whom the dura mater is not densely adherent to the inner table of skull, bleeding tends to spread thinly over large areas, making an expectant treatment possible despite the overall volume of the clot. Associated fractures in the children let the EDH to drain out in the sub-galeal space and spontaneous resolution [22].
Location of hematoma is also an important factor. It is known that temporal EDHs cause acute neurological deterioration more frequently than hematomas located elsewhere because of midbrain distortion. The only temporal EDH treated conservatively by Pang et al. needed evacuation on 8th day after injury [20]. Also, one temporal EDH managed conservatively in our series had to be operated on day 6th. This we conclude that spontaneous resolution tends to occur in patients with non-temporal EDHs since brain distortion is better tolerated in these areas.
Some authors believe that the presence of intradural lesions contraindicated the nonsurgical management of EDHs [20]. We found that patients with associated small contusions may not require operation, as have others [17]. In our study, an associated brain contusion or small SDH were found in 6 of our patients with EDH. All of them were managed conservatively and recovered uneventfully. Probably, brain edema due to underlying contusion prevents expansion of EDH.
Amongst various theories that have been proposed to explain the underlying mechanisms of rapid spontaneous resolution is the existence of potential communication with a fracture between intracranial and extracranial spaces. The increased intracranial pressure creates a pressure gradient between EDH and extracranial soft tissue spaces so that hematoma is forced out of extradural space through fracture-line [22]. Also, second probable mechanism reported is the pressure-induced redistribution of the EDH secondary to brain swelling along the inner table of the cranial vault. However, dissipation of the hematoma seems harder due to tenacious adhesions between the dura matter and skull. Another theory emphasises that the extracranial blood may be pushed into the extradural space through a fracture line, due to an increased extracranial sub-galeal interstitial pressure and the pressure gradient after injury. Hence, as the sub-galeal interstitial pressure decreases, the blood from the intracranial extradural space leaks back [23]. With regard to time course of resolution, it occurred within 90 days in the largest cases of Pang et al [20]. In our series, resolution occurred within average time of 38.5 days (range from 15 to 75 days). The resorptive process in these EDHs was characterised by progressive decreases in size and clot density.
The option of conservative management of EDH in children relies on several specific pathophysiological mechanisms. First, young children in particular tolerate an acute increase in intracranial pressure more than adults because they have unfused cranial sutures, open fontanels, large extracerebral spaces and large basal cisterns. Second, the origin of EDH in children is often venous, whereas in adults, it is mainly caused by arterial bleeding [13]. In our series we had nine patients under the age of 18. Also in elderly, changes of brain atrophy on CT scan seems to give enough space for EDH to accommodate without any mass effect.
Evolution of the EDH over time significantly influences its treatment, since patients who are diagnosed less than 6 h after the trauma are at a higher risk of subsequent deterioration and require evacuation, whereas patients with normal initial brain CT and delayed EDH and the patients who had delayed presentation usually after 24–36 h who present with mild symptoms can be managed conservatively with careful neurological evaluation and repeat brain CT [24]. In our study, we had 15 patients whose CT scan was done within 6 h of trauma and only one patient deteriorated and was subsequently operated with uneventful recovery. Thus, patients diagnosed early do not always necessitate surgical intervention.
Long term follow-up of patients with conservatively managed EDH is important to prove the validity of this approach. All patients in this report showed a follow-up without problems up to 6 months and 13 of 19 up to one year. The follow-up period ranged from 4 to 40 months. Cayli et al [19] compared the results of surgical and conservative management related to single photon emission computed tomography (SPECT) and neuropsychological tests at 3 and 6 months. They concluded that minimally symptomatic or asymptomatic EDH causes no pathological SPECT findings or neuropsychological impairment. Our observation also reported no neuropsychological problems in the patients on follow-up.
Conclusions
Radiologically significant EDH can be managed conservatively. Our retrospectively collected data from 19 patients with initially normal neurological findings and significant EDH on CT demonstrated safe and successful conservative treatment in 18 patients, safe and successful conversion in treatment with subsequent evacuation of EDH in one patient. If conservative treatment in radiologically significant EDH is considered, it is mandatory to monitor the patient round the clock, put them on radiological surveillance, and there should be a possibility to perform an operation at any time in case of neurological deterioration. GCS on admission > 13, midline shift of < 5 mm on CT location of EDH and volume of EDH < 30 ml on CT plays a key role in decision making and prevents patients from pain and morbidities associated with neurosurgical procedures and in turn leads to optimum utilisation of hospital resources.
Follow-up at 6 months showed no neuropsychological problem. So, we conclude that even a dreaded entity like extradural haemorrhage can be managed conservatively in selected cases with strict clinical and radiological surveillance. We have called them ‘’Benign extradural haemorrhages’’.
Abbreviations
- CT:
-
Computed tomography
- EDH:
-
Extradural haemorrhage
- SPECT:
-
Single photon emission computed tomography
- EDH:
-
Epidural hematoma
- GCS:
-
Glasgow coma scale
- CT:
-
Computerised tomography
- ICP:
-
Intracranial pressure
References
McKenzie EJ. Epidemiology of influences: current trends and future challenges. Epidemiology Rev. 2000;22(1):112–9.
Pandey S, Sharma V, Shinde N, et al. Bilateral occipital extradural hematoma in a child. J Pediatr Neurosci. 2015;10(3):270–2. https://doi.org/10.4103/1817-1745.165701.
Mezue WC, Ndubuisi CA, Chikani MC, et al. Traumatic extradural hematoma in enugu Nigeria. Niger J Surg. 2012;18(2):80–4. https://doi.org/10.4103/1117-6806.1031113.
Mishra SS, Nanda N, Deo RC. Extradural hematoma in an infant of 8 months. J Pediatr Neurosci. 2011;6(2):158–60. https://doi.org/10.4103/1817-1745.92855.
Fadalla T, Jalaleldean B, Suliman M, et al. Post-traumatic bilateral synchronous acute extradural hematomas: a case report and review of literature. Ann Med Surg (Lond). 2022;1275:103377. https://doi.org/10.1016/j.amsu.2022.103377.
Singh S, Sameer P, Paul D, et al. Contralateral acute extradural hematoma following decompressive craniectomy for subdural hematoma evacuation: a rare complication and a short literature review. Neurol India. 2022;70(3):1230–1. https://doi.org/10.4103/0028-3886.349721.6.
Soon WC, Marcus H, Wilson M. Traumatic acute extradural haematoma - Indications for surgery revisited. Br J Neurosurg. 2016;8:1–2.
Lim J, Housley SB, Drumsta D, Spiro RM. Chronic epidural hematoma presenting with diplopia. Surg Neurol Int. 2021;12:420.
Gean AD, Fischbein NJ, Purcell DD, Aiken AH, Manley GT, Stiver SI. Benign anterior temporal epidural hematoma:indolent lesion with a characteristic CT imaging appearance after blunt head trauma. Radiology. 2010;257(1):212–8.
Liu JT, Tyan YS, Lee YK, Wang JT. Emergency management of epidural haematoma through burr hole evacuation and drainage. A preliminary report. Acta Neurochir. 2006;148(3):313–7.
Greenberg MS. Epidural Haematoma. In: Handbook of Neurosurgery, 6th edn. New York: Thieme (2010)
Babu ML, Bhasin SK, Kumar A. Extradural hematoma-an experience of 300 cases. JKScience. 2005;7(4):205–7.
Jamous MA, Aziz HA, AlKaisy F, Eloqayli H, Azab M, Al-Jarrah M. Conservative management of acute epidural hematoma in a pediatric age group. Pediatr neurosurgery. 2009;445(3):181–4.
Ciurea AV, Kapsalaki EZ, Comam TC, Roberts JL, Robinson JS 3rd, Tascu A, Brehar F, Fountas KN. Supratentorial epidural hematoma of traumatic etiology in infants. Childs Nerv Syst. 2007;23:335–41.
Servadei F, Piazza G, Seracchioli A, Acciarri N, Pozzati E, Gaist G. Extradural hematoma: an analysis of the changing characteristics of patients admitted from 1980 to 1986: diagnosis and therapeutic implication in 158 cases. Brain Inj. 1988;2:87–100.
McLauren R, Towbin R. Post- traumatic hematoma. In: Mclauren R, Schult L, Veres J, Episten E, editors. Pediatric Neurosurgery. 2nd ed. Philadelphia: Saunders; 1989. p. 277–89.
Ericson K, Hakansson S. Computed tomography of epidural hematomas: Association with intracranial lesions and clinical correlation. Acta Radiol (Diagn). 1981;22:513–9.
Illinogwrth R, Shawdon H. Conservative management of intracranial extradural hematoma presenting late. J Neurol Neurosurg Psychiatry. 1983;46:558–60.
Cayli S, Beskonakli E, Bestepe E, Okay O, Naldoken S, Taskin Y. Asymptomatic or minimally symptomatic traumatic epidural hematomas: comparison of the results of surgical and conservative management related to SPECT and neuropsychological tests: preliminary results. Neurosurgery Rev. 1998;21:226–331.
Pang D, Horton JA, Herron JM. Neurosurgical management of extradural hematomas in children. J Neurosurg. 1983;59:958–71.
Bullock MR, Chest R, Ghajar J, Gordon D, Hartle R, Newweu DW, et al. Guidelines for the surgical management of traumatic brain injury. Neurosurgery. 2006;53:327–9.
Pozzati E, Tognetti F. Spontaneous healing of acute extradural hematomas: Study of twenty-two cases. Neurosurgery. 1986;18(6):696–700.
Bhat AR, Raswan US, Kirmani AR. Intracranial extradural hematoma: Spontaneous rapid decompression- not resolution. J Pediatr Neurosci. 2015;10:266–9.
Knuckey NW, Gelbard S, Epstein MH. The management of asymptomatic epidural heatomas: a prospective study. J Neurosurg. 1989;70:392–6.
Acknowledgements
We thank Dr Aaliya fayaz for her typographical assistance
Funding
No funding was obtained for this study.
Author information
Authors and Affiliations
Contributions
SHA-conception, design of the work, KK-the creation of new software used in the work, MF the acquisition, analysis and interpretation of data, SSC-drafted the work.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was approved by institutional ethics committee of SKIMS. A written consent to participate in study was taken form the patient or next of kin.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Arif, S.H., Kareim, K., Fayaz, M. et al. Benign extradural haemorrhage: scope of conservative trial. Egypt J Neurosurg 39, 9 (2024). https://doi.org/10.1186/s41984-024-00267-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41984-024-00267-8