Abstract
Background
Increase pressure on arteries branching points and curves (hyperdynamic theory) is the most popular theory to explain the aneurysms formation that augmented by the observation of high incidence of anomalies (either A1 aplasia or hypoplasia) and the anterior communicating artery (ACoA) aneurysms. It still underestimated the correlation between these anatomical anomalies and aneurysm occurrence and its rupture. We aim to estimate the incidence and type of anatomical anomalies of the anterior cerebral circulation, including the A2 segment in patients with ACoA aneurysms and their predictive value for aneurysm occurrence and rupture parallel to the risk of hypertension. Also, we study the impact of these anomalies on the configuration of the aneurysm, including the neck and size.
Results
A1 hypoplasia and aplasia were significantly higher in AcoA aneurysms group than in the control group (P < 0.001 and 0.002, respectively). These anomalies have no significant statistical difference between rupture and unruptured ACoA aneurysms. A2 anomalies were insignificantly different between both groups.
Conclusion
Congenital anomalies in the A1 segment (hypoplasia and aplasia) have a significant predictive value for AcoA aneurysms formation, with no predictive value for the aneurysm rupture. Concomitantly, A2 anomalies have no significant risk for AcoA aneurysms formation and rupture.
Similar content being viewed by others
Introduction
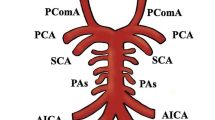
Anterior communicating (ACoA) complex considers a common site of cerebral aneurysms with an incidence of 30% [1]. This anatomical complex includes five main arterial vessels (right and left A1, right and left A2, and ACo artery), besides related branches: frontopolar, orbitofrontal, recurrent artery of Heubner and perforators [2].
Anomalies include aplasia or hypoplasia of the A1 segment or single and triple A2 segment of the anterior cerebral artery (ACA). Recent studies involving computational fluid dynamics modeling presented significant flow-dependent variations in formation of ACoA aneurysm [3, 4]. Castro et al. [5] showed an association between the variation of A1 artery blood flow, ACoA aneurysm development and rupture, they described the significance of asymmetric blood flow as a cause of aneurysm formation in animal models. Hashimoto et al. recorded an experimental model of ACoA aneurysm formation by inducing hemodynamic stress through a unilateral or bilateral carotid occlusion in hypertensive rats [6].
Sheering stress at the arterial wall can lead to activation of many molecular mechanisms which include nitric oxide synthase and apoptosis of smooth muscle cells in the vessel wall leading to aneurysm formation [7] also, providing similar changes by anatomical alternations in humans. Multiple studies described A1 segment anomalies as the one most commonly accompanying ACoA aneurysm, this frequency ranges from 41.33% up to 50% [8]. Bazowski et al. showed anomaly incidence of anterior communicating artery complexes harboring aneurysm at 37.7% [9]. Charbal et al. by using angiography, represent a significant association between anomalies of A1 segment of the ACA and ACoA aneurysm formation [10].
The correlation between aneurysm occurrence and its rupture risk in association to the anatomical anomalies is still underestimated. We look to estimate the incidence and types of anomalies of the anterior cerebral circulation, including the A2 segment in patients with ACoA aneurysms, and to explain their association with aneurysm occurrence and its rupture parallel to the risk of hypertension. We also studied the effect of these anomalies on the configuration of the aneurysm, including the aneurysm size and its neck.
Methods
This study included 210 patients with ACoA aneurysms diagnosed between January 2012 and December 2020 at two different departments (Regensburg University and Sohag University hospital).
ACoA aneurysms group: we included 210 patients with an established diagnosis of ACoA aneurysms (ACoA aneurysms group). These patients were further divided into ruptured (n = 148) and unruptured (n = 62) groups.
We excluded the cases with poor diagnostic cerebral angiography or 3D CT angiography (3D CTA) image quality, evidence of vasospasm, or incomplete catheter angiograms.
Control group: we included as a control group age and sex-matched, 486 patients with a negative study of ACoA aneurysm who underwent DSA and/or CTA for other medical indications during the same period.
We collected data on the following variables: age, sex, medical history (hypertension), and radiological findings. We got information regarding medical history from the patient’s medical reports.
Imaging and definitions: it gained imaging using a machine brand: Philips, model: incisive 128 slices, manufacturer name: Philips, country of origin: made in India, multi-row Computed Tomography using the following study parameters: 120 kV, 74 mA, 120 mA; rotation time: 0.75; slice thickness: 3 mm; pitch: 1.5. We injected intravenously patients with an iodine contrast medium to achieve angiographic images. We examined in three planes: coronal, sagittal, and transverse the maximum intensity (MIP) and volume rendering (VR) reconstructions. We carefully examined each part of the ACoA complex and measured the internal diameter of each artery.
Aneurysm size was determined based on the greatest diameter, while the sides were determined based on how the aneurysm was best represented on the angiogram and whether it was visible only from the ipsilateral side by the contrast agent.
We classified the ACoA complex anomalies into the following types: right A1 hypoplasia, left A1 hypoplasia, right A1 aplasia, left A1 aplasia, Azygous A2 and triple A2.
We classified as hypoplastic an arterial segment size less than 75% of the contralateral vessel or less than 1 mm in diameter. At least two senior physicians meticulously examined all images. If conflicting opinion on particular patients occurred, extraction from our sample was done (Fig. 1).
Statistical analysis: (SPSS v26, year: 2019, of IBM Inc, Chicago, IL, USA did statistical analysis). We presented quantitative variable number one as mean and standard deviation (SD) and compared between the two groups using unpaired Student's t-test. Number two as frequency and percentage (%) analyzing by using the Chi-square test or Fisher's exact test when appropriate. We used odds ratios (OR) to determine the statistical association between ACoA complex anomalies and aneurysm occurrence and its rupture. We considered statistically significant a two-tailed P value < 0.05.
Results
Demographic data: patients’ characteristics (age, sex and hypertension) were insignificantly different between ACoA aneurysms group and the control group (Table 1).
Anterior communicating artery (ACoA) complex anomalies and the risk of ACoA aneurysms formation: right A1 hypoplasia, left A1 hypoplasia, right A1 aplasia and left A1 aplasia were significantly higher in ACoA aneurysms group than the control group (P < 0.001, < 0.001, 0.001 and 0.002, respectively). A2 anomalies with different forms (azygous or triple A2) were insignificantly different in both ACoA aneurysms group and the control group (P 0.431) (Table 2) (Figs. 2, 3, 4).
Anterior communicating artery (ACoA) complex anomalies and the risk of aneurysm rupture: patients’ characteristics (age, sex and hypertension) were insignificantly different between unruptured group and rupture group (Table 3).
Congenital anomalies (A1 hypoplasia, A1 aplasia and A2 anomalies) were insignificantly different between unruptured group and rupture group (Table 4).
Impact of ACoA complex anomalies on the aneurysms morphology: in our study, no significant effect of the AcoA complex anomalies on the aneurysm configuration was observed regarding the aneurysm size and its neck (Table 5) (Fig. 5).
Discussion
The percentage of reports of the anterior communicating artery (ACoA) aneurysms in several studies is about 25% of all cerebral aneurysms. The high prevalence of aneurysms at this anatomical site, make it is crucial to assess the risk of aneurysm initiation at this site [11, 12].
Demographic distribution: we found that patients’ characteristics (age, sex and hypertension) were insignificantly different between ACoA aneurysms group and control group. Our findings were not parallel to Zhang et al. [13] who enrolled 160 cases with Acom aneurysms and 66 control subjects with no aneurysms. They found that women of 50 to 70 years were more vulnerable to ACoA aneurysm formation than men, so it significantly associated ACoA aneurysm with patient age. This difference could be justified by recruiting healthy patients and ethnic consideration.
Correlation between ACoA complex anomalies and the aneurysm formation: in the present study, right A1 hypoplasia left A1 hypoplasia, right A1 aplasia and left A1 aplasia were significantly higher in ACoA aneurysms group than control group (P < 0.001, < 0.001, 0.001 and 0.002, respectively) and A2 with its different forms was insignificantly different between both groups. In line with our finding, Rinaldo et al. [14] observed that of 145 patients who presented with aneurysmal subarachnoid hemorrhage after rupture of an ACoA aneurysm, 31 (21.4%) had a hypoplastic or absent A1 segment. He observed a high associated between hypoplastic or absent A1 segment and increased rate of radiologic infarction (OR = 2.54, 95% CI 1.02–6.43; P = 0.0466).
In a parallel line, Krzyżewski et al. [15] also reported a significantly higher incidence of hypoplastic A1 segment of the anterior cerebral artery (24% versus 7%; P < 0.01) and aplastic A1 segment of the anterior cerebral artery (12% versus 3%; P = 0.03) in a 50 patients with ACoA aneurysm and 100 patients sex- and age-matched control group. Concomitantly, they also reported A2 segment anomalies to be a potential predictor of ACoA aneurysm (6% versus 1%; P = 0.07). In a parallel line, frequency of A1 segment anomalies in aneurysm patients was 37.7% in a study done on Polish population [16].
Krasny et al. [17] observed 141 patients with aneurysm of the ACoA; aneurysm group (63%) showed variations (hypoplasia or aplasia) of the A1 segment in compared with 48 patients in the control group (24%) (P < 0.001). They reported a statistically significant relation between hypoplastic A1 and poor outcome (postoperative morbidity) [18].
Anomalies as a risk factor for aneurysm rupture: regarding patients’ characteristics, we found that age, sex and hypertension were insignificantly different between unruptured group and rupture group. However, we reported demographic factors, including sex and age, to associate them with rupture of intracranial aneurysms [19, 20]. Additionally, Jinjin et al. [21] found that patients with ruptured ACoA aneurysms were younger than those with unruptured ACoA aneurysms and Matsukawa et al. confirmed that [22]. They found that patients with ruptured ACoA aneurysms were significantly younger (a higher proportion were younger than 60 years of age); larger recruited sample size and different ethnic population could justify their different results. Ye et al. disagreed with our results by observing that higher proportion of patients with unruptured ACoA had hypertension than patients with ruptured ACoA (56.5% versus 19.5%, P = 0.002). Further, they reported that hypertension was a risk factor for aneurysm formation, growth and rupture [23].
In the present study, congenital anomalies (A1 hypoplasia, A1 aplasia and A2) were insignificantly different between unruptured group and rupture group. Consistent with our results, Stojanovi´c et al. [24] detected that 36% ruptured aneurysms, associated with hypoplasia of the A1 segment localized on the ACoA with no statistical significance, found between the hypoplasia of the A1 segment and a ruptured aneurysm.
Impact of ACoA complex anomalies on the aneurysm configuration: in the current study, irrelevant effect of the ACoA anomalies on the aneurysm size and neck, the most constant observation is that aneurysms in the anomalies bearing vessels have a trend toward a wide aneurysm neck (4 mm or more) and the aneurysm fundus directed towards the hypoplastic or aplastic vessels. These findings are in line with the hyperdynamic theory and Rhoton rules for the aneurysm formation.
Previous studies have shown a significant difference in the middle cerebral artery (MCA) bifurcation angle between patients with and without MCA aneurysms, showing that the MCA bifurcation angle plays an important role in MCA aneurysm formation. We have reported the ACoA aneurysms to be to associate with the smaller angle formed between the A1 and A2 segments of the anterior cerebral artery at the ACoA complex [25, 26]. Despite we observe this relation, we recommend a statistical study of the angle between A1 and A2 segment parallel to the origin of the recurrent artery of Heubner.
Conclusion
Anomalies of the A1 segment (hypoplasia, aplasia) have a highly valuable tool for prediction of ACoA aneurysms formation, with no significant risk for the aneurysm rupture. Anomalies of the A2 segment play no role in the development of ACoA aneurysms and subsequent rupture. Further, these anomalies have no effect on the aneurysm configuration apart from fundus direction toward the affected segment.
Availability of data and materials
Available.
Abbreviations
- ACoA:
-
Anterior communicating artery
- DCA:
-
Diagnostic cerebral angiography
- 3D CTA:
-
3D CT angiography
- MI:
-
Maximum intensity
- VR:
-
Volume rendering
- SD:
-
Standard deviation
- OR:
-
Odds ratios
- MCA:
-
Middle cerebral artery
- ACA:
-
Anterior cerebral artery
References
Feigin VL, Rinkel GJ, Lawes CM, Algra A, Bennett DA, van Gijn J, et al. Risk factors for subarachnoid hemorrhage: an updated systematic review of epidemiological studies. Stroke. 2005;36:2773–80.
Meling TR. Lawton’s seven aneurysms: tenets and techniques for clipping. Neurosurgery. 2011;68:E1774.
Inagawa T. Risk factors for the formation and rupture of intracranial saccular aneurysms in Shimane, Japan. World Neurosurg. 2010;73:155–64 (discussion e23).
Agrawal A, Jagetia A, Bodeliwala S, Singh D, Dutta G, Shah A. Intraoperative microsurgical anatomy of the anterior communicating artery complex harbouring an anterior cerebral territory aneurysm. Neurol India. 2019;67:823–8.
Castro MA, Putman CM, Cebral JR. Patient-specific computational fluid dynamics modeling of anterior communicating artery aneurysms: a study of the sensitivity of intra-aneurysmal flow patterns to flow conditions in the carotid arteries. Am J Neuroradiol. 2006;27:2061.
Aoki T, Nishimura M. The development and the use of experimental animal models to study the underlying mechanisms of CA formation. J Biomed Biotechnol. 2011;2011: 535921.
Sorokin V, Vickneson K, Kofidis T, Woo CC, Lin XY, Foo R, et al. Role of vascular smooth muscle cell plasticity and interactions in vessel wall inflammation. Front Immunol. 2020;11:3053.
Jabbarli R, Reinhard M, Roelz R, Kaier K, Weyerbrock A, Taschner C, et al. Clinical relevance of anterior cerebral artery asymmetry in aneurysmal subarachnoid hemorrhage. J Neurosurg. 2017;127:1070–6.
Chen J, Li M, Zhu X, Chen Y, Zhang C, Shi W, et al. Anterior communicating artery aneurysms: anatomical considerations and microsurgical strategies. Front Neurol. 2020;11:1020.
Kancheva AK, Velthuis BK, Ruigrok YM. Imaging markers of intracranial aneurysm development: a systematic review. J Neuroradiol. 2021;49:219.
Ujiie H, Liepsch DW, Goetz M, Yamaguchi R, Yonetani H, Takakura K. Hemodynamic study of the anterior communicating artery. Stroke. 1996;27:2086–93 (discussion 94).
Brilstra EH, Rinkel GJ, van der Graaf Y, van Rooij WJ, Algra A. Treatment of intracranial aneurysms by embolization with coils: a systematic review. Stroke. 1999;30:470–6.
Zhang XJ, Gao BL, Hao WL, Wu SS, Zhang DH. Presence of anterior communicating artery aneurysm. We associated it with age, bifurcation angle, and vessel diameter. Stroke. 2018;49:341–7.
Rinaldo L, McCutcheon BA, Snyder KA, Porter AL, Bydon M, Lanzino G, et al. A1 segment hypoplasia associated with cerebral infarction after anterior communicating artery aneurysm rupture. J Neurosurg Sci. 2019;63:359–64.
Krzyżewski R, Tomaszewska I, Lorenc N, Kochana M, Goncerz G, Klimek-Piotrowska W, et al. Variations of the anterior communicating artery complex and occurrence of anterior communicating artery aneurysm: A2 segment consideration. Folia Med Cracov. 2014;54:13–20.
Klimek-Piotrowska W, Kopeć M, Kochana M, Krzyżewski RM, Tomaszewski KA, Brzegowy P, et al. Configurations of the circle of Willis: a computed tomography angiography based study on a Polish population. Folia Morphol. 2013;72:293–9.
Krasny A, Nensa F, Sandalcioglu IE, Goricke SL, Wanke I, Gramsch C, et al. Association of aneurysms and variation of the A1 segment. J Neurointerv Surg. 2014;6:178–83.
Alawamry AME, Taha MM, Abdelbary TH, Bessar AA, Farid M. Role of preoperative computed tomography angiographic anatomical considerations and their intraoperative interpretations in prediction of outcome in microsurgical clipping of ruptured anterior communicating aneurysm. Egypt J Neurosurg. 2021;36:5.
Greving JP, Wermer MJ, Brown RD Jr, Morita A, Juvela S, Yonekura M, et al. Development of the PHASES score for prediction of risk of rupture of intracranial aneurysms: a pooled analysis of six prospective cohort studies. Lancet Neurol. 2014;13:59–66.
Aarhus M, Helland CA, Wester K. Differences in anatomical distribution, gender, and sidedness between ruptured and unruptured intracranial aneurysms in a defined patient population. Acta Neurochir. 2009;151:1569–74.
Liu J, Chen Y, Lan L, Lin B, Chen W, Wang M, et al. Prediction of rupture risk in anterior communicating artery aneurysms with a feed-forward artificial neural network. Eur Radiol. 2018;28:3268–75.
Matsukawa H, Uemura A, Fujii M, Kamo M, Takahashi O, Sumiyoshi S. Morphological and clinical risk factors for the rupture of anterior communicating artery aneurysms. J Neurosurg. 2013;118:978–83.
Xia N, Liu Y, Zhong M, Zhuge Q, Fan L, Chen W, et al. Smoking associated with increased aneurysm size in patients with anterior communicating artery aneurysms. World Neurosurg. 2016;87:155–61.
Stojanović NN, Kostić A, Mitić R, Berilažić L, Radisavljević M. Association between circle of Willis configuration and rupture of cerebral aneurysms. Medicina. 2019;55:338.
Sadatomo T, Yuki K, Migita K, Imada Y, Kuwabara M, Kurisu K. Differences between middle cerebral artery bifurcations with normal anatomy and those with aneurysms. Neurosurg Rev. 2013;36:437–45.
Ingebrigtsen T, Morgan MK, Faulder K, Ingebrigtsen L, Sparr T, Schirmer H. Bifurcation geometry and cerebral artery aneurysms. J Neurosurg. 2004;101:108–13.
Acknowledgements
Not applicable.
Funding
No.
Author information
Authors and Affiliations
Contributions
Author contributions to the study and manuscript preparation include the following. KMS: conception and design; BGH: acquisition of data; AKK: analysis and interpretation of data; MAA: drafting the article. All authors: critically revising the article and reviewed submitted version of manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Ethics approval is obtained at Sohag faculty of medicine ethical committee in June 2020 and consent to participate is obtained for all patients.
Consent for publication
Consent for publication is obtained from the authors.
Competing interests
The authors report no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hassanin, B.G., Abdelhameid, A.K., Abbas, M.A. et al. Predictive value of the anterior communicating artery (ACoA) complex variations for the incidence and rupture of ACoA aneurysms. Egypt J Neurol Psychiatry Neurosurg 59, 158 (2023). https://doi.org/10.1186/s41983-023-00758-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41983-023-00758-9