Abstract
Background
Multiple sclerosis (MS) is widely known to cause brain atrophy due to neurodegeneration. Neuroimaging investigations revealed a direct link between brain atrophy detected by MRI and impairment. Transcranial sonography is a safe, feasible and cost-effective imaging technique. In this study, we aimed to validate B-mode transcranial sonography (TCS) as a marker of neurodegeneration in MS. This study is the first study directed to set cut-off point for brain parenchyma measurement in Egyptian population. An observational, case–control study, conducted on 125 subjects; divided into 2 groups: first group, 71 healthy volunteers and patients’ group (54 patients) with relapsing–remitting multiple sclerosis (RRMS). All studied subjects were assessed using B-mode TCS. Transcranial sonography findings were categorized into: first, assessment of brain atrophy parameters by measuring diameter (cm) of third ventricle and both frontal horns of lateral ventricles. Then, the echogenicity of thalamus and brainstem structures (substantia nigra (SN), red nucleus) planimetric surface area (cm2) were assessed along with their abnormal echogenicity (cm2), while brainstem raphe was assessed semi-quantitatively for its echogenicity (intact or interrupted).
Results
Quantitative measures of brain atrophy: in normal control group the third ventricle diameter mean was 0.2 cm (± 0.08), right and left frontal horn diameter were 0.3 cm (± 0.13) and 0.28 cm (± 0.1), respectively, brain atrophy parameters could differentiate between MS patients and normal control group as a statistically significant (p< 0.001) larger ventricular diameter was found in MS patients. On the other hand, assessment of brain stem structures and thalamus showed no statistical significance between MS patients and normal control except in surface area of both red nuclei.
Conclusions
Brain parenchymal sonography may be used as a tool in assessment of neurodegeneration in MS patients.
Similar content being viewed by others
Background
Transcranial sonography (TCS) has long been regarded as a tool used exclusively to monitor hemodynamics of cerebral circulation [1]. That is why its application in displaying brain parenchyma was met with a degree of doubt, until it was proven and reproduced by many investigators in different neurodegenerative disorders, especially Parkinson disease (PD) and other atypical Parkinsonian syndromes [2]. The first consensus guidelines on the standardized procedure for B-mode TCS of the brain parenchyma were documented by the European Society of Neuro-sonology and Cerebral Hemodynamics (ESNCH) during their 9th Meeting in Wetzlar, Germany [3].
Using B-mode TCS of brain parenchyma, the ventricular system can be displayed, and its diameters measured, in addition to monitoring echogenicity and the surface area of basal ganglia (caudate, lentiform, putamen and thalamus), midbrain structures (substantia nigra, red nucleus, brainstem raphe) and the dentate nucleus of the cerebellum [3, 4]. This can be done in a reliable, cost-effective, and non-invasive way, allowing for frequent follow-ups without the fear of repeated radiological exposures [5].
In this study, we aim to validate B-mode transcranial sonography (TCS) of the brain parenchyma as a marker of brain atrophy, and to establish the cut-off point of the ventricular diameter and parenchymal echogenicity (substantia nigra, red nucleus and thalami) in the Egyptian population.
Methods
The current study is an observational, case–control study conducted on 125 subjects; age range from 18 to 50 with both males and females being included. The study population was divided into 2 groups: the first (patients) group represents (54 adult Egyptian patients with relapsing–remitting multiple sclerosis (RRMS), according to the Revised-McDonald’s criteria 2010 [6], recruited from the MS unit of the Neurology Department at Kasr AlAiny Hospital, Cairo University. Their ages ranged from 18 to 50 years (29.22 ± 8.15 SD) and included both sexes. The second group included 71 patients aged between 19 and 50 years (30.7 ± 7.6 SD), sex-matched healthy volunteers, enrolled from family members or friends of the probands, the hospital staff or patients admitted at other Hospital Departments, as Ophthalmology Department; meeting the selection criteria as a control group. The study took place from April 2015 to November 2015. All the participants gave their informed written consent to participate in the study and the research was approved by research ethics committee of Faculty of Medicine, Cairo University. The MS group included patients with a diagnosis of MS of the relapsing–remitting subtype (RRMS) [6]. Patients included should be in remission for at least one month prior to evaluation. Patients with progressive forms of MS, either primary or secondary progressive subtypes, were excluded from this study.
Participants were subjected to: clinical assessment, including neuro-status evaluation, using modified Expanded Disability Status Scale (EDSS) (for the MS patients’ group) done by a certified EDSS rater. Radiological assessment: using B-mode transcranial sonography (TCS) for brain parenchyma. Subjects enrolled were 125, those who completed study to the end were 105 participants (Fig. 1).
The TCS studies took place in the Neurosonology Unit in the Neurology Department, Cairo University and were performed by a single experienced certified neurosonographer (European Society of Neuro-sonology and Cerebral Hemodynamics “ENSCH”), who was blind to the subjects’ clinical data throughout the entire study. The studies were carried out using a high-resolution ultrasonography instrument (PHILIPS IU22 x MATRIX, California, US, L 1-5 transducer, equipped with a 2.5 MHz Phased array transducer). It should be stressed that system-specific image-processing technologies influence distinct measurements such as the assessment of echogenic areas of small brain structures. That is why normal ranges, especially for substantia nigra (SN) echogenic areas, need to be obtained separately for each ultrasound system.
The system was adjusted according to the ultrasound system settings for brain parenchyma assessment using TCS recommended in Walter and Školoudík [4]. We applied tissue harmonic imaging (THI), rather than the conventional imaging mode, to increase the tissue contrast and therefore enable an easier delineation of small echogenic structures—the SN. Via the transtemporal window, the sonographic assessment was carried out in 2 planes: the mesencephalic brainstem plane and ventricular plane (plane of the thalamus) [4]. The mesencephalic brainstem plane which is the quality criterion of adequate midbrain visualization is the butterfly-shaped, midbrain transection surrounded by the highly echogenic basal cisterns and is completely displayed, as shown in (Fig. 2). Here the following structures are visible: brainstem raphe (BR), substantia nigra (SN), and red nucleus (RN) (Figs. 2, 3 and 4), while in the ventricular plane (plane of the thalamus) landmark structure is represented by the highly echogenic pineal gland, due to its calcification (Fig. 5), and structures assessed are the bilateral thalami, the third ventricle and bilateral frontal horns.
Transcranial sonography findings were categorized into: i. assessment of brain atrophy parameters by measurement of diameter (cm) including third ventricle and both frontal horns of lateral ventricles. ii. assessment of the echogenicity for brainstem structures (SN, red nucleus) and thalamus (TH), which were assessed for their planimetric surface area (cm2) [3, 4] along with their abnormal echogenicity (cm2). In addition, brainstem raphe was assessed semi-quantitatively for its echogenicity (intact or interrupted).
Rating of echogenicity (normal or hyperechogenic): to rate SN echogenicity the 75th and 90th percentile of measures in healthy control volunteers were used as a reference, the larger of bilaterally measured SN echogenic sizes was used for classification according to the consensus guidelines as follows: normal echogenic: measured area is < the 75th percentile. Moderately hyperechogenic (H1): measured area is between the 75th and the 90th percentile. And markedly hyperechogenic (H2): measured area is ≥ the 90th percentile [3].
On the other hand, to mark SN echogenicity as reduced (SN hypoechogenicity), we used the 10th percentile of bilateral sum of SN surface area healthy control volunteer as a cut-off point. Values below this cut-off point were regarded as SN hypoechogenicity [7,8,9]. The reason for using the sum of bilateral measurement (SNsA) in cases of SN hypoechogenicity was attributed to limitation of image resolution, when very small echogenic signals of SN were present. The value for both RN and thalamic hyperechogenicity were set in the same way as that used with SN hyperechogenicity, using the 75th and 90th percentiles [3].
Rating the echogenicity of brainstem raphe (semi-quantitatively) was done according to the consensus guideline that recommends the discrimination of only of two grades (normal and reduced echogenicity) [3], as follows: normal (intact: visualized as a highly echogenic continuous line with an echogenicity identical to that of the adjacent red nucleus) and reduced: the echogenic line of the brainstem raphe is either interrupted or not visible at bilateral insonation.
Statistical analysis: according to Walter and colleagues study (2009), the mean difference between the two groups was 1.7 and SD for the third ventricle width was 2.8 and 2.4 mm for patients and controls, respectively. Considering a level of significance of 95% and power of 90%, the minimum sample size required is 104 patients. A drop out of 20% was considered to give the final required sample size of 125. Statistical Package for Social Sciences (SPSS), version 22.0, was used for data management and data analysis. Mean ± standard deviation described quantitative variables (numbers were approximated to next 2 decimals) and median with range when appropriate (distribution did not follow normality). Number and percentages described qualitative data using the Chi-square or Fisher exact tested proportion independence. For comparing mean values of 2 independent groups, parametric and non-parametric T test were used. P value is always 2-tailed and significant at 0.05 level. Correlation analysis was used to show magnitude of association between numerical variables (TCS measures and different cognitive and disability measures). One way to summarize the validity of a continuous numerical data (TCS dimensions) to discriminate MS cases from healthy control is to plot sensitivity against 1—specificity for different cut-off values. The resulting curve is called the receiver operating characteristic (ROC) curve; ROC analysis was done to obtain cut-off points for ventricular diameters, radiologically discriminating between patients with MS and healthy control in order to use it for prediction of physical disability in patients with MS as a variable in the logistic regression equation.
Results
Quantitative measures of brain atrophy in normal control group: third ventricle diameter mean was 0.2 cm (SD 0.08), right frontal horn (FH) diameter was 0.3 cm (SD 0.13) and left frontal horn (FH) diameter was 0.28 cm (SD 0.1).
Surface area of deep grey matter (DGM) (assessed using planimetric method) in normal control group: thalamic measures; right thalamus surface area mean was 4.4 cm2 (SD 0.97) and left thalamus surface area mean was 4.7 cm2 (SD 1.24); substantia nigra measures: right substantia nigra surface area mean was 0.15 cm2 (SD 0.05) and left substantia nigra surface area mean was 0.15 cm2 (SD 0.06); and red nucleus measures: right red nucleus surface area mean was 0.05 cm2 (SD 0.03) and left red nucleus surface area mean was 0.06 cm2 (SD 0.03).
It is worth mentioning that within the normal control group, there was inter-side significant positive correlation between different parameters, either for right and left FH diameters (r = 0.6, p < 0.0001) or for DGM echogenic sizes (Rt and Lt SN: r = 0.63, p < 0.0001, Rt and Lt RN: r = 0.56, p < 0.0001, Rt. and Lt. Th.: r = 0.45, p < 0.0001) suggesting reliability of the measurement. Age at onset of MS symptoms; in patients included in this study; ranged from 12 to 46 with median 21.5 and mean 23.3 (SD 7.37), disease duration ranged from 0.25 to 22 with median 4.74 and mean 5.94 (SD 4.47); the total number of attacks ranged from 1 to 415 with median 3 and mean 4.17 (SD 2.89), mean EDSS was 2.34 (SD 1.12).
The MS patients’ group demonstrated larger ventricular diameter than the control group across the measurement of the 3 ventricular diameters, indicating more occurrence of brain atrophy. The inter-group difference was statistically significant. However, assessment of brainstem structures (SN, red nucleus) and thalamus, by planimetric surface area (cm2) showed only the surface area of both red nuclei and Right SN differed significantly between MS patients’ group and control group, with MS patients displaying a larger surface area of these structures (Table 1).
Diameter of the left FH showed significant positive correlation with the disease duration and total EDSS. Otherwise, neither age, age at onset, disease duration nor total EDSS showed any significant correlation with other parameters of brain atrophy (Table 2). Significant positive correlation was found between the cerebral functional system (FS) and the diameter of the third ventricle, while the pyramidal and bowel, and bladder functional steps were positively correlated to the diameter of the Left FH (Table 3).
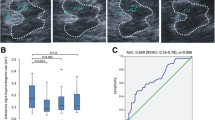
ROC curve analysis: ROC analysis for the third ventricular diameter showed an Area Under Curve (AUC) of 0.81 [95% confidence interval (CI) 0.72–0.89] and it was set at above a cut-off point of 0.23 cm; the third ventricular diameter was capable of discriminating between RRMS and healthy control with a sensitivity of 81.6%, specificity of 71.4%, with a positive predictive value (PPV) of 71.4% and a negative predictive value (NPV) of 81.6% (p < 0.001*).
ROC analysis for the diameter of the right FH showed an AUC of 0.87 [95% confidence interval (CI) 0.80–0.94] and it was set that above a cut-off point of 0.36 cm; the diameter of the Right FH can discriminate between RRMS and healthy control subjects with a sensitivity of 93.8%, specificity of 71.4%, PPV of 73.8% and a NPV of 93.0% (p < 0.001*).
ROC analysis for the diameter of the left FH showed an area under curve (AUC) of 0.85 [95% confidence interval (CI) 0.77–0.92] and it was set that above a cut-off point of 0.37 cm, the diameter of left FH can discriminate between RRMS and healthy control subjects with a sensitivity of 72.9%, specificity of 80.4%, PPV of 76.1% and NPV of 77.6% (p < 0.001*).
Echogenicity of the grey matter: the cut-off values for echogenicity of the 3 DGM structures (SN, RN and thalamus) were defined first in the control subjects. This was done at 2 levels; > 75th percentile and > 90th percentile per the larger side of bilateral measurement, to classify the structure as moderately hyperechogenic (H1 = 75–90th percentiles) or markedly hyperechogenic (H2 ≥ 90th percentile) (Table 4).
Then, upon comparison of these values between patients and control subjects, the only significant difference was for the RN, at both > 75th and 90th percentile, indicating that patients with MS displayed a greater occurrence of both moderate and marked RN hyperechogenicity than the control group (Table 5).
The cut-off value of SN hypoechogenicity was set in the healthy control subjects at a value < 10th percentile of sum of bilateral SN surface area, which was 0.15 cm2. Values below this cut-off point are considered to represent SN hypoechogenicity. Within the group of MS patients, only 2 displayed SN hypoechogenicity (4.17%), whereas 6 control subjects displayed this echo-feature (10.53%).
As for echogenicity of brain stem raphe, the inter-group difference was statistically significant, with 7 MS patients showing interrupted raphe (14.6%), with none of the control subjects displaying the same echo-feature (p = 0.002).
Discussion
The application of TCS in displaying brain parenchyma has been verified in several neurodegenerative diseases [2,3,4]. Furthermore, the application of B-mode TCS of the brain parenchyma has been established. The reliability of the TCS measurements was confirmed by the presence of significant inter-side positive correlation between the paired structures in the control group. The transtemporal window was sufficient in all subjects assessed, owing to their age. This is in favor of the use of TCS in patients with MS, thus permit evaluation of parameters of brain atrophy and neurodegeneration.
Significant brain atrophy was found in patients with MS, even early in the disease course and with relatively mild physical disability emphasizing the hidden neurodegenerative component of MS pathology reported to occur as early as in the first clinical event (CIS) [10]. These findings are in concordance with other several studies which documented ventricular enlargement in early MS, using either MRI or TCS [11,12,13].
Affection of the DGM during early MS has been described in literature [10, 14,15,16,17]. The apparent WM affection seen with conventional magnetic resonance imaging (MRI) could only partially explain the degree of disability (both motor and non-motor). However, the pathology affecting normal appearing grey matter (NAGM) and normal appearing white matter (NAWM) was unmasked by more sophisticated MRI sequences [18, 19] TCS [20] and by histopathology [21].
In the present study, patients with MS displayed significantly increased echogenicity of right and left RN and right SN. Likewise, Haider and Colleagues [21] have denoted that hypothalamus was the most prominent DGM to be affected by focal demyelination and that thalamus was more prominently affected by neurodegeneration, whereas caudate nucleus suffered both processes. Similarly, the affection of other DGM as SN and RN, which was only marginally addressed in few studies in MS, could be interpreted [22].
The pathological processes involving SN and RN, in terms of increase of their planimetric size and consequently hyperechogenicity, reflect excess iron deposition [23, 24]. This supports the idea of iron having a role in neurodegeneration in MS and may explain our findings of significant RN hyperechogenicity in MS patients in comparison to the control group. This may represent a useful application of TCS in monitoring early and even pre-clinical conversion of clinical isolated syndrome (CIS) to clinical definite multiple sclerosis (CDMS), before the occurrence of a second clinical event, which may warrant early introduction of disease modifying therapy. Another possible hypothesis includes a compensatory trial to overcome the affection of pyramidal tract through adaptive reorganization of the red nucleus connections [25].
In this study, the lack of significant apparent thalamic atrophy in MS patients, in comparison to the control, is somewhat misleading, as indirect evidence for the presence of thalamic atrophy is the significant third ventricular enlargement in MS patients relative to the healthy controls. Although thalamic atrophy was reported in different studies, even since the early phases of MS, this was assessed using MRI [26, 27]. This may suggest that the assessment of thalamus using TCS is not the best option for detecting its atrophy and that MRI-based assessment may be more informative.
Since visually based semi-quantitative measurement may be subjective to a large degree of variability and that planimetric method is much more objective, we aimed to define our own cut-off points. Our results calculated a cut-off of SN moderate and marked hyperechogenicity ≥ 0.19 and 0.22 cm2, respectively. This is close to other research group cut-off values using similar Phillips system, who reported ≥ 0.20 and ≥ 0.27 cm2 for moderate and marked hyperechogenicity, respectively [28,29,30].
Because hyperechogenicity of RN and thalamus were not assessed by planimetric method before with no cut-off values found for them in literature, we calculated a cut-off value for moderate and marked hyperechogenicity of both RN and thalamus. This highlights that the current work not only represents the first TCS study of brain parenchyma in the Egyptian population, but also the first planimetric assessment of echogenicity of RN and thalamus with cut-off values.
Brainstem raphe consists of multiple nuclei (mainly serotonergic, which are part of the infra-tentorial GM; our results showed that interrupted raphe was found in 14.6% of MS patients. The TCS finding of interrupted raphe was described in 50–70% of patients with depressive disorders as a reflection of central serotonergic system alteration [31]. However, in our study, depression was an exclusion criterion. Hence, loss of serotonergic neurons in the rostral nuclear complex of the raphe is believed to be secondary to pathological changes taking place in the target areas of these projections [32, 33].
Diameter of the left FH showed significant positive correlation to the disease duration, total EDSS, pyramidal and bowel and bladder functional steps; whereas diameter of 3rd ventricle showed significant positive correlation to the cerebral functional system score. This denotes the relation between brain atrophy and physical disability even in the relapsing–remitting stage. Also, Sailer and colleagues [33] noted the presence of focal atrophy in the bilateral frontal and temporal cortices early in the disease course and additionally in the motor cortex in patients with more advanced disease [34], which is in concordance with our results of significant correlation of FH width to the disease duration. Correlation of ventricular diameters to physical disability was reported in different studies, either using TCS [13, 20, 35, 36] or MRI [37, 38]. Some of these studies have correlated ventricular diameters as assessed by TCS to that assessed by MRI and found strong significant correlation between both methods.
Abnormalities in the WM may precede clinical onset by several years, while those in the GM appear to coincide more closely with first symptom occurrence. They may explain the correlation of the FH to the disease duration and not the 3rd ventricle based on more proximity of the frontal horn to the corpus callosum, which is commonly and early affected in MS [39]. However, the exact pattern of atrophy evolution in MS is not exactly clear, with conflicting results among different research groups, mostly due to different patient populations, sample sizes and the techniques used [11].
In conclusion, TCS can help in early detection of structural brain changes in MS patients, allowing for better assessment and management plan. It may be used as a quick and easy tool for frequent routine follow-up in this type of chronic patients.
Availability of data and materials
The corresponding author takes full responsibility for the data, has full access to all the data; and has the right to publish any and all data separate and apart from any sponsor.
Abbreviations
- TCS:
-
Transcranial sonography
- MS:
-
Multiple sclerosis
- SN:
-
Substantia nigra
- PD:
-
Parkinson disease
- ESNCH:
-
European Society of Neuro-sonology and Cerebral Hemodynamics
- RRMS:
-
Relapsing–remitting multiple sclerosis
- THI:
-
Tissue harmonic imaging
- EDSS:
-
Expanded Disability Status Scale
- BR:
-
Brainstem raphe
- RN:
-
Red nucleus
- TH:
-
Thalamus
- ROC:
-
Receiver operating characteristic
- FH:
-
Frontal horn
- DGM:
-
Deep grey matter
- AUC:
-
Area under curve
- CI:
-
Confidence interval
- MRI:
-
Magnetic resonance imaging
- NAGM:
-
Normal appearing grey matter
- NAWM:
-
Normal appearing white matter
- CIS:
-
Clinical Isolated Syndrome
- CDMS:
-
Clinical Definite Multiple Sclerosis
References:
Levi C, Selmes C, Chambers B. Transcranial ultrasound: clinical applications in cerebral ischaemia. Aust Prescr. 2001;24:137–40.
Berg D, Becker G, Zeiler B, Tucha O, Hofmann E, Preier M, et al. Vulnerability of the nigrostriatal system as detected by transcranial ultrasound. Neurology. 1999;53:1026–31.
Walter U, Behnke S, Eyding J, Niehaus L, Postert T, Seidel G, et al. Transcranial brain parenchyma sonography in movement disorders: state of the art. Ultrasound Med Biol. 2007;33:15–25.
Walter U, Školoudík D. Transcranial sonography (TCS) of brain parenchyma in movement disorders: quality standards, diagnostic applications and novel technologies. Ultraschall der Medizin Eur J Ultrasound. 2014;35:322–31.
Stern MB. Introductory remarks on the history and current applications of TCS. Int Rev Neurobiol. 2010;90:2–5.
Polman CH, Reingold SC, Banwell B, Clanet M, Cohen JA, Filippi M, Fujihara K, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol. 2011;69:292–302.
Godau J, Schweitzer KJ, Liepelt I, Gerloff C, Berg D. Substantia nigra hypoechogenicity: definition and findings in restless legs syndrome. Mov Disord. 2007;22:187–92.
Godau J, Wevers A-K, Gaenslen A, Di Santo A, Liepelt I, Gasser T, et al. Sonographic abnormalities of brainstem structures in restless legs syndrome. Sleep Med. 2008;9:782–9.
Schmidauer C, Sojer M, Seppi K, Stockner H, Högl B, Biedermann B, et al. Transcranial ultrasound shows nigral hypoechogenicity in restless legs syndrome. Ann Neurol. 2005;58:630–4.
Dalton CM. Early development of multiple sclerosis is associated with progressive grey matter atrophy in patients presenting with clinically isolated syndromes. Brain. 2004;127:1101–7.
Bermel RA, Bakshi R. The measurement and clinical relevance of brain atrophy in multiple sclerosis. Lancet Neurol. 2006;5:158–70.
Muller M, Esser R, Kotter K, Voss J, Muller A, Stellmes P. Third ventricular enlargement in early stages of multiple sclerosis is a predictor of motor and neuropsychological deficits: a cross-sectional study. BMJ Open. 2013;3:e003582–e003582.
Muller M, Esser R, Kötter K, Voss J, Muller A, Stellmes P. Width of 3. Ventricle: reference values and clinical relevance in a cohort of patients with relapsing remitting multiple sclerosis. Open Neurol J. 2013;7:11–6.
Calabrese M, Reynolds R, Magliozzi R, Castellaro M, Morra A, Scalfari A, et al. Regional distribution and evolution of gray matter damage in different populations of multiple sclerosis patients. PLoS ONE. 2015;10:1–12.
Fisher E, Lee J-C, Nakamura K, Rudick RA. Gray matter atrophy in multiple sclerosis: a longitudinal study. Ann Neurol. 2008;64:255–65.
Geurts JJ, Barkhof F. Grey matter pathology in multiple sclerosis. Lancet Neurol. 2008;7:841–51.
Fisniku LK, Chard DT, Jackson JS, Anderson VM, Altmann DR, Miszkiel KA, et al. Gray matter atrophy is related to long-term disability in multiple sclerosis. Ann Neurol. 2008;64:247–54.
Chard D, Miller D. Grey matter pathology in clinically early multiple sclerosis: evidence from magnetic resonance imaging. J Neurol Sci. 2009;282:5–11.
Walter U, Wagner S, Horowski S, Benecke R, Zettl UK. Transcranial brain sonography findings predict disease progression in multiple sclerosis. Neurology. 2009;73:1010–7.
Haider L, Simeonidou C, Steinberger G, Hametner S, Grigoriadis N, Deretzi G, et al. Multiple sclerosis deep grey matter: the relation between demyelination, neurodegeneration, inflammation and iron. J Neurol Neurosurg Psychiatry. 2014;85:1386–95.
Stankiewicz J, Panter SS, Neema M, Arora A, Batt CE, Bakshi R. Iron in chronic brain disorders: Imaging and neurotherapeutic implications. Neurotherapeutics. 2007;4:371–86.
Hill MJ. Iron and proteins of iron metabolism in the central nervous system. In: Ponka P, Schulman HM, Woodworth RC, Richter GW, editors. Iron transport and storage illustrate. Milton Park: Taylor and Francis; 1990. p. 316–27.
Blazejewska AI, Al-Radaideh AM, Wharton S, Lim SY, Bowtell RW, Constantinescu CS, Gowland PA. Increase in the iron content of the substantia nigra and red nucleus in multiple sclerosis and clinically isolated syndrome: a 7 Tesla MRI study. J Magn Reson Imaging. 2015;41:1065–70.
Jang SH, Kwon HG. Change of neural connectivity of the red nucleus in patients with striatocapsular hemorrhage: a diffusion tensor tractography study. Neural Plast. 2015;2015:10–2.
Henry RG, Shieh M, Amirbekian B, Chung S, Okuda DT, Pelletier D. Connecting white matter injury and thalamic atrophy in clinically isolated syndromes. J Neurol Sci. 2009;282:61–6.
Houtchens MK, Benedict RHB, Killiany R, Sharma J, Jaisani Z, Singh B, et al. Thalamic atrophy and cognition in multiple sclerosis. Neurology. 2007;69:1213–23.
Huang Y-W, Jeng J-S, Tsai C-F, Chen L-L, Wu R-M. Transcranial imaging of substantia nigra hyperechogenicity in a Taiwanese cohort of Parkinson’s disease. Mov Disord. 2007;22:550–5.
Kim JY, Kim ST, Jeon SH, Lee WY. Midbrain transcranial sonography in Korean patients with Parkinson’s disease. Mov Disord. 2007;22:1922–6.
Mehnert S, Reuter I, Schepp K, Maaser P, Stolz E, Kaps M. Transcranial sonography for diagnosis of Parkinson’s disease. BMC Neurol. 2010;10:9.
Becker G, Becker T, Struck M, Lindner A, Burzer K, Retz W, et al. Reduced echogenicity of brainstem raphe specific to unipolar depression: a transcranial color-coded real-time sonography study. Biol Psychiatry. 1995;38:180–4.
Stüber C, Pitt D, Wang Y. Iron in multiple sclerosis and its noninvasive imaging with quantitative susceptibility mapping. Int J Mol Sci. 2016;17:100.
Rub U, Del Tredici K, Schultz C, Thal DR, Braak E, Braak H. The evolution of Alzheimer’s disease-related cytoskeletal pathology in the human raphe nuclei. Neuropathol Appl Neurobiol. 2000;26:553–67.
Sailer M, Fischl B, Salat D, Tempelmann C, Schönfeld Ariel M, Busa E, et al. Focal thinning of the cerebral cortex in multiple sclerosis. Brain. 2003;126:1734–44.
Berg D, Mäurer M, Warmuth-Metz M, Rieckmann P, Becker G. The correlation between ventricular diameter measured by transcranial sonography and clinical disability and cognitive dysfunction in patients with multiple sclerosis. Arch Neurol. 2000;57:1289–92.
Kallmann B-A, Sauer J, Schließer M, Warmuth-Metz M, Flachenecker P, Becker G, et al. Determination of ventricular diameters in multiple sclerosis patients with transcranial sonography (TCS). J Neurol. 2004;251:30–4.
Miller DH. Measurement of atrophy in multiple sclerosis: pathological basis, methodological aspects and clinical relevance. Brain. 2002;125:1676–95.
Radue EW, Bendfeldt K, Mueller-Lenke N, Magon S, Sprenger T. Brain atrophy: an in-vivo measure of disease activity in multiple sclerosis. Swiss Med Wkly. 2013;143:1–11.
Taylor JS, Reddick W. Dynamic contrast-enhanced mr imaging in musculoskeletal tumors. In: Jackson A, Buckley DL, Parker GJM, editors. Dynamic contrast-enhanced magnetic resonance imaging in oncology. Berlin: Springer; 2005. p. 215–37.
Ajitomi S, Fujimori J, Nakashima I. Usefulness of two-dimensional measurements for the evaluation of brain volume and disability in multiple sclerosis. Mult Scler J Exp Transl Clin. 2022;8(1):20552173211070748.
Acknowledgements
Not applicable.
Funding
No funds were received to fulfill this work.
Author information
Authors and Affiliations
Contributions
NAK: designed and conceptualized the study, drafting the manuscript, performing the ultrasound. SH: study conception, design and final revision of manuscript. HA: study conception and design and final revision of manuscript. NS: material preparation and data collection. AO: conception of the work, drafting the manuscript. MM: conception of the work, manuscript revision, data collection, paper submission. All authors have agreed to conditions noted on the Authorship Agreement Form and have read and approved the final version submitted. The content of the manuscript has not been published, or submitted for publication elsewhere.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All procedures performed in the study were in accordance with the ethical standards of the Faculty of Medicine, Cairo University research and ethical committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. We obtained approval from research ethics committee of Faculty of Medicine, Cairo University on 17/11/2014. Written informed consent was obtained from participants for participation. We obtained approval from research ethics committee.
Consent for publication
Not applicable.
Competing interests
None of the authors has any competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kishk, N.A., Hamdy, S., Amer, H. et al. Validation of brain parenchyma sonography as a marker of neurodegeneration in multiple sclerosis. Egypt J Neurol Psychiatry Neurosurg 59, 134 (2023). https://doi.org/10.1186/s41983-023-00732-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41983-023-00732-5