Abstract
Background
The aim was to evaluate the protective effect of hydroalcoholic extract of Anacyclus pyrethrum root (APE) against pentylenetetrazole (PTZ) drug which is used for inducing epileptic seizures in animal model.
Results
50 male rats were divided: control (without any intervention), positive control 1st (received PTZ 60 mg/kg, IP), first experimental group (PTZ + Extract 500 mg/kg, gavages, 30 min before PTZ), positive control 2nd (PTZ + Phaclofen, 200 µg/µl, ICV), and second experimental group (PTZ + extract 500 mg/kg, gavage, 30 min before PTZ + Phaclofen 200 µg/µl, ICV). Several parameters were assessed during 20 min and followed up for 1.5 h. Then, the data were analyzed. APE with a dose of 500 mg/kg increased the latency time of seizures in the first experimental group, compared to the positive control 1st, also, comparison of different groups in terms of Seizure Score at the 1st time (severity of first attack) had no significant difference (P-value = 0.51, P-value = 0.34). The mean of seizure attacks (event number) was significant between the first and second positive control groups (P-value = 0.01) and also between the second positive control and the first experimental group (P-value = 0.011). Significant changes were observed in the mean score of the first and second positive control groups (P-value = 0.001) and the first experimental and second positive control groups (P-value = 0.003). In addition, the second experimental group had significant changes compared to the first positive control group (P-value = 0.014), However, no significant changes were observed between the positive control and experimental groups in terms of the severity of seizures.
Conclusion
Results have shown both blocked GABAergic receptors A and B involved in epileptic seizures. In addition, APE root increased delay time of epileptic seizures, as well as reduces epileptic seizure in dose response state.
Similar content being viewed by others
Background
The history of epileptic disease goes back to about 2000 B.C, and was called “falling sickness” [1]. Despite the wide range of knowledge in the field of neurodegenerative diseases, including epilepsy, and extensive research in finding the mechanism of the disease, and discovery of anti-epileptic drugs, to date 50 million people suffer from this illness worldwide [2, 3]. Epilepsy is a multifactorial disease, and its etiology includes genetic, structural, brain tumors /trauma, CNS infections, or inborn error of metabolism [4, 5]. Epileptic seizures happen due to behavioral alternations in the neuronal circuits. It can lead to changes in the structure and function of the brain cells [4, 5]. Pentylenetetrazole (PTZ) is a chemical substance used to induce epileptic seizures in animal model [5]. It induces both acute and chronic (kindling) epileptic effects [5,6,7]. Single high injection dose of PTZ has shown to induce acute epileptic convulsion [5,6,7]. However, the mechanism of PTZ is not clear, and it acts as a selective antagonist of the GABAA receptor [3, 8]. PTZ has been suggested to block chloride entrance through binding with active site of GABA [8, 9]. Imbalance of inhibitory and excitatory systems might occur as a result of chloride ion reduction in the brain. This imbalance leads to convulsion or seizure [5, 9,10,11]. Phaclofen or phosphonobaclofen, as a selective blocker of GABAB receptors, binds to K channel by G protein that leads to convulsion [10, 12, 13]. Therefore, by blocking both receptors of GABAergic system by PTZ as a selective antagonist of GABAA receptors and Phaclofen as a selective antagonist of GABAB receptors was tried to determine the possible mechanism of APE on GABAergic system. Recently, people tend to take traditional remedies more than synthetic drugs, because they believe that such herbal medicines have fewer side effects and are cheaper than synthetic drugs [14]. Anacyclus pyrethrum with different names, such as Aqarqarhaa in Greek, Persian, Indianan and Arabic, or Pellitory in English and Akarkara in Hindu, is considered a valuable herb in traditional medicine [15]. It has therapeutic effects, such as aphrodisiac, analgesia (tooth pain), anti-inflammatory, anti-rheumatism, and brain tonic [15]. Anacyclus pyrethrum plant contains several phytocomponents, such as tannins, [16] flavonoids, terpenoids, essential oils, inulin, coumarins, alkaloid pellitorine, anacyclin, phenylethylamine, etc. [17, 18]. This plant can be harvested twice a year (June and April), but it might contain different components according to the time of harvest [15, 16]. Toxicity studies in animal model have shown that consumption of Anacyclus pyrethrum plant up to 2 g per kg/body weight is safe [14, 18, 19]. Administration of the ethanolic extract of Anacyclus pyrethrum was previously reported to cure epileptic seizures induced by maximum electric shock [15, 18]. The aim of the present research was to evaluate the protective effect of hydroalcoholic extract of Anacyclus pyrethrum root in animal epileptic model treated with pentylenetetrazole drug, as a gold standard for induced epileptic seizure and also to evaluate the possible mechanism of this plant on GABAergic system.
Methods
Plant material
The dried roots of Anacyclus pyrethrum were purchased from a local market, and a voucher specimen (No: PM1090-) by faculty of pharmacy, Shiraz University of Medical Sciences, Shiraz, Iran, was deposited in the herbarium.
Preparation of extract
After cleaning and drying in air, the root of Anacyclus pyrethrum was ground and powdered. The second step in preparing hydro-alcohol extract of the roots of Anacyclus pyrethrum was percolation with ethanol 70% which was poured in the percolator device. Then, the extract was concentrated using rotatory evaporator and dried in vacuum oven.
Animals used
Fifty male Sprague Dawley rats (200–250 g) aged 3 months were purchased from animal laboratory of Shiraz University of Medical Sciences randomly and divided into 5 groups, 10 per group. As described by Wan Nor Arifin and Wan Mohd Zahiruddin, in animal studies, which are examined in five groups, the optimal sample size is a maximum of 3–5 rats [20]. Due to the severe intervention and the possibility of death, twice the maximum number of samples was selected. It should be noted that nine rats were excluded from the study due to various reasons, such as improper placement of the implanted cannula, cannula detachment, non-induction of seizure symptoms after PTZ injection and death. Thus, the exact number of rats in each group was as follows: control = 10, positive control 1st = 8, positive control 2nd = 9, experiment 1st = 7, experiment 2nd = 7. The animals had free access to standard food (chow) and water. They were housed in standard cages with a 12-h light/dark cycle and maintained at 25 ± 3.0 °C temperature. The plexiglass cages were 46 cm in length by 25 cm in width by 15 cm in height; cage bedding consisted of heat-treated pine shavings spread at a depth of 4 cm. The individual rat was considered the experimental unit within the study.
Methods to prevent bias
All rats were identified by earmarks and numbered accordingly. Random numbers were generated using the standard = RAND() function in Microsoft Excel. Animals from experimental group were always treated and assessed first, followed by control group. Test time was between 9 am and 5 pm and each animal tested at a different time each test day.
Evaluation of anticonvulsant activity
Epilepsy was induced chemically with PTZ (Sigma Chemical Co.), as described by Vohora et al. [21]. PTZ (as a blocker of GABA-A receptor), (60 mg/kg, i.p.), and phaclofen (Sigma Chemical Co) as a blocker of GABA-β receptor (200 μg/μl, icv.) as standard convulsing agents were used to induce epilepsy in rats for comparison. The control animals were kept without any intervention. The first positive control group received single dose of intraperitoneal injection of 60 mg/kg PTZ dissolved in normal saline to induce epilepsy [22]. The second positive control group received intracerebroventricular injection of 200 μg/µL phaclofen and intraperitoneal injection of 60 mg/kg PTZ. The first experimental group was fed with 500 mg/kg APE 30 min before PTZ injection [22]. The second experimental group was fed with 500 mg/kg APE 30 min before PTZ injection and ICV injection of 200 μg/µL phaclophen. Hydroalcoholic extract of Anacyclus pyrethrum was dissolved in distilled water and administered orally by gavage. It is noted that the epileptic seizures were induce in the positive control groups but the same volume of distilled water was used instead of APE. Mortality was observed after 24 h.
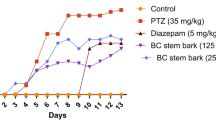
It should be noted that we used a dose of 50 mg/ml of PTZ, but did not receive any feedback. Then, the appropriate dosage 60 mg/kg of PTZ was used for the study as the dose which produces 100% generalized tonic clonic seizures with minimal mortality. In addition, in the case of hydroalcoholic extract of APE, a dose of 100 and 500 mg/kg was used for the study; however, the dose of 500 mg/kg was the appropriate dosage. Therefore, we used this dose for the rest of the study (Fig. 1).
The onset and duration of convulsions were noted and recorded. The APE ability to prevent the convulsion activity or delay the latency or onset of the seizure was considered as an index of anti- convulsant activity. The following parameters were assessed: latency time, number of events, seizure intensity (total sum scores in each rat instead of attack) and sum of scores (total sum of all the seizure scores for each rat). The parameters were assessed during 20 min and due to the presence of convulsive movements in some animals after this time, they were followed up for 1.5 h. The control animals were kept without any intervention. Attacks strength was determined by revised Racine’s scoring system [23].
ICV injection
The heads of the animal were fixed by mouth and ear bars of a stereotaxic instrument (Stoelting Co.). A stainless steel cannula was placed in the right lateral ventricle according to the Paxinos rat brain atlas guides (bregma: + 0.3 mm anterior, − 1.0 mm right lateral ventricle and − 3.0 mm ventral). Then, the implanted cannula was secured to the surface of the skull bone through micro-jewelry screws and dental acryl cement. At the end of the surgery, animals were housed into an individual cage, and 7 days after recovery, Phaclofen drug was injected into the right lateral ventricle via Hamilton syringe (2 μl/animal). Giemsa dye injection was used to assess the cannula place[24]. The rats were anesthetized by intraperitoneal injection of 80 mg/kg ketamine (Sigma Aldrich Co.)
Statistical analyses
The data were analyzed by SPSS software (Version: 22). The statistical tests used were ANOVA and Tukey and non-parametric tests. P-values < 0.05 were considered as statistically significant.
Results
Anacyclus pyrethrum root extract with a dose of 500 mg/kg increased the latency time of seizures in the first experimental group, relative to positive control 1st, but was not significant (P-value = 0.51) (Fig. 2). In addition, comparison of different groups in terms of Seizure Score at the 1st time (severity of first attack) had no significant difference (P-value = 0.34) (Fig. 3). Significant changes were observed in the mean of seizure attacks of the first and second control positive groups (P-value = 0.010) and also between the second control positive and first experimental group (P-value = 0.010), but no significant changes were observed between the second experimental group compared to the first and second positive control groups (P-value = 0.23 and P-value = 0.49) (Fig. 4). In addition, significant changes were observed in the mean scores of the first control positive with the second control positive (P-value = 0.001); between the first experimental group and 2nd control positive group (P-value = 0.003); and between the first positive control and first experimental group with the 2nd experimental group, respectively (P-value = 0.014, P-value = 0.032) (Fig. 5). However, there was no significant change between the 2nd experimental group and the 2nd control positive. (P-value = 0.82) (Fig. 5). The other measured parameter was the severity of epilepsy; no significant changes were found between the positive control and experimental groups in the severity of seizures (P-value = 0.17) (Fig. 6).
Discussion
After stroke, epilepsy is the 2nd neurological disorder in the world. The wide spectrum of systemic side effects, as well as cognitive impairments, are due to anti-epileptic drugs [21]. PTZ is a blocker of the GABA-A receptor. It induces convulsing effects after one single injection (acute method) or repeated administrations (chronic method). It performs its effects through GABAergic, glutamatergic and cholinergic systems [25]. In contrast to synthetic drugs, medical plants are used by patients more owing to their wide pharmacological effects and structural variety as well as low cost [22].
According to the results of this study, hydroalcoholic extract of Anacyclus pyrethrum root could averagely increase the seizure latency about 11 s compared with the first positive group; however, this increase was not statistically significant (P-value = 0.51) (Fig. 2). In addition, comparing different groups based on the seizure score for the 1st time did not have any significant difference (P-value = 0.34), and, averagely, the first seizures seen among the groups took score one (Fig. 3).
For precise understanding of the effect of Anacyclus pyrethrum plant on the GABAergic system and dissociation of its effect on GABA-A and GABA-B subunits, phaclofen drug which is a selective antagonist of GABA B was injected to the animals as intracerebroventricular (ICV) [12]. As expected, in the second positive control group where the animals received phaclofen besides PTZ, they had more events and also higher scores compared with the first positive control group (PTZ alone) (P-value = 0.01 and P-value = 0.01) (Figs. 4 and 5). This increase is due to the fact that in addition to inhibition of GABA-A receptors through PTZ, GABA-B receptors were blocked in the second positive control group.
This indicates that both GABA-A and GABA-B receptors play a role in seizure inhibition. In addition, by restraining GABA neurotransmitter, the irritability rate of the neurons would increase significantly and lead to an increase in the number of seizure attacks.
Comparison of the experimental group number one and the first positive control group did not reveal any significant variance regarding the number of events (P-value = 1), but these changes were significant compared to second positive control group (P-value = 0.01) (Fig. 4). In addition, comparison of second experimental group with first and second positive control groups showed that this plant was able to reduce the number of seizure events; however, these changes were not statistically significant (P-value = 0.49, P-value = 0.23) (Fig. 4).
Probably, using doses higher than 500 mg/kg of Anacyclus pyrethrum root extract as the single dose or daily gavage of this extract before the seizure induction may show more protection of itself compared with seizures, and decrease the number of events significantly, as in a study by Zaidi et al., good anticonvulsant effects have been obtained from a dose of 800 mg/kg of the extract [26].
It is also possible that in case the duration between giving the extract by gavage to the animal and PTZ injection as intra-peritoneal is more than 30 min, the probability of oral absorption, and consequently, the effect of the extract in the animal body would get higher, where there would be a need for performing more studies to find out the preciseness of these hypotheses.
As to the mean scores, according to the results, changes between the control groups one and two were statistically significant (P-value = 0.001); also, there were significant changes between the experimental group one and control group two (P-value = 0.003). Comparison of the experimental group two with the first positive control group showed that the difference was also statistically significant (P-value = 0.014). In addition, significant changes were seen between the experimental groups one and two (P-value = 0.032) (Fig. 5). Probably, better results could be reached through increasing the dosage and duration of giving the extract to the animal by gavage.
Another variable evaluated was the severity of epilepsy; no significant difference was seen among the groups (Fig. 6). In fact, PTZ causes seizure through GABAA receptor [25, 27]. When this receptor is active, its chloride channel is open and leads to chlorine ion flow and nervous hyperpolarization [8].
Pentylenetetrazole decreases the receptor’s activities through connection to its chlorine channel, and in this way its epileptogenesis effects are induced [28, 29]. This can suggest that one of the possible mechanisms of Anacyclus pyrethrum root restraining effect on seizure would be through its effect on the GABAergic system.
Although the second experimental group did not have any significant differences regarding the number of events and mean score, hydroalcoholic extract of Anacyclus pyrethrum root has been capable of generating more decrease in these two parameters compared with the first experimental group and first positive control group in which GABAA has been blocked only due to the PTZ effect. Therefore, possibly hydroalcoholic extract of Anacyclus pyrethrum root exerts its anti-epileptic effect mostly through affecting GABAB receptors. Better results may be reached using a higher dose of the extract.
This plant extract contains flavonoid factors such as apigenin [30, 31]. Apigenin connects to benzodiazepine receptors [32], known as fortifiers of the chloride ion flow, and causes an increase in chlorine conduction through affecting the receptor’s chloride channel (GABAA) [33, 34], which might be one of the possible mechanisms of this plant for dealing with seizures derived from PTZ. Therefore, it is possible that Anacyclus pyrethrum anti-epileptic effects are related to the presence of flavonoid compounds [35, 36].
It has been determined that Anacyclus pyrethrum exerts its anti-oxidant effects because of the presence of polyphenol compounds such as flavonoid which are anti-oxidant compounds [37]. Polyphenol flavonoids derived from plants perform anti-oxidant activities through mechanisms, such as ROS inhibition and lipid peroxidation [30]. Therefore, these compounds might be effective in induction of hydroalcoholic extract of Anacyclus pyrethrum root anti-epileptic effects. Of course, this issue needs further investigation and research.
According to scientific reports, cyclooxygenase restraint has been proved by the alkamides extracted from Anacyclus pyrethrum [22, 38], it could be proposed that maybe some Anacyclus pyrethrum anti-epileptic effects are due to cyclooxygenase enzyme inhibition. Activation of cyclooxygenase enzyme would lead to increase in free radicals synthesis [39, 40]. Peroxynitric and hydroxide are the main peroxidases that are formed after epilepsy derived from PTZ [40, 41].
The reason why the hydroalcoholic extract of Anacyclus pyrethrum root in a dose of 500 mg/kg could not significantly decrease the seizure latency and also epilepsy severity may be due to the fact that extract anti-epileptic substances did not have enough strength in this field, or their concentration in the volume used was low. Moreover, it is possible that using a larger sample size can give better results. Therefore, it is suggested that future studies be performed with concentrations above 500 mg/kg of the extract and a larger sample size.
Conclusions
Results of this study have shown that both GABAA and GABAB receptors play a role in the seizure inhibition, and administration by oral gavage of hydroalcoholic extract of Anacyclus pyrethrum root could decrease the numbers of events and total score and increase delay time of epileptic seizures with a dose of 500 mg/kg; however, these changes were not statistically significant, and performing complementary research for finding the effective components on the anticonvulsant activity of Anacyclus pyrethrum root is suggested.
Availability of data and materials
The data sets used and/or analyzed during the current study are available from the corresponding author on reasonable request. All materials used in this study are properly included in “Methods” section.
Abbreviations
- APE:
-
Anacyclus pyrethrum Extract
- CNS:
-
Central nervous system
- EXT:
-
Extract
- GABA:
-
Gama-aminobutyric acid
- ICV:
-
Intracerebroventricular
- IP:
-
Intraperitoneal
- Min:
-
Minute
- PTZ:
-
Pentylenetetrazole
- ROS:
-
Reactive oxygen species
- = RAND():
-
Random, the Excel RAND function returns a random number between 0 and 1
References
Shin EJ, Jeong JH, Chung YH, Kim WK, Ko KH, Bach JH, Hong JS, Yoneda Y, Kim HC. Role of oxidative stress in epileptic seizures. Neurochem Int. 2011;59(2):122–37.
Strine TW, Kobau R, Chapman DP, Thurman DJ, Price P, Balluz LS. Psychological distress, comorbidities, and health behaviors among US adults with seizures: results from the 2002 National Health Interview Survey. Epilepsia. 2005;46(7):1133–9.
Rabiei Z. Anticonvulsant effects of medicinal plants with emphasis on mechanisms of action. Asian Pac J Trop Biomed. 2017;7(2):166–72.
Shorvon SD. The etiologic classification of epilepsy. Epilepsia. 2011;52(6):1052–7.
Turker S, Severcan M, Ilbay G, Severcan F. Epileptic seizures induce structural and functional alterations on brain tissue membranes. Biochimica et Biophysica Acta (BBA) Biomembranes. 2014;1838(12):3088–96.
Dhir A. Pentylenetetrazol (PTZ) kindling model of epilepsy. Curr Protoc Neurosci. 2012. https://doi.org/10.1002/0471142301.ns0937s58.
Dastgheib M, Moezi L. Acute and chronic effects of agomelatine on intravenous penthylenetetrazol-induced seizure in mice and the probable role of nitric oxide. Eur J Pharmacol. 2014;736:10–5.
Huang R-Q, Bell-Horner CL, Dibas MI, Covey DF, Drewe JA, Dillon GH. Pentylenetetrazole-induced inhibition of recombinant γ-aminobutyric acid type A (GABAA) receptors: mechanism and site of action. J Pharmacol Exp Ther. 2001;298(3):986–95.
Kalueff A. Mapping convulsants’ binding to the GABA-A receptor chloride ionophore: a proposed model for channel binding sites. Neurochem Int. 2007;50(1):61–8.
Kerr DI, Ong J, Prager RH, Gynther BD, Curtis DR. Phaclofen: a peripheral and central baclofen antagonist. Brain Res. 1987;405(1):150–4.
Naseer MI, Shupeng L, Kim MO. Maternal epileptic seizure induced by pentylenetetrazol: apoptotic neurodegeneration and decreased GABA B1 receptor expression in prenatal rat brain. Mol Brain. 2009;2(1):20.
Bowery N, Bettler B, Froestl W, Gallagher J, Marshall F, Raiteri M, et al. International Union of Pharmacology. XXXIII. Mammalian γ-aminobutyric acidB receptors: structure and function. Pharmacol Rev. 2002;54(2):247–64.
Soltesz I, Haby M, Leresche N, Crunelli V. The GABAB antagonist phaclofen inhibits the late K+-dependent IPSP in cat and rat thalamic and hippocampal neurones. Brain Res. 1988;448(2):351–4.
Tauheed A, Hamiduddin AA. Aqarqarha (Anacyclus pyrethrum dc.) A potent drug in unani medicine: a review on its historical and phytopharmacological perspective. J Pharm Sci Innov. 2017;6(1):22–7.
Usmani A, Khushtar M, Arif M, Siddiqui MA, Sing SP, Mujahid M. Pharmacognostic and phytopharmacology study of Anacyclus pyrethrum: an insight. J Appl Pharm Sci. 2016;6:144–50.
Elazzouzi H, Soro A, Elhilali F, Bentayeb A, El Belghiti MA, Zair T. Phytochemical study of Anacyclus pyrethrum (L.) of Middle Atlas (Morocco), and in vitro study of antibacterial activity of pyrethrum. Adv Natl Appl Sci. 2014;8(8):131–41.
Sujith K, Darwin R, Suba V. Toxicological evaluation of ethanolic extract of Anacyclus pyrethrum in albino wistar rats. Asian Pac J Trop Dis. 2012;2(6):437–41.
Gautam O, Verma S, Jain S. Anticonvulsant and myorelaxation activity of Anacyclus pyrethrum DC.(Akarkara) root extract. Pharmacologyonline. 2011;1(1):121–5.
Kumar VK, Lalitha K. Acute oral toxicity studies of Anacyclus pyrethrum DC root in albino rats. Int J Pharm Pharm Sci. 2013;5(4):675–8.
Arifin WN, Zahiruddin WM. Sample size calculation in animal studies using resource equation approach. Malays J Med Sci. 2017;24(5):101–5.
Ali A, Ahmad FJ, Pillai K, Vohora D. Evidence of the antiepileptic potential of amiloride with neuropharmacological benefits in rodent models of epilepsy and behavior. Epilepsy Behav. 2004;5(3):322–8.
Pahuja M, Mehla J, Reeta K, Joshi S, Gupta YK. Root extract of Anacyclus pyrethrum ameliorates seizures, seizure-induced oxidative stress and cognitive impairment in experimental animals. Epilepsy Res. 2012;98(2–3):157–65.
Lüttjohann A, Fabene PF, van Luijtelaar G. A revised Racine’s scale for PTZ-induced seizures in rats. Physiol Behav. 2009;98:579–86.
Paxinos G, Watson C. figures: coronal sections of the brain. In: Paxinos G, Watson C, editors. The rat brain in stereotaxic coordinates. 6th ed. New York: Elsevier Academic Press; 2007. (Figs. 13-97).
Bikjdaouene L, Escames G, Camacho E, León J, Ferrer JM, Espinosa A, et al. Effects of some synthetic kynurenines on brain amino acids and nitric oxide after pentylenetetrazole administration to rats. J Pineal Res. 2004;36(4):267–77.
Zaidi SMA, Pathan SA, Singh S, Jamil S, Ahmad FJ, Khar RK. Anticonvulsant, anxiolytic and neurotoxicity profile of Aqarqarha (Anacyclus pyrethrum) DC (Compositae) root ethanolic extract. Pharmacol Pharm. 2013;4(07):535.
Sahu S, Dutta G, Mandal N, Goswami AR, Ghosh T. Anticonvulsant effect of Marsilea quadrifolia Linn. on pentylenetetrazole induced seizure: a behavioral and EEG study in rats. J Ethnopharmacol. 2012;141(1):537–41.
Olsen RW. Drug interactions at the GABA receptor-ionophore complex. Annu Rev Pharmacol Toxicol. 1982;22(1):245–77.
Korpi ER, Gründer G, Lüddens H. Drug interactions at GABAA receptors. Prog Neurobiol. 2002;67(2):113–59.
Kalim MD, Bhattacharyya D, Banerjee A, Chattopadhyay S. Oxidative DNA damage preventive activity and antioxidant potential of plants used in Unani system of medicine. BMC Complement Altern Med. 2010;10(1):77.
Selles C, Benali O, Tabti B, Larabi L, Harek Y. Green corrosion inhibitor: inhibitive action of aqueous extract of Anacyclus pyrethrum L. for the corrosion of mild steel in 0.5 M H2SO4. J Mater Environ Sci. 2012;3(1):206–19.
Svenningsen AB, Madsen KD, Liljefors T, Stafford GI, van Staden J, Jäger AK. Biflavones from Rhus species with affinity for the GABAA/benzodiazepine receptor. J Ethnopharmacol. 2006;103(2):276–80.
Hevers W, Lüddens H. The diversity of GABA A receptors. Mol Neurobiol. 1998;18(1):35–86.
Avallone R, Zanoli P, Puia G, Kleinschnitz M, Schreier P, Baraldi M. Pharmacological profile of apigenin, a flavonoid isolated from Matricaria chamomilla. Biochem Pharmacol. 2000;59(11):1387–94.
Kahnberg P, Lager E, Rosenberg C, Schougaard J, Camet L, Sterner O, et al. Refinement and evaluation of a pharmacophore model for flavone derivatives binding to the benzodiazepine site of the GABAA receptor. J Med Chem. 2002;45(19):4188–201.
Fernández SP, Wasowski C, Loscalzo LM, Granger RE, Johnston GA, Paladini AC, et al. Central nervous system depressant action of flavonoid glycosides. Eur J Pharmacol. 2006;539(3):168–76.
Sudha K, Rao AV, Rao A. Oxidative stress and antioxidants in epilepsy. Clin Chim Acta. 2001;303(1–2):19–24.
JalayerNaderi N, Niakan M, Khodadadi E. Determination of antibacterial activity of Anacyclus pyrethrum extract against some of the oral bacteria: an in vitro study. J Dent -Shiraz Univ Med Sci. 2012;13(2):59–63.
Julou L, Guyonnet J, Ducrot R, Fournel J, Pasquet J. Ketoprofen (19.583 RP)(2-(3-benzoylphenyl)-propionic acid). Main pharmacological properties—outline of toxicological and pharmacokinetic data. Scandinavian J Rheumatol Suppl. 1976;1976:33–44.
Garavand S, Keramati K, Zendehdel M, Jadidoleslami M, Garavand S. Effect of intracerebroventricular injection of flunixin meglumine on PTZ-induced seizures in male rats. Physiol Pharmacol. 2010;14(1):34–40.
Oliveira MS, Furian AF, Royes LFF, Fighera MR, Fiorenza NG, Castelli M, et al. Cyclooxygenase-2/PGE2 pathway facilitates pentylenetetrazol-induced seizures. Epilepsy Res. 2008;79(1):14–21.
Acknowledgements
The authors wish to thank Fasa University of Medical Sciences for financial support of this project. This work was conducted by M. Adloo as a part of required fulfillment for M.D. degree. The authors wish to thank Mrs M. Ahmadifar and Mr A. Mostafavinia Medical Student of Fasa University of Medical Sciences for solving problems during project techniques, and Dr. Nasrin Shokrpour at the Research Consultation Center (RCC) of Shiraz University of Medical Sciences for her invaluable assistance in editing this manuscript.
Funding
This research was supported by the Fasa University of Medical Sciences, Fasa, Iran. The funder played no role in the design of the study; collection, analysis, and interpretation; and in writing the Manuscript.
Author information
Authors and Affiliations
Contributions
MA has involved in the practical implementation of the project, the analysis of data, as student thesis. MB has collaborated on the part of practical implementation of the project, which led to the creation of two initial slides. MBS has involved in the subject and the design of the project, the monitoring of the proper performance of the work and the analysis of data and project management and writing the article. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The animal procedures were approved by The Animal Experimentation Ethics Committee of the Fasa University of Medical Sciences (Ethics Code: 92034), and under the guidelines of “Principles of Laboratory Animal Care” (NIH publication No. DH/HA&P/8/2/3).
Consent for publication
Not applicable.
Competing interests
Authors declared they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Adloo, M., Bahadori, M. & Shojaeifard, M.B. The impact of hydroalcoholic extract of Anacyclus pyrethrum plant on epileptic seizure induced by pentylenetetrazole in male rat. Egypt J Neurol Psychiatry Neurosurg 58, 72 (2022). https://doi.org/10.1186/s41983-022-00497-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41983-022-00497-3