Abstract
Objectives
Epilepsy is a neurological disorder resulting from excessive electrical discharge in the brain. Bombax costatum (BC) is an herb being used in African traditional medicine for the treatment of seizures. This study evaluated the possible anti-convulsant potential of stem bark ethanolic extract of BC on PTZ-induced kindling in rats.
Methods
Thirty-five Wistar rats were grouped into five (n = 7) and received normal saline, 35 mg/kg of PTZ, 5 mg/kg diazepam followed by 35 mg/kg PTZ after 30 min and BC stem back ethanolic extract at 125 mg/kg and 250 mg/kg followed by 35 mg/kg of PTZ intraperitoneally after 30 min. BC was administered orally daily while normal saline and PTZ were given intraperitoneally every other day for 26 days. Seizure activity was evaluated using the Racine scale, cognitive abilities through modified elevated plus maze and anxiety through forced swimming test. Further, the levels of GABA and oxidative stress biomarkers were also evaluated from the rat’s brain homogenate.
Results
Pretreatment with BC significantly reduced (p < .05) the seizure score and increased GABA level in BC treated rats when compared to PTZ alone treated rats. The first transfer latency of PTZ alone treated rats was significantly increased (p < .05) relative to the control rats and rats pretreated with diazepam and BC extract. Pretreatment with BC extract at 250 mg/kg was shown to significantly increase (p < .05) the activities of catalase, reduced glutathione, and superoxide dismutase compared to the PTZ alone treated rats.
Conclusions
Conclusively, BC was found to prevent seizure, avert neurodegeneration, and enhance cognition in PTZ-treated rats by regulating GABA level and enhancing antioxidant activity. Therefore, BC could be explored further for possible development of antiseizure agents.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Epilepsy is a non-communicable disorder of the central nervous system which occurs due to excessive electrical discharges in the brain cells [1]. Clinical features of epilepsy were recorded as far back as 4000 BC, which is characterized by recurrent seizures which are involuntary movements involving either the whole body or part of the body associated with or without loss of consciousness [2, 3]. Epilepsy has high incidence among age group of less than 2 years and older than 65 years and seizures can vary from a simple jerky movement of a muscle to severe/prolonged involuntary movements involving various regions in the body [4, 5]. When the neuronal membrane properties are altered by hypoxia, alkalosis, hypoglycemia, and abnormal neurotransmitter activity, it can lead to excessive release of synaptic neurotransmitters causing seizure [6, 7]. The immediate period after occurrence of an epileptic seizure is termed as a postictal state and which shows features such as headache, confusion, dysphasia, memory loss, short-term paralysis, or deep sleep [4]. The most common comorbidities associated with epilepsy are depression and anxiety which are psychiatric disorders more often affecting people with drug resistant epilepsy and cognitive impairment [8]. The cumulative effect of these comorbidities is impaired quality of life, which affects an individual more than the epileptic seizure [9]. Epilepsy affects 50 million people worldwide and is presently viewed as a global health problem with serious physical and economic challenges which needs urgent attention [5].
Animal model of epilepsy can be developed through kindling, a process of administering repetitive focal stimulation using chemical or electrical impulses to the brain, thus altering its the function, and producing epileptic-like convulsion [10]. Pentylenetetrazol (PTZ) is a drug initially used as circulatory and respiratory stimulant which in high doses led to convulsions, as discovered by a Hungarian American neurologist and psychiatrist [11]. PTZ is an antagonist of gamma aminobutyric acid (GABA) receptors used to induce acute seizures in rodents upon administration of single dose; however, PTZ produces significant pathologic changes or spontaneous epilepsy only when administered repetitively (kindling) [12, 13]. Administration of PTZ produces a reliable discriminative stimulus which is mainly mediated by the GABA-A receptor and affects the GABAergic and glutamatergic systems in different regions of the brain, including the hippocampus [14, 15]. GABA is a principal inhibitory neurotransmitter in the cerebral cortex regulating its intricate balance between excitatory and inhibitory tone and any shift in this balance results in seizure [16, 17]. GABA is produced from its precursor glutamate by action of enzyme glutamate decarboxylase and any excessive release of glutamate and impaired uptake which occurs as part of ischemic cascade leads to excitotoxicity [17]. Furthermore, developmental disruption of glutamate receptor expression and Glutamic acid has been implicated in epileptic seizures [18, 19].
Bombax costatum Pellegr. and Vuillet (BC) is a medicinal plant which belongs to the family Bombacaceae and is widely used in Africa and has been gaining popularity for its medicinal properties [20]. Different parts of BC plant have been used in traditional medicine of various countries in West Africa for treating wide range of illness [21]. The root and stem bark possess diuretic, wound healing, severe headache, and anti-epileptic properties [20]. Reports from recent studies showed that the extracts from stem bark of BC have antioxidant and anti-inflammatory properties, and leaves have anti-convulsant effects [22, 23].
Various research has been done on the phytochemical constituents of different parts of the plant and pharmacological effects including the anti-convulsant effects (anti-convulsive and anti-epileptic are synonymously used) on acute episodes of seizure [23, 24]. Because of wide geographical distribution in Africa and its easy availability, the anti-convulsant effect of BC has been studies on various animal models of epilepsy [23]. Furthering the research on its anti-convulsive properties, this study for the first time aims to identify and assess comorbid psychiatric conditions and cognitive impairments on PTZ-induced model of epilepsy, to ascertain the therapeutic potentials of stem bark ethanolic extract of BC. The anti-epileptic effects were evaluated by behavioral changes including anxiety and cognitive abilities, observing the histopathological changes, measuring the levels of oxidative stress markers, excitatory and inhibitory neurotransmitters in the hippocampus and temporal lobe.
Materials and methods
Plant material
Bombax costatum Pellegr. and Vuillet stem bark were collected from the University of Maiduguri staff quarters, Maiduguri, Borno State. It was authenticated by a botanist in the Department of Biological Sciences, University of Maiduguri, Nigeria, with voucher number (UM/HAH/2021/002) [24]. The phytochemical screening and the toxicity of the extract were reported earlier [24]. It was air dried, grinded and 600 g was soaked in 1500 ml of absolute ethanol for 4 days with occasional agitations. The solution was filtered and evaporated using an oven at low temperature to get the ethanol extract.
Drugs and chemicals
Pentylenetetrazol and ethanol were purchased from Sigma-Aldrich (St. Louis, USA). Diazepam and ketamine injection were purchased from a Pharmacy in Maiduguri, Nigeria.
Experimental animals
Thirty-five (35) male Wistar rats were purchased and kept at the animal house of the Department of Biochemistry, University of Maiduguri for 2 weeks to acclimatize with 12 h dark/light cycle and free access to food and water. The study protocol was approved by the Postgraduate Board, University of Maiduguri. All the animals used were handled with care and exposed to minimum stress during the conduct of experiments following the ARRIVE guidelines and in accordance with the National Guideline for Laboratory Animal Care [25].
Experimental design
Following the completion of acclimatization, the rats were randomly divided into five groups with seven rats per group and treated as follows: Group 1 (normal) rats were administered normal saline intraperitoneally on alternate days throughout the experiment. Group 2 rats were given 35 mg/kg of PTZ intraperitoneally on alternate days. Group 3 rats were given diazepam 5 mg/kg body weight intraperitoneally daily and on alternate days co-administered with 35 mg/kg body weight of PTZ given intraperitoneally after 30 min. Groups 4 and 5 rats were administered with stem bark ethanolic extract of BC orally at 125 mg/kg and 250 mg/kg daily, and on alternate days, they were co-administered 35 mg/kg of PTZ intraperitoneally after 30 min. The experiments were conducted for 26 days, and seizure activity was evaluated using the Racine scale [26] as follows: stage 0, no response; stage 1, restlessness and twitching; stage 2, head nodding, head clonus, and myoclonic jerks; stage 3, unilateral forelimb clonus; stage 4, rearing with bilateral forelimb clonus; stage 5, generalized tonic-colonic seizure with falling. Kindling was considered complete when the rats display seizure score of 4 or above for 3 consecutive administrations [26, 27]. At the end of the 26 days, the rats were subjected to behavioral tests, and after which they were euthanized, and their brain tissues harvested for histology and biochemical assays.
Modified elevated plus maze (mEPM)
Elevated plus maze apparatus was a plywood structure which is plus ( +) shaped and consisted of two open arms (50 cm, length × 10 cm, width) opposite to each other and two enclosed arms (50 cm, length × 10 cm, width × 40 cm, height) also opposite to each other. The central square (10 × 10 cm) linked all the four arms to the middle and the whole apparatus stands on its legs at a height of 50 cm above floor. The experiment was performed in a dark room illuminated only through one window. On day 1 of the experiment, which is the acquisition session, rats were individually placed at the far end of the open arm facing away from the central square. The time spent by the rat to reach either of the enclosed arms was recorded is referred as initial transfer latency (ITL). The rats were then allowed to explore the apparatus for 20 s before they were returned to their home cage. When a rat failed to enter either of the enclosed arms after 90 s, it was guided gently to it and allowed to explore for 20 s. Rats were recorded to be inside either of the enclosed arms when all their four paws had crossed an imaginary line between the center square and the enclosed arm. Twenty-four hours after the acquisition session, the first transfer latency for the rats was evaluated. Similarly, rats were placed at one end of the open arm facing opposite the central square, the time taken by the rat to turn and enter either of the enclosed arms is recorded as first transfer latency (TL1) [28]. In between trials, the apparatus was cleaned with 70% alcohol to avoid olfactory queue.
Forced swimming test (FST)
The FST was carried-out according to the method described by Slattery [29] with minor modification. A day before the main test, the rats underwent a pre-test individually in a transparent cylinder having dimensions of being 20 cm wide and 50 cm high and filled with water at 25 °C, at a depth of 30 cm and allowed to swim for 15 min to acclimatize. Subsequently, on the day of the actual test, the same procedure was followed but the swimming time being 5 min, and the activities of the rats were recorded with a video camera and analyzed later. The duration of immobility of the rats when placed in water during the 5-min test was recorded. Immobility was defined as the absence of movement by the rats except the minor movement of their limbs to keep their heads above the water.
Estimation of biochemical parameters
The levels of oxidative stress markers (catalase, reduced glutathione, and superoxide dismutase) and neurotransmitters (glutamate and GABA) were evaluated from the supernatant obtained brain homogenate. The method described by Guna et al. [30] was followed to determine the level of MDA, while Hassanzadeh et al. [31] method was followed to evaluate the levels of superoxide dismutase (SOD), glutathione (GSH), and catalase (CAT) and the determination of neurotransmitters GABA and glutamate was performed as mentioned in the manufacturer’s manual of the kits (Shanghai Coon Koon Biotech Co., Ltd China).
Histology cortex and hippocampus
The whole brain tissue of rats was harvested and fixed in Bouin’s solution for 1 week and then processed for histological examination. The brain tissues were dehydrated in a series of graded alcohol, embedded in paraffin wax, and sectioned coronally at 5 µm using microtome [32]. The sectioned tissues were mounted on glass slides, stained by hematoxylin and counter stained by eosin dyes, and then viewed under light microscope (MBJXISO-COPE, Los Angeles, USA) and the images were snapped using digital camera (M500, X64, version 3.7) [33].
Statistical analysis
One-way ANOVA was conducted using GraphPad Prism 10.0 (San Diego, USA). Statistical significance was considered at p < 0.05 and the results expressed as mean ± SEM (standard error of the mean).
Results
The effects BC stem bark extract on the development of kindling in PTZ-induced rats
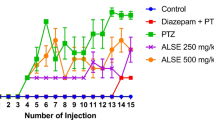
The seizure score of the control group of rats was zero throughout the experimental period. The group of rats, which received only PTZ, showed a gradual increase in the seizure score after the third dose which reached 4.3 after the eighth dose and resulted in kindling by the tenth dose. However, the groups of rats which had been co-administered with BC stem bark extract showed significantly reduced the seizure score, with a maximum score of 2.5 for rats co-administered 125 mg/kg and 3.0 for the rats co-administered 250 mg/kg of extract, respectively. The group receiving diazepam also showed similar results as the group of rats which received BC stem bark extract. The scores remained low even after the thirteenth dose without developing kindling (Fig. 1).
The effects of BC stem bark extract on cognition and depressive-like behaviors in PTZ-kindled rats
The analysis of results of mEPM to study the effects of BC stem bark extract on cognitive abilities in PTZ-kindled rats revealed a statistically significant increase (p < 0.05) of ITL in group of rats administered only PTZ compared to the control group which did not show any significant increase in ITL. However, no statistically significant differences were observed in the ITL in rats of groups co-administered with the diazepam and BC stem bark when compared to the PTZ alone treated group. However, the TL1 of rats of group receiving only PTZ showed statistically significant increase (p < 0.05) when compared to rats of control group, group administered with diazepam, and groups which received BC stem bark extract (Fig. 2).
The results of FST to evaluate of effects of BC stem bark extract on depressive-like behaviors in PTZ-kindled rats showed a statistically significant increase (p < 0.05) of immobility time in PTZ alone treated group when compared to the control group of rats. No significant change (p > 0.05) was observed in the immobility time of diazepam and BC stem bark co-administered rats compared to rat group receiving only PTZ (Fig. 2).
Neurotransmitters and antioxidant activity
Analysis of glutamate level in the brain revealed that there was statistically significant increase (p < 0.05) in group of rats which received only PTZ compared to the rats of control group. Though, a non-significant decrease (p > 0.05) was observed in the glutamate level of BC stem bark pretreated rats compared to the PTZ alone treated rats. No significant change (p > 0.05) was observed in the glutamate level of BC stem bark extract pretreated rats compared to the control (Fig. 3). On the other hand, a significant increase (p < 0.05) was observed in GABA level of BC stem bark extract 250 mg/kg pretreated group of rats when compared to PTZ alone treated rats, which was also observed in group receiving diazepam (Fig. 3). A significant decrease (p < 0.05) in catalase, reduced glutathione, and superoxide dismutase activities were noted in PTZ alone treated rats compared to the control group. While pretreatment with BC stem bark extract at dosage of 250 mg/kg was shown to have significant increase (p < 0.05) the activities of catalase, reduced glutathione, and superoxide dismutase compared to the PTZ alone treated rats which was comparable to group receiving diazepam (Fig. 4). Pretreatment with BC stem bark extract at 125 mg/kg did not significantly increase (p > 0.05) catalase and superoxide dismutase activities, but it significantly increased (p < 0.05) reduced glutathione activity related to the PTZ alone treated rats (Fig. 4).
Histology
The hippocampal histology showed normal neurons in the CA1 and CA3 regions of the hippocampus of the control group of rats, while both regions displayed severe degeneration of neurons in PTZ-treated rats. However, both regions revealed mild degeneration in rats pretreated with BC stem bark extract at 125 mg/kg with mild to moderate degeneration in rats pretreated with BC stem bark extract at 250 mg/kg (Fig. 5). The histology of the temporal lobe displayed normal neurons in the control and rats pretreated with BC stem bark extract at 125 mg/kg. The temporal lobe of PTZ-treated rats and rats pretreated with BC stem bark extract at 250 mg/kg showed mild to moderate degeneration, respectively (Fig. 6).
The effect of BC stem bark extract on the hippocampal histology of PTZ-treated rats. Black and white arrows indicate degenerating and normal neurons, respectively. a = control, b = 35 mg/kg PTZ, c = 5 mg/kg diazepam, d = 125 mg/kg BC, e = 250 mg/kg BC. BC = Bombax costatum, H&E stain, × 200 magnification
The effect of BC stem bark extract on the temporal lobe histology of PTZ-treated rats. Black and white arrows indicate degenerating and normal neurons, respectively. a = control, b = 35 mg/kg PTZ, c = 5 mg/kg diazepam, d = 125 mg/kg BC, e = 250 mg/kg BC. BC = Bombax costatum, H&E stain, × 200 magnification
Discussion
The present study was carried out to evaluate the pharmacological properties of ethanol extract of BC stem bark on PTZ-kindled rat model of epilepsy. In this study, the seizure score was evaluated to ascertain if BC could prevent the onset and progression of kindling in PTZ-kindled rats. During induction of epilepsy through the process of kindling, the rat groups which received BC stem bark extract were not completely kindled, while this was observed in all dosages of the extract. The results showed that pretreatment of the rats with BC prevented the development of kindling even after 13 successive PTZ injections with a maximum seizure score of 3 observed. These results were similar to reports of previous studies on BC stem bark, leaf, and root extracts in prevention of PTZ-induced acute seizure and serve as an anticonvulsant agent in epileptic rats [23, 34].
In the present study, mEPM was performed to evaluate cognitive behaviors of the PTZ-kindled rats. Pretreatment of the rats with BC extract showed significant decreases of the first transfer latency in mEPM which is an indicator of learning and memory in the rats. A study conducted by Bello et al. [34] reported similar results after administration of chloroform extract of BC to PTZ-kindled rat model. The result obtained in this study further confirmed the cognitive enhancing effect of BC extract.
FST was carried out to evaluate antidepressant-like behaviors in PTZ-kindled rats pretreated with BC. However, BC administration could not prevent depressive-like behavior in kindled rats as their immobility time in FST showed significant increase compared to the control. The results are in line with previous reports suggesting that some antiepileptic drugs/compounds may contribute to postictal depression [35, 36]. Though a different study revealed that aqueous extract of BC stem bark has an antidepressant effect [20], However, the present study has shown that the ethanol extract of BC stem has less effect on depressive-like behaviors in PTZ-kindled rats. The present study revealed that BC extract prevented seizure progression and enhanced cognition in PTZ-induced rats. Therefore, combination of ethanol extract of BC with antidepressants may result in a better outcome in fighting against epilepsy.
In this study, GABA and glutamate levels in the rat’s brain were estimated to ascertain the role of these neurotransmitters in seizure progression and the effect of BC in regulating them. BC extract at the high dose significantly increases the level of GABA. This agrees with results of previous research which showed similar occurrences in kindled rats treated with chloroform extract of BC [34]. In the present study, pretreatment with BC reduced the glutamate level when compared to the PTZ alone treated rats, although the decrease was not statistically significant. Glutamate plays a critical role in anxiety and memory as well as the growth and development of neurons and over-secretion and under-secretion could result in seizure, anxiety, and cognitive impairment [37]. High brain glutamate level is associated with recurrent seizure and drug resistance in epileptic patients [38, 39]. It is suggestive that the phytochemical constituents of BC extract should be evaluated further on glutamate level in PTZ-kindled rat model.
The present study further checked the pharmacological potential of BC on oxidative stress in PTZ-kindled rats. The results revealed an increase in antioxidants activity (catalase, reduced glutathione, and superoxide dismutase) in BC pretreated rats suggesting that BC could enhance antioxidant activity in the brain. The result of this study supports previous reports on different parts (leaf, root, and stem bark) of BC which showed to possess antioxidant properties [40, 41]. Some studies have reported the role of antioxidants in preventing or delaying the onset and progression of neurogenerative diseases [42, 43], and extracts of BC could be a potential agent to treat epilepsy and neurodegenerative disease.
The hippocampus is the part of the brain responsible for memory and learning, and studies have shown that substances that prevent degeneration of hippocampus usually enhance learning [42, 44]. The present study evaluated the effect of BC on the histology of hippocampus and temporal lobe of PTZ-kindled rats. It was found that at dosage of 125 mg/kg BC prevented neurodegeneration. The BC extract also attenuated cognitive impairment and oxidative stress in the rats, however, it does not show more effects on depressive-like activities in the rats. Finally, BC extract prevents neurodegeneration besides upregulating the levels of GABA in the rat’s brain.
Conclusion
BC as an herb, which has been used in traditional medicine, has shown to have protective mechanism is reducing the occurrence of epilepsy in PTZ-kindled rats by normalizing the levels of neurotransmitters in the brain, attenuating the oxidative stress leading to reduced neurodegeneration, which has been substantiated with increase in the viable neurons seen on histology of the hippocampus and temporal lobe. It could be concluded that BC has the potential for therapy of epilepsy. Therefore, further studies are recommended for possible development of BC as anti-seizure remedy.
Data availability
No datasets were generated or analysed during the current study.
Abbreviations
- BC:
-
Bombax costatum
- CA1:
-
Cornu ammonis 1
- PTZ:
-
Pentylenetetrazol
- CA2:
-
Cornu ammonis 2
- GABA:
-
Gamma aminobutyric acid
- mEPM:
-
Modified elevated plus maze
References
Subota A, Pham T, Jette N, Sauro K, Lorenzetti D, Holroyd-Leduc J. The association between dementia and epilepsy: A systematic review and meta-analysis. Epilepsia. 2017;58(6):962–72.
Cooper K, Gosnell K. Adult health nursing-e-book. Elsevier Health Sciences; 2022.
Mantoan Ritter L, Nilsson C. Seizures and epilepsy in dementia: diagnosis and management. Management of patients with dementia: the role of the physician. 2021;251–290.
Huether SE, McCance KL. Understanding pathophysiology-e-book. Elsevier Health Sciences; 2015.
WHO. Epilepsy: Fact sheet. 2017. http://www.who.int/mediacentre/factsheets/fs999/en/. Accessed January 2024.
Farine AP. Seizure and Epilepsy. In: Thompson T, Mathias P, editors. Lyttle’s Mental health and disorders. 3rd ed. Philadelphia: Elsevier; 2003. p. 297–303.
Sen A, Capelli V, Husain M. Cognition and dementia in older patients with epilepsy. Brain. 2018;141(6):1592–608.
Monteagudo Gimeno E, Sánchez-González R, Raduà-Castaño J, Fortea González L, Boget Llucià T, Carreño Martínez M, Donaire Pedraza A, Bargalló Alabart N, Setoain Perego X, Rumià Arboix J, Bulbena Vilarrasa A. Association between depressive and anxious symptoms with cognitive function and quality of life in drug-resistant epilepsy. 2022. Available at SSRN 4273802.
Chakravarty K, Shukla G, Poornima S, Agarwal P, Gupta A, Mohammed A, Ray S, Pandey RM, Goyal V, Srivastava A, Behari M. Effect of sleep quality on memory, executive function, and language performance in patients with refractory focal epilepsy and controlled epilepsy versus healthy controls–A prospective study. Epilepsy Behav. 2019;92:176–83.
Muhammad A, Hamman LL, Chiroma SM, Attah MOO, Dibal NI. Adansonia digitata L. stem bark attenuates epileptic seizure, depression, and neurodegeneration by mediating GABA and glutamate in pentylenetetrazol- kindled rats. J Pharmacopuncture. 2023;26(4):327–37.
Sheng F, Chen M, Tan Y, Xiang C, Zhang M, Li B, Su H, He C, Wan J, Li P. Protective effects of otophylloside n on pentylenetetrazol-induced neuronal injury in vitro and in vivo. Front Pharmacol. 2016;7(25):224.
Shimada T, Yamagata K. Pentylenetetrazole-induced kindling mouse model. J Vis Exp. 2018;136:e56573.
Mohamed HK, Sohair A, Eltony SA. Effect of acute pentylenetetrazol injection induced epileptic seizures on rat dentate gyrus at different postnatal ages. Anat Cell Biol. 2020;53:84–94.
Jung ME, Lal H, Gatch MB. The discriminative stimulus effects of pentylenetetrazol as a model of anxiety: recent developments. Neuroscience Biobehaviour Review. 2002;26(4):429–39.
Zhao X, Cheng P, Xu R, Meng K, Liao S, Jia P, Zheng X, Xiao C. Insights into the development of pentylenetetrazole-induced epileptic seizures from dynamic metabolomic changes. Metab Brain Dis. 2002;37:2441–55.
Treiman DM. GABAergic mechanisms in epilepsy. Epilepsia. 2001;42:8–12.
Sapolsky R. Biology and human behavior: the neurological origins of individuality. 2nd ed. The Teaching Company; 2005. p. 19–20.
Shinohe A, Hashimoto K, Nakamura K, Tsujii M, Iwata Y, Tsuchiya KJ, Sekine Y, Suda S, Suzuki K, Sugihara GI, Matsuzaki H. Increased serum levels of glutamate in adult patients with autism. Prog Neuropsychopharmacol Biol Psychiatry. 2006;30(8):1472–7.
Glushakov AV, Glushakova O, Varshney M, Bajpai LK, Sumners C, Laipis PJ, Embury JE, Baker SP, Otero DH, Dennis DM, Seubert CN. Long-term changes in glutamatergic synaptic transmission in phenylketonuria. Brain. 2005;128(2):300–7.
Lea Blondelle KD, Simplice FH, Hervé NA, Eglantine KW, Roland RN, Linda DK, Balbine KN, Désiré GN, Guillaume CW, Alin C. Antidepressant, anti-amnesic and vasoprotective effect of Bombax costatum Pellegr. & Vuillet aqueous stem bark extract on chronic mild unpredictable stress induced in rat. J Ethnopharmacol. 2022;293:115315.
Das G, Shin HS, Ningthoujam SS, Talukdar AD, Upadhyaya H, Tundis R, Das SK, Patra JK. Systematics, phytochemistry, biological activities and health promoting effects of the plants from the subfamily bombacoideae (family Malvaceae). Plants. 2021;10(4):651.
Antwi-Adjei M, Yeboah KO, Oppong-Kyekyeku J, Osafo N. Inflammation modulating activity of the hydroethanol stem bark extract of Bombax costatum in murine models. Scientifica. 2022.
Nazifi AB, Ahmed A, Hassan FI, Mohammed N, Danbala AA, Odoma S. Comparative anticonvulsant activity of leaf, stem bark and root bark extracts of Bombax costatum Pellegr. and Vuillet in acute models of epilepsy. Trop J Nat Prod Res (TJNPR). 2020;4(10):844–9. https://doi.org/10.26538/tjnpr/v4i10.30.
Bello AM, Malgwi IS, Adegoke SH, Abubakar AU, Ibrahim BM, Chiroma SM. Oral acute toxicity study on stem bark extracts of Bombax costatum Pellegr. and Vuillet on wistar albino rats. Bull Natl Res Cent. 2022;46(1):254.
Institute of Laboratory Animal Resources (US). Committee on Care, Use of Laboratory Animals. Guide for the care and use of laboratory animals. US Department of Health and Human Services, Public Health Service, National Institutes of Health. 1986.
Racine RJ. Modification of seizure activity by electrical stimulation. II. Motor seizure. Electroencephalogr Clin Neurophysiol. 1972;32(3):281–94.
Audu HA, Ahmed A, Zirahei JV, Dibal NI, Chiroma SM. Anogeissus leiocarpus (DC.) Guill and Perr ameliorates pentylenetetrazole-induced seizure/cognitive impairment in rats via inhibition of oxidative stress. Adv Tradit Med. 2023;4:1199–208.
Chiroma SM, Baharuldin MT, Taib CN, Amom Z, Jagadeesan S, Adenan MI, Moklas MA. Protective effect of Centella asiatica against D-galactose and aluminium chloride induced rats: behavioral and ultrastructural approaches. Biomed Pharmacother. 2019;109:853–64.
Slattery DA, Cryan JF. Using the rat forced swim test to assess antidepressant-like activity in rodents. Nat Protoc. 2012;7(6):1009–14.
Guna V, Saha L, Bhatia A, Banerjee D, Chakrabarti A. Anti-oxidant and antiapoptotic effects of berberine in pentylenetetrazole-induced kindling model in rat. J Epilepsy Res. 2018;8:66–73.
Hassanzadeh P, Arbabi E, Atyabi F, Dinarvand R. Ferulic acid exhibits antiepileptogenic effect and prevents oxidative stress and cognitive impairment in the kindling model of epilepsy. Life Sci. 2017;179:9–14.
Sarangi SC, Pattnaik SS, Katyal J, Kaleekal T, Dinda AK. An interaction study of Ocimum sanctum L. and levetiracetam in pentylenetetrazole kindling model of epilepsy. J Ethnopharmacol. 2020;249:11238.
Usman HA, Chiroma SM, Zirahei JV, Dibal NI. Adansonia digitata L. fruit shell prevents aluminum-induced cognitive impairment and depression in mice. Brain Behav Immun Integr. 2023;2:100014.
Bello AM, Salami HA, Malgwi IS, Chiroma SM. Stem bark chloroform extract of Bombax costatum Pellegr. & Vuillet exhibit anticonvulsant and neuroprotective effects in pentylenetetrazole-induced seizures in rats. Ann Pharm Fr. 2023;2(81):233–47.
Hoppe C, Elger CE. Depression in epilepsy: a critical review from a clinical perspective. Nat Rev Neurol. 2011;7(8):462–72.
Elger CE, Johnston SA, Hoppe C. Diagnosing and treating depression in epilepsy. Seizure. 2017;44:184–93.
Chen TS, Huang TH, Lai MC, Huang CW. The role of glutamate receptors in epilepsy. Biomedicines. 2023;11(3):783.
Sarlo GL, Holton KF. Brain concentrations of glutamate and GABA in human epilepsy: a review. Seizure. 2012;91:213–27.
Wang W, Wu Y, Li X, Li L, Sun K, Yan S. Altered plasma glutamate and glutamine levels in patients with drug-resistant and drug-responsive symptomatic focal epilepsy. Neurosci J. 2021;26(4):315–22.
Bi TE, Monnet TY, Ya KC, Ekissi ES, Bedel J, Fagbohoun PL. Phytochemicals compound and antioxidant activity study using different solvents and drying mode of flower Bombax costatum Pellgr and Vuillet (Bombacaceae) from Côte d’Ivoire. Eur J Biotechnol Biosci. 2019;7:38–48.
Oraebosi MI, Good GM, Chia T, Oyeniran OI. Bombax Costatum extract abrogates piroxicam-mediated hepatic and gastric toxicities in rats. Ann Pharm Fr. 2020;6(78):507–14.
Mahdi O, Chiroma SM, Hidayat Baharuldin MT, Mohd Nor NH, Mat Taib CN, Jagadeesan S, Devi S, Mohd Moklas MA. Win 55, 212–2 attenuates cognitive impairments in alcl3+ d-galactose-induced alzheimer’s disease rats by enhancing neurogenesis and reversing oxidative stress. Biomedicines. 2021;9(9):1270.
Mailafiya MM, Abubakar K, Chiroma SM, Danmaigoro A, Zyoud TY, Rahim EBA, ... & Zakaria ZAB. Curcumin‐loaded cockle shell‐derived calcium carbonate nanoparticles ameliorates lead‐induced neurotoxicity in rats via attenuation of oxidative stress. Food Sci Nutr. 2023;11(5):2211–2231.
Chiroma SM, Baharuldin MT, Mat Taib CN, Amom Z, Jagadeesan S, Ilham Adenan M, Mahdi O, Moklas MA. Centella asiatica protects d-galactose/AlCl3 mediated Alzheimer’s disease-like rats via PP2A/GSK-3β signaling pathway in their Hippocampus. Int J Mol Sci. 2019;20(8):1871.
Acknowledgements
The authors acknowledge the contributions made by Pharm. S.S Chiroma of Pharmacy Council of Nigeria and Dr A.B Nazifi of Bayero University Kano for their support towards this project. Additionally, Dr Saravanan Jagadeesan of Tayloy’s University Malaysia is also appreciated for the editing the manuscript.
Author information
Authors and Affiliations
Contributions
SSMC, NID, HSB: Concept and design; HSB, JVZ, SHG: Methodology and Validation; SMC, NID, HSB: Resources; HSB, NID: Writing original draft; SMC, NID, SHG, JVZ: Supervision and Project Administration; All authors reviewed and agreed to publish the manuscript.
Corresponding author
Ethics declarations
Ethical approval
The study protocol was approved by the Postgraduate Board, University of Maiduguri. All the animals used were handled with care and exposed to minimum stress during the conduct of experiments following the ARRIVE guidelines and in accordance with the National Guideline for Laboratory Animal Care [25].
Competing interests
The authors declare no competing interests.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Buba, H.S., Garba, S.H., Zirahei, J.V. et al. Bombax costatum Pellegr. & Vuillet attenuates pentylenetetrazol-induced seizure and cognitive impairment in rats via inhibition of oxidative stress. Nutrire 49, 28 (2024). https://doi.org/10.1186/s41110-024-00271-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41110-024-00271-w