Abstract
Background
The fall armyworm, Spodoptera frugiperda (J. E. Smith) (Lepidoptera: Noctuidae), is a polyphagous insect pest species that travel great distances every summer to disperse. They mostly feed on maize and over 350 other crops. In this investigation, two entomopathogens: Beauveria bassiana and Heterorhabditis taysearae, were utilized to test the efficacy of each against S. frugiperda separately and then as a group by inoculating H. taysearae two days after fungal infection.
Results
Evaluations were done on mortality percentage, infective juvenile (IJ) production, and conidia production. The LT50 continually reduced with increases in nematode and fungus concentrations. For the LC50 value, the H. taysearae isolate was 289 IJs/larva after 96 h of treatment, while for B. bassiana isolate, it was 106 CFU/ml after 144 h of treatment. Dual infections with B. bassiana and H. taysearae had a beneficial effect on pest mortality, resulting in 83% mortality, and caused a significant increase in conidia production while utilizing the nematode or fungal separately caused decrease in mortality (63 and 73%, respectively).
Conclusions
The study’s findings indicated a quicker time to death and suggested that combining a moderately pathogenic fungal isolate with nematodes could raise the mortality rate. The mutually beneficial relationship between B. bassiana and H. taysearae controls S. frugiperda.
Similar content being viewed by others
Background
Heterorhabditidae and Steinernematidae are two families of obligate insect parasites that belong to the entomopathogenic nematodes (EPN). These are attractive possibilities for biological control tactics against economically significant insect pests because of their tremendous reproductive potential, ability to produce large quantities, and low toxicity to non-target animals and the environment (Abd El Azim and Khashaba 2021).
Regarding Hussain et al. (2009), there is a wealth of data showing that entomopathogenic fungi (EPF), such as Beauveria bassiana, are detrimental to a range of pest species. EPF dig tunnels through the insect host’s cuticle to reach the hemocoel. The fungus grows its infectious spore stage on the host’s cuticle. The insect is then killed by the fungus that forms inside its body (Safavi 2013).
Molecular identification is the most accurate technique for determining phylogenetic relationships because morphological identification is unreliable for classifying novel isolates into specific species. Using genome sequencing of different parts of the EPNs, this technique can identify nuclear genes such as the 18S rRNA gene, internal transcribed spacers (ITS1 and ITS2), the 5.8S and 28S rRNA genes, and ribosomal genes such as the 18S rRNA gene (Stock 2009; Abd El Azim 2022).
Combining biocontrol treatments can significantly improve pest management, according to mounting data. Their greater effectiveness might be explained by additive or synergistic effects. According to a theory on synergistic interactions, one agent may cause stress to the target insect or change its behavior (such as eating or moving), increasing its susceptibility to the other agent (Ansari et al. 2008). Since nematodes primarily target soil-dwelling pests, mutual applications with EPF would seem to be a feasible way to increase levels of pest control (Acevedo et al. 2007).
More than 350 plant species, including cotton, rice, sorghum, millet, maize, sugarcane, and vegetable crops, are consumed by the Fall Armyworm (FAW), Spodoptera frugiperda (J. E. Smith) (Lepidoptera: Noctuidae), an insect pest that inhabits the tropical and subtropical regions of the Americas and recently in Africa and Asia. Larval stage is the harmful and important polyphagous pest’s stage (Montezano et al. 2018). If S. frugiperda is not controlled properly, it causes large yields’ losses. In May 2019, S. frugiperda was recorded for the first time in maize field in Aswan Governorate, Upper Egypt (Dahi et al. 2020).
According to Thakur et al. (2022), in a bioassay greenhouse experiment, the mixture of native EPNs, EPF, and EPB demonstrated a strong virulence toward Spodoptera litura. According to the study, the most effective biological control strategy, dealing with local pest issues, may involve combining three entomopathogens (H. bacteriophora + B. bassiana and H. bacteriophora + Bacillus thuringiensis) in a synergistic blend.
In the present study, the nematode Heterorhabditis taysearae alone and in combination with EPF isolate B. bassiana (MN337282) was used against the FAW to assess influences on mortality, time to death, and host colonization.
Methods
Sampling
Fifteen soil samples (from 20 cm deep) from an area cultivated with peanuts in the Ismailia Egyptian Governorate (N 30 27″ 11.2″, E 32 16′ 20.1″) were collected in 2021. Samples from different locations in the field were brought into the laboratory and placed in a plastic container holding 250 g of the subsample stored at 16 °C and were homogenized before the extraction of EPNs.
The Galleria mellonella (Galleria trap) method of insect-baiting was used to process the soil samples (Bedding and Akhurst 1975). Six instar G. mellonella larvae were placed inside 250 g perforated plastic boxes and then embedded in the soil sample. Samples of soil were kept at 25 °C. Dead G. mellonella exhibiting signs of an EPN’s infection were removed and replaced every five days. This process lasted five days to complete. Infectious juveniles (IJs) were gathered throughout the course of the next few days and stored at 16 °C in distilled water after the dead G. mellonella larvae were moved to White traps (White 1927).
Nematode identification
Molecular characterization of the isolate
The ITS rDNA sequences were used to molecularly characterize the isolate. DNA extraction from EPN was described by Kary et al. (2009). After being ground up in 20 μl of 1 × PCR solution, the nematodes were placed into a 1.5 ml tube, which was sterilized, frozen, and then filled with 20 μl of the same solution. 5 μl of 60 μg/ml Proteinase K was added to the tube after a 15 min incubation period at 70 °C and a 60 °C thawing period. The tube was incubated at 65 °C for two hours and then heated to 95 °C for fifteen min. Following a 15 min centrifugation at 16,000 rpm, the nematode DNA-containing supernatant was gathered and kept at 70 °C until required. To determine the quantity and quality of DNA, a spectrophotometer and a 1% agarose gel electrophoresis were utilized.
Using a 25 μl reaction mix containing 2 μl of the DNA suspension, 2.5 μl of 10X PCR buffer, 2.5 μl of MgCl2 (25 mM), 2 μl of the dNTP combination (10 mM of each dNTP), 0.6 U of Taq DNA polymerase, 13.4 μl of dd H2O, and 1 μl of the forward primer, the ITS region of the nematode rDNA was amplified by PCR. AB28:5′-ATATGCTTAAGTTCAGCGGGT3 and 1 μL of the reverse primer TW81:5-GTTTCCGTAGGTGAACCTGC-3' (Joyce et al. 1994). Following a 5-min initial denaturation at 94 °C, the ITS rDNA region was subjected to 30 cycles of 94 °C for 1 min, 64 °C for 1 min, and 72 °C for 1 min. One final extension took place at 72 °C for 5 min. Next, using the Wizard® SV Gel and PCR Clean-Up System Kit (Promega) and the manufacturer’s instructions, the product was placed onto a 2% agarose gel and purified.
Sequence-specific primers and a BigDye Terminator Cycle Sequencing Ready Reaction Kit (PE Applied Biosystems, USA) were used by the Macrogen Inc. service located in South Korea to sequence PCR data on an ABI PRISM 310 Genetic Analyzer (PE Applied Biosystems, USA) in both directions. An NCBI BLAST (Basic Local Alignment Search Tool) search yielded around 700 base pairs of sequences (National Centre for Biotechnology Information). The isolate’s Heterorhabditis sequences were found in the NCBI database, and they were compared to the Heterorhabditis sequences found.
Phylogenetic analysis
The phylogeny.fr platform (http://www.phylogeny.fr/phylogeny.cgi) was used to create a phylogenetic tree (Dereeper et al. 2008). The program Muscle was used to align the ITS sequences. The PhyML program’s maximum likelihood approach was utilized to reconstruct the phylogeny, and the bootstrapping technique was employed to assess the internal branch reliability (100). The application TreeDyn was used to render the trees.
The utilization of insects, nematode, and fungi isolates
FAW larvae were grown in Egypt at the Faculty of Agriculture, Cairo University. They were raised in a laboratory and kept in the dark at 26 ± 2 °C. Corn leaves (Zea mays) were used to raise the larvae in glass jars.
Dutky et al. (1964) reported that G. mellonella larvae in their last instar were infected using the recently isolated EPN. Two weeks following their harvest, the infected juveniles (IJs) that came out of White traps were kept to use. After that, they were kept at 16 °C in distilled water. At 28 ± 1 °C for 14 days, in complete darkness, 90 mm Petri plates containing 50 μg/ml chloramphenicol and 50 μg/ml streptomycin (Meyling and Eilenberg 2007) were cultured for the fungal isolate B. bassiana (MN337282), which had previously been isolated by Khashaba (2021). The 0.01% Triton X-100 aqueous solution was used to collect and suspend the conidia. The overall spore count was determined microscopically with a hemocytometer. Dilutions in series were made for different concentrations.
Analyzing of biological activity of isolated nematodes and fungi on larvae of Spodoptera frugiperda
Nematode isolate
After being collected, the 4th instar larvae of S. frugiperda were placed into plastic cups with 50 g of moist sand and 10% distilled water w/w. One larva was contained in each cup, and the median lethal concentration (LC50) for EPN and EPF was determined, using 0,100,200,300, and 400 IJs/larvae in nematode concentration–response experiments. Twenty larvae were used as the control group in two separate tests on each concentration of nematodes. Following that, they were maintained in a dark growth environment at 26 ± 2 °C. The larval death rate was determined after 48, 72, 96, and 120 h by calculating the color shift, which in cases of Heterorhabditis is typically reddish-purple. Larval mortality persisted for 5 days.
Fungus isolate
Conidia from a 14-day-old culture were cultured on SDAY, collected and suspended in 0.01% Triton X 100, after the agar surface was scraped. The resultant suspension was filtered to remove any last bits of hyphal debris. The primary conidial concentrations were found using a light microscope and hemocytometer (108). After that, 20 larvae were evaluated with two duplicates of each of the three fungal dilutions, which were adjusted to 105, 106, and 107 spores/ml, using sterile 0.01% Triton X 100 solution from the main concentration. All that was given to the control cups was sterile distilled water. Cups were incubated at 25 ± 2 °C, following inoculation. In the study, the rates of larval mortality were recorded at 72, 96, 120, 144, and 168 h after the hardening of larvae caused by the fungus or the formation of conidiophores. After that, to promote sporulation, the larvae were placed in sterile Petri plates with damp filter paper.
Nematode–fungus interactions
To ascertain the interactions between the calculated LC50 of entomopathogenic nematode and fungal isolate, the EPNs were started two days after the EPF infection. After 144 h of infection, the mortality rate was calculated. Every 24 h after infection, larvae were checked, and symptoms and fatalities were recorded. To promote sporulation, cadavers exhibiting signs of fungal infection, such as stiffening or the appearance of conidiophores, were placed in sterile Petri plates on moist filter paper. Larvae exhibiting changes in pigmentation suggestive of an EPN infection were cultured in Petri dishes on white traps to harvest EPNs.
Data analysis
Probit analysis was used to determine the lethal time, and concentration values for LT50 and LC50 were calculated according to Finney (1971) using LDPline software.
Results
Fifteen soil samples were taken from the governorate of Ismailia’s peanut field. A single Heterorhabditis isolate (7%), identified by the morphological characteristics of deceased larvae with a brown/black color, was obtained.
Molecular characterization
A 700 bp PCR product containing the ITS1-5.8S-ITS2 sequences was used to do a BLAST against the nucleotide collection (nr/nt) database to compare the EPN isolate to other noteworthy species that were kept in Gen-Bank. The discovered isolate exhibited 99% identity with H. taysearae based on sequence homology, and it has been deposited under the accession number Heterorhabditis taysearae EAG2 isolate (MW544011).
Phylogenetic analysis
The NCBI-acquired EPN sequences of the “H. taysearae” group were compared to the ITS sequences of the nematodes isolated from the positive sample using BLAST as a match. The novel isolate was categorized alongside other H. taysearae isolates in a clade. Using the Phylogeny.fr program, they were added together and placed in a clade alongside additional isolates of H. taysearae that were found in the GenBank database (Fig. 1). H. taysearae (EF043443) from Ireland and H. taysearae (KC633186) from the Gaza Strip belong to the same clade, according to the phylogenetic tree.
EPFs and EPNs bioassay
There were variations in the mortality rates of the larvae for both the single and combined entomopathogens. Within five days, H. taysearae caused mortality in larvae, where after 120 h, the concentration of 400 IJs/ larvae caused the greatest larval mortality (63%) and 100 IJs/ larvae caused the lowest one (16%) after 96 h. Within 7 days, the fungal isolate B. bassiana caused death of larvae. At 168 h, the concentration of 1 × 107 CFU/ml had the highest mortality percentage (73%), while the concentration of 1 × 105 CFU/ml had the lowest mortality percentage (28%) at 168 h. LT50 and LC50 values were calculated (Tables 1 and 2), which showed the effects of pathogens individually. It was shown that LT50 steadily reduced with increases in nematode and fungus. B. bassiana isolate demonstrated virulence, with LT50 values of 95 h at a concentration of 107 CFU/ml, while H. taysearae was virulent with LT50 values of 66 h at 400IJs/larvae. For the LC50 value, the H. taysearae isolate was 289 IJs/larva after 96 h of treatment, while for B. bassiana isolate, it was 106 CFU/ml after 144 h of treatment.
Entomopathogenic nematode and fungi interactions
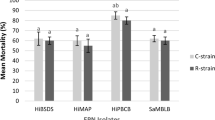
Heterorhabditis taysearae was inoculated two days after B. bassiana treatment at a concentration of 289 IJs/larva for H. taysearae isolate, while for B. bassiana isolate was 106 CFU/ml according to LC50 value, the two entomopathogens worked in concert to suppress the 4th instar larvae of FAW. The combined effect was 83% mortality rate at 144 h, which was higher than what was observed when the two entomopathogens were inoculated separately (Fig. 2).
Emergence of infections and symptoms after various treatments
By day 10, 60% of the cadavers for B. bassiana produced conidia, and 75% of the infected larvae showed evidence of fungal colonization. Eighty percent of the larvae of the nematode H. taysearae showed symptoms of infection, and by day six, IJs had emerged from seventy percent of the cadavers. At the dual infection, 70% of the larvae developed conidia after B. bassiana was infected 48 h before H. taysearae, of which 50% produced conidia. Thirty percent of the cadavers had IJs and showed signs of nematode infection.
Discussion
Fifteen soil samples were taken from the Governorate of Ismailia’s peanut field. Based on the physical characteristics of dead larvae (brown/black color), one isolate of Heterorhabditis was found (7%). The soil sample was positive for the occurrence of Heterorhabditis. ITS sequences were used to identify the nematodes that were isolated from the positive sample down to the species level. According to the results obtained, the isolate EAG2 (MW544011) indicated a 99% similarity with Heterorhabditis taysearae. Because of several characteristics, including the sandy clay loam type of the soil sample and the temperature range (23°–25°), it was anticipated that the recovery rate for EPNs in this region would be high. The results agree with Shamseldean and Abd Elgawad (1994) who first isolated H. taysearae from Egyptian sandy soil at Behera Governorate.
Heterorhabditis taysearae EF043443 and KC633186 are all members of the same clade, according to the phylogenetic tree, as was the isolate H. taysearae EAG2 (MW544011). Given the vast geographic separations between the “taysearae subgroup” nematode occurrence sites (Irand, Gaza Strip, and Egypt), these occurrences may be caused by “latitudinal clades,” as described by Dolgin et al. (2008). H. taysearae isolates from all over the world may therefore belong to “latitudinal clades.”
In the present study, the percentage of S. frugiperda larvae that died rose with time and in response to an increase in pathogen concentration. Similar findings were also reported by Garcia et al. (2008) as the mortality % of fall armyworm larvae positively correlated with H. indica concentration. Also, Ansari et al. (2008) demonstrated that administering EPFs and EPNs simultaneously or at short intervals of two days with other pests can create favorable interactions that are synergistic. The results agree with the outcomes of Degaga and Degaga (2023) showing that FAW larvae could potentially be controlled by 5 fungal isolates found in maize fields and showed mortality rates ranging from the highest (96.67%) and lowest (80.0%). Also, Shinde et al. (2022) evaluated 2 native strains of H. indica against larval instars of S. frugiperda, and both strains showed great potential in managing S. frugiperda under laboratory conditions.
According to Koppenhofer and Grewal (2005), an additive effect occurs when two agents combine to act independently of one another with no interaction, whereas synergistic or antagonistic effects occur when the interaction between the agents makes the combination more or less effective in controlling pests. In the work of Ansari et al. (2008), one of the primary causes of a synergistic interaction was the larvae’s initial fungal infection, which made them weaker and prevented them from feeding normally. Additionally, by creating a stressful environment, altering behavior, and releasing more CO2 in reaction to fungal colonization, the fungus made the host more vulnerable to nematodes.
This work demonstrated that 83% control of S. frugiperda 4th instar larvae can be achieved by using B. bassiana and H. taysearae in combination. Both B. bassiana with H. taysearae provided a significant control when used alone but efficacy increased in combination. This is in line with the findings of Ansari et al. (2010), who found that combining M. anisopliae V275 with S. kraussei resulted in 100% control over BVW infesting strawberries in grow bags during the winter, as opposed to a single application. Additionally, in all combined treatments, Ibrahim et al. (2019) demonstrated synergistic interaction between the nematode and fungus species. Shapiro et al. (2004) found that there was antagonism between the fungi Paecilomyces fumoroseus and B. bassiana and the nematodes that caused dual infections of Curculio caryae (Coleoptera: Curculionidae). Also, Usman et al. (2020) observed additive interactions when EPNs S. riobrave and S. carpocapsae were combined with EPFs M. brunneum and I. javanica when tested on the fruit fly (Rhagoletis pomonella).
Conclusion
The combination of nematodes and fungi applied together in biocontrol provided a promising approach that may outperform ecologically benign means of controlling a variety of insect pests more than single applications. The economic feasibility will always be the deciding factor, although public concern about the environmental effects of conventional insecticides and the emergence of infections resistant to pesticides may encourage it.
Availability of data and materials
All datasets are presented in the main manuscript.
Abbreviations
- EPF:
-
Entomopathogenic fungi
- FAW:
-
Spodoptera frugiperda: S. frugiperda
- B. bassiana :
-
Beauveria bassiana
- H. taysearae :
-
Heterorhabditis taysearae
References
Abd El Azim AM (2022) Efficacy of the entomopathogenic nematode isolate Heterorhabditis taysearae to control the cotton leafworm, Spodoptera littoralis (Boisd.)(Lepidoptera: Noctuidae). Egypt J Biol Pest Control 32:4
Abd El Azim AM, Khashaba EHK (2021) Genetic variability among three Egyptian isolates of Heterorhabditis indica using a new marker technique (SCoT). Egypt J Biol Pest Control 31:71. https://doi.org/10.1186/s41938-021-00419-0
Acevedo JPM, Samuels RI, Machado IR, Dolinski C (2007) Interactions between isolates of the entomopathogenic fungus Metarhizium anisopliae and the entomopathogenic nematode Heterorhabditis bacteriophora JPM4 during infection of the sugar cane borer Diatraea saccharalis (Lepidoptera: Pyralidae). J Invertebrate Pathol 96:187–192
Ansari M, Shah F, Butt T (2008) Combined Use of Entomopathogenic Nematodes and Metarhizium Anisopliae as a New Approach for Black Vine Weevil, Otiorhynchus Sulcatus. Control Entomol Exp Et Appl 129:340–347
Ansari M, Shah F, Butt T (2010) The Entomopathogenic Nematode Steinernema kraussei and Metarhizium anisopliae Work Synergistically in Controlling Overwintering Larvae of the Black Vine Weevil, Otiorhynchus sulcatus, in Strawberry Grow bags. Biocontrol Sci Technol 20:99–105
Bedding RA, Akhurst RJ (1975) A simple technique for the detection of insect parasitic nematodes in soil. Nematologica 21:109–110
Dahi HF, Salem SAR, Gamil WE, Mohamed HO (2020) Heat Requirements for the Fall Armyworm Spodoptera frugiperda (J. E. Smith) (Lepidoptera: Noctuidae) as a New Invasive Pest in Egypt. Egypt Acad J Biolog Sci 13(4):73–85
Degaga AH, Degaga EG (2023) Evaluating the effectiveness of isolated fungi against the Fall Armyworm (Spodoptera frugiperda). J Life Sci Biomed 13(1):17–24
Dereeper A, Guignon V, Blanc G, Audic S, Buffet S, Chevenet F, Dufayard JF, Guindon S, Lefort V, Lescot M, Claverie JM, Gascuel O (2008) Phylogeny.fr: robust phylogenetic analysis for the non-specialist. Nucleic Acids Res. https://doi.org/10.1093/nar/gkn180.Epub
Dolgin ES, Fe’Lix MA, Cutter AD (2008) Hakuna Nematoda: genetic and phenotypic diversity in African isolates of Caenorhabditis elegans and C. briggsae. Heredity 100(3):304–315. https://doi.org/10.1038/sj.hdy.6801079
Dutky SR, Thompson JV, Cantwell GE (1964) A technique for the mass propagation of the DD-1 36 nematode. J Insect Pathol 6(41):7–422
Finney DJ (1971) Probit analysis. Cambridge University Press, Cambridge, p 333
Garcia Luiz C, Raetano CG, Leite LG (2008) Application Technology for the Entomopathogenic Nematodes Heterorhabditis indica and Steinernema sp. (Rhabditida: Heterorhabditidae and Steinernematidae) to Control Spodoptera frugiperda (Smith) (Lepidoptera: Noctuidae) in Corn. Neotropical Entomol 37(3):305–311
Hussain A, Tian MY, He YR, Ahmed S (2009) Entomopathogenic fungi disturbed the larval growth and feeding performance of Ocinara varians (Lepidoptera: Bombycidae) larvae. Insect Sci 16(6):511–517
Ibrahim SAM, Taha MA, Salem HHA (2019) Initial fungal infection reduce the penetration and reproduction rate of Steinernema riobravae in Galleria mellonella. Egypt Acad J Biolog Sci 12(1):101–109
Joyce SA, Burnell AM, Powers TO (1994) Characterization of Heterorhabditis isolates by PCR amplification of segments of mtDNA and rDNA genes. J Nematol 26:260–270
Kary NE, Niknam G, Griffin CT, Mohammadi SA, Moghaddam MA (2009) Survey of entomopathogenic nematodes of the families Steinernematidae and Heterorhabditidae (Nematoda: Rhabditida) in the north-west of Iran. Nematol 11(1):107–116. https://doi.org/10.1163/156854108X398453
Khashaba EHK (2021) Inoculation and colonization of isolated entomopathogenic fungi Beauveria bassiana in rice plants, Oryza sativa L. through seed immersion method. Egypt J Biol Pest Control 31:92
Koppenhofer AM, Grewal PS (2005) Compatibility and interactions with agrochemicals and other biocontrol agents. In: Grewal PS, Ehlers R-U, Shapiro-Iian D (eds) Nematodes as biocontrol agents. CABI Publishing, Wallingford, pp 362–381
Meyling NV, Eilenberg J (2007) Methods for isolation of entomopathogenic fungi from the soil environment. Lab Manual 32:818–890
Montezano DG, Sosa-Gómez DR, Specht A, Roque-Specht VF, Sousa-Silva JC, Paula-Moraes SV, Peterson JA, Hunt TE (2018) Host plants of Spodoptera frugiperda (Lepidoptera : Noctuidae) in the Americas. African Entomol 26(2):286
Safavi SA (2013) In vitro and in vivo induction, and characterization of Beauvericin Isolated from Beauveria bassiana and Its Bioassay on Galleria mellonella Larvae. J Agr Sci Tech 15:1–10
Shamseldean MM, Abd-Elgawad MM (1994) Natural occurrence of insect pathogenic nematodes (Rhabditida: Heterorhabditidae) in Egyptian Soils Afro-Asian. J Nematol 4(2):151–154
Shapiro-Ilan DI, Jackson M, Reilly CC, Hotchkiss MW (2004) Effects of combining an entomopathogenic fungi or bacterium with entomopathogenic nematodes on mortality of Curculio Caryae (Coleoptera: Curculionidae). Biol Control 30:119–126
Shinde SP, Ingole DB, Biradar VK, Gokte-Narkhedkar N, Lavhe NV, Thube SH, Shah V, Prasad YG (2022) Efficacy of native strains of entomopathogenic nematode, Heterorhabditis indica against the fall armyworm, Spodoptera frugiperda (J. E. Smith) (Lepidoptera: Noctuidae) from India. Egypt J Biol Pest Control. https://doi.org/10.1186/s41938-022-00638-z
Stock SP (2009) Molecular approaches and the taxonomy of insect-parasitic and pathogenic nematodes. In: Stock SP, Vandenburg J, Glazer I, Boemare N (eds) Insect pathogens: molecular approaches and techniques. CAB International Press, Wallingford, pp 71–100
Thakur N, Tomar B, Sharma S, Kaur S, Sharma S, Yadav AN (2022) Hesham A (2022) Synergistic effect of entomopathogens against Spodoptera litura (Fabricius) under laboratory and greenhouse conditions. Egypt J Biol Pest Control 32:39. https://doi.org/10.1186/s41938-022-00537-3
Usman M, Gulzar S, Wakil W, Wu S, Piñero JC, Leskey TC, Nixon LJ, Oliveira-Hofman C, Toews MD, Shapiro-Han D (2020) Virulence of entomopathogenic fungi to Rhagoletis pomonella (Diptera: Tephritidae) and interactions with entomopathogenic nematodes. J Econ Entomol 113(6):2627–2633. https://doi.org/10.1093/jee/toaa209
White GF (1927) A method for obtaining infective nematode larvae from cultures. Science 66:302–303
Acknowledgements
We would like to thank Prof. Dr. Reda E. Moghaieb (Department of Genetics, Faculty of Agriculture, Cairo University) for helping and also Prof. Dr. Ehab M. Bakr (Acarology Department, Plant Protection Research institute) for helping in statistical analysis.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
E. K. participated in the work and manuscript writing. A. A. participated in the work and manuscript writing. G.K. participated in the work and manuscript writing. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing Interests
On behalf of all authors, the corresponding author states that there is no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Abd El Azim, A.M., Khashaba, E.H.K. & El Kady, G.A. Effectiveness study of the dual application of new Indigenous entomopathogenic nematode isolate Heterorhabditis taysearae and entomopathogenic fungi Beauveria bassiana against armyworm (Spodoptera frugiperda). Egypt J Biol Pest Control 34, 41 (2024). https://doi.org/10.1186/s41938-024-00804-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41938-024-00804-5