Abstract
Background
Temperature alters host suitability for the development of parasitoids through direct (thermal effect) and indirect (parental effect) pathways. The effects of three temperature regimes on the development and survival of two parasitoid species, Citrostichus phyllocnistoides (Narayanan) and Cirrospilus ingenuus Gahan (Eulophidae: Hymenoptera) of the citrus leafminer, Phyllocnistis citrella Stainton (Lepidoptera: Gracillariidae) was evaluated. The experiment was conducted at 20, 25, and 30°C temperatures with 65 ± 2% relative humidity (R.H.) and 16h: 8h (L: D) photoperiod.
Results
In C. phyllocnistoides, the pre-ovipositional period was longer at 20°C, while non- significant difference was observed in the pre-ovipositional period of C. ingenuus under the effect of different temperatures (P > 0.05). The ovipositional period of C. phyllocnistoides and C. ingenuus was higher at 20°C and gradually decreased by increasing the temperature. Non- significant (P > 0.05) difference was found in post-ovipositional period of both parasitoid species. Both species exhibited the maximum fecundity at 25°C, while, the minimum fecundity was recorded at 30°C. However, the adult longevity of both parasitoid species was highest at 20°C and gradually decreased by increasing the temperature. In both parasitoids, the parasitism rate was highest at 25°C.
Conclusion
This study highlighted the importance of thermal effects on some parasitoid species of insect pests to predict the future of trophic dynamics in global warming situations.
Similar content being viewed by others
Background
The citrus leafminer (CLM), Phyllocnistis citrella Stainton (Lepidoptera: Gracillariidae) is a devastating pest of citrus (Arshad et al. 2018a). It is native to Southeast Asia (Clausen 1931). This micro-lepidopterous species was reported for the first time in 1856 by Calcutta (Indo-Pak subcontinent) (Stainton 1856). Since the last two decades of the twentieth century, this pest has invaded the Americas and Mediterranean basin (Vercher et al. 2005) along with citrus growing tropical and sub-tropical areas of the world (Kerns and Wright 2001).
Adults of P. citrella are minute moths (2 mm long) having wings fringed with long hairs (Mustafa et al. 2014a). Females oviposit single eggs on the young, tender citrus leaves (5–45 mm long) about 24 h after mating (Amalin et al. 2002). Newly hatched larvae immediately bore a mine and enter into the tiny leaf below the epidermal layer (Mansour and Braham 2020). There are four larval instars of P. citrella. The first three instars feed on the sap and epidermal cells, while the fourth instar is prepupae, which is a non-feeding stage (Beattie and Hardy 2004). The prepupae spin a silken cocoon to form a pupal chamber, usually protected by folding the leaf margin (Chermiti et al. 2001). Phyllocnistis citrella is multivoltine and has nearly continuous generations. In tropical conditions, this pest has up to 15 generations per year (Mustafa et al. 2014b).
Phyllocnistis citrella is regarded as a serious threat to citrus cultures for causing high economic losses (Ujiye 2000; Dahmane and Chakali 2022). Infested leaves become deformed, chlorotic and cause a reduction in photosynthetic rate (Arshad et al. 2019). Besides, mining provides an entry hole to the canker bacterium (Xanthomonas axonopodis pv. citri) by opening the leaf cuticle and facilitate its access to the plant (Gottwald et al. 2007). Previous studies have demonstrated that P. citrella damage has no economic impact on the mature trees under Mediterranean conditions (Diez et al. 2006), but it causes significant economic losses in citrus nurseries, young plantations, and top-grafted trees (De Prins and De Prins 2005).
Citrus growers mostly rely on the repeated applications of synthetic insecticides for the control of P. citrella (Amiri 2007; Elazab et al. 2021). However, the effective chemical control of P. citrella is difficult because the larvae and pupae are protected inside the mines or chambers, respectively (Beattie and Hardy 2004). Furthermore, the regular use of insecticides has disrupted the non-targeted natural enemies and led to resistance in the populations of P. citrella against insecticides (Elekcioğlu 2017). The chemical control seemed a costly and short-term solution for the management of P. citrella (Mafi and Ohbayashi 2010). On the other hand, biological control is an effective and promising option for controlling P. citrella over the long term (Kalaitzaki et al. 2021). Several studies have reported a significant reduction in the P. citrella population governed by the indigenous natural enemies (Elekcioğlu 2017).
Citrostichus phyllocnistoides (Narayan) (Hymenoptera: Eulophidae) is a primary ecto-parasitoid of P. citrella around the world (Tsagkarakis et al. 2013). Elekcioğlu and Uygun (2013) reported C. phyllocnistoides causing up to 51% parasitism in Turkey. Similarly, Cirrospilus ingenuus Gahan (Hymenoptera: Eulophidae) is another solitary ecto-parasitoid of P. citrella (Hoy and Nguyen 2003). This parasitoid is native to Asia and commonly found in China, Australia, India, Japan, Indonesia, Oman, Thailand, Malaysia and Taiwan (Zhu et al. 2002). Both parasitoids were released in several countries for the classical biological control of P. citrella (Elekcioğlu and Uygun 2013). In Pakistan, Arshad et al. (2018b) reported both C. phyllocnistoides and C. ingenuus from the Sargodha region of Punjab province.
Knowledge of the thermal effect on natural enemies of insect pests is crucial to develop a cost-effective mass rearing programs, as it has the potential to use in augmentative biological control of insect pests. This study aimed to evaluate the effect of temperature on the ovipositional periods, fecundity, longevity, and parasitization of C. phyllocnistoides and C. ingenuus on P. citrella under controlled laboratory conditions.
Methods
The study was conducted at the Pak-China Joint Research Centre for Citrus Diseases and Insect Pests Management, College of Agriculture, University of Sargodha, Sargodha, Pakistan.
Rearing of Phyllocnistis citrella
Leaves infested with P. citrella were collected from the citrus plantations around the vicinity of the College of Agriculture, University of Sargodha (32°07′53.6"N, 72°41′09.3"E) for rearing in the laboratory. The collected leaves were kept under controlled climatic conditions at 26 ± 2°C temperature, 60 ± 5% RH. and 16h: 8h (L:D) photoperiod until the adults' emergence. After emergence, the adults were transferred into Perspex cages (50 × 50 × 100 cm, Shenzhen Yijin, Guangdong, China), provided with young citrus plants pruned to a height of 40–50 cm for easy handling. The plants were planted in 0.5-L plastic pots filled with fertile sand. Every 10 days, three plants were exposed to adult P. citrella moths for 2–3 days for egg-laying. Following that period, the moths were removed from the cages and infested plants were kept undisturbed in rearing chambers until adult emergence. Adults P. citrella moths were fed on a mixture of honey and water (1:3).
Rearing of parasitoids
Two parasitoids of P. citrella; C. phyllocnistoides and C. ingenuus were studied. Parasitods were obtained from the colony reared in the laboratory. The Perspex cages (50 × 50 × 100 cm) were used for the rearing of each parasitoid. For the emergence of adult wasps, the parasitized P. citrella larvae or pupae were kept under the same conditions as for rearing P. citrella. After emergence, the adult parasitoids were released in the cages with a ratio of 1:1 of both male and female wasps. Honey water (10% w/v) soaked filter paper strips were placed in cages to feed the adult wasps. P. citrella infested plants were provided in each cage for the oviposition of gravid female wasps. After oviposition, the plants were kept undisturbed under controlled conditions for the emergence of the next generation. A continuous supply of infested plants was provided to get a maximum population of parasitoids.
Temperature regimes
The experiments were performed under completely randomized design (CRD) in growth chamber (BIOBASE, BJPX-A400) at three different temperature levels, 20, 25, and 30°C ± 1°C at 65 ± 2% relative humidity (R.H.) and 16h: 8h (L:D) photoperiod.
Ovipositional period, fecundity and adults’ longevity of parasitoids
Citrus leaves infested with third instar P. citrella larvae were placed in Petri plates (140 mm diameter) lined with water-soaked filter papers. A total of 5 pairs of each parasitoid was tested by considering each pair as one replicate. One pair of adult parasitoids was offered 10 larvae of P. citrella at 48 h intervals. After 48 h, a new set of the host larvae were provided. Adult parasitoids were fed on small droplets of honey water (10% w/v) solution deposited on the leaves. The hosts and parasitoids were observed three times every day under a stereomicroscope. The evidence of stinging and feeding of parasitoids on P. citrella larvae ranged from barely detectable markings or very small black dot left after ovipositor insertion. The data of pre-ovipositional, ovipositional, and post-ovipositional periods, fecundity, and adults' longevity were determined by daily observations (Elekcioğlu 2017). The duration between the start of the experiment and the day when oviposition began was calculated as the pre-ovipositional period. The period between the first and last egg-laying of the parasitoids was considered as the ovipositional period. While the period between final egg-laying and the parasitoid’s death was recorded as the post-ovipositional period.
Parasitism rate
A total number of parasitoids and P. citrella adults emerged from the parasitized and unparasitized individuals, respectively were counted from the experiment to determine the parasitization rate at different temperatures. The percent parasitism was calculated using the formula suggested by Li et al. (2018).
Data analysis
Data were subjected to one-way analysis of variance (ANOVA) at α = 0.05. Prior to the analyses, data were transformed through square-root transformation to normalize the variance after checking the normality of residuals and homogenized with the Shapiro–Wilk test. Original means were separated using the Fisher’s least significant difference (LSD) multiple range test. All the data were evaluated by using Minitab 17.0 software.
Results
Ovipositional periods
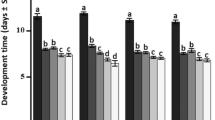
The temperature had significant effects on the ovipositional period of both C. phyllocnistoides (F2,12 = 68.5, P < 0.001) and C. ingenuus (F2,12 = 32.7, P < 0.001), while non- significant effect was observed on post-ovipositional (F2,12 = 2.00, P = 0.178 for C. phyllocnistoides and (F2,12 = 3.45, P = 0.065) periods of both parasitoids (Fig. 1). Some of the females of both parasitoids started egg-laying on the 1st day right after being released on the P. citrella larvae, while some of them within 2 days. The pre-ovipositional period of C. phyllocnistoides was longer at 20°C (2.2 ± 0.178 days), while non-significant difference was observed in the pre-ovipositional period of C. ingenuus under the effect of different temperatures (F2,12 = 2.92, P = 0.092). In both C. phyllocnistoides and C. ingenuus, the maximum duration of ovipositional was recorded at 20°C (11.40 ± 0.357 days and 11.20 ± 0.521 days, respectively). It gradually decreased by increasing the temperature. C. phyllocnistoides had a short post-ovipositional period (< 1day) as the females died soon after their final egg-laying, while C. ingenuus survived for more 1.5 to 3 days after laying their last eggs (Fig. 1).
Effect of temperature on the pre-oviposition, oviposition and post-oviposition period (means ± S.E.) of Citrostichus phyllocnistoides and Cirrospilus ingenuus reared on Phyllocnistis citrella. means within the same parasitoid sharing similar letters are not significantly different (α = 0.05; LSD test). ** shows the significance (P < 0.05) and ns = shows the non-significance (P > 0.05) among two parasitoids for each temperature regime
Fecundity and longevity
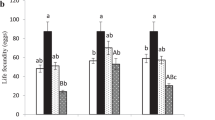
Fecundity rate of C. phyllocnistoides (F2,12 = 14.5, P < 0.001) and C. ingenuus (F2,12 = 53.0, P < 0.001) was significantly affected with different temperatures (Table 1). Females of both parasitoids deposited a maximum number of viable eggs at 25°C. Fecundity rate of C. phyllocnistoides and C. ingenuus was 17.0 ± 0.938 and 17.6 ± 0.606 eggs, respectively at 25°C, reduced by the increasing temperature to 30°C. On the other hand, change in temperature also significantly affected the longevity of both parasitoids (F2,12 = 31.5, P < 0.001 for C. phyllocnistoides and F2,12 = 45.7, P < 0.001 for C. ingenuus). In both parasitoids, longevity showed a contrary trend to fecundity as it gradually decreased by increasing the temperature (Fig. 2). Maximum longevity of C. phyllocnistoides adults (14.2 ± 0.521 days) was recorded at 20°C (20 > 25 > 30°C). Similarly, the longevity of C. ingenuus at 20°C was higher than the rest (16.80 ± 0.657 days, 20 > 25 > 30°C) (Fig. 2).
Effect of temperature on adult longevity (days) (means ± S.E.) of Citrostichus phyllocnistoides and Cirrospilus ingenuus reared on Phyllocnistis citrella, means within the same parasitoid sharing similar letters are not significantly different (α = 0.05; LSD test), ** shows the significance (P < 0.05) among two parasitoids for each temperature regime
Parasitism rate
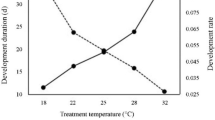
The highest parasitization of C. phyllocnistoides (F2,12 = 14.6, P < 0.001) and C. ingenuus (F2,12 = 30.7, P < 0.001) was observed at 25°C which was significantly different from the other temperatures. The parasitization rate of both parasitoids showed a similar trend by changing the temperature i.e. 25 > 30 > 20°C (Fig. 3). Temperature showed a negative and strong relation with longevity (R2 = 0.78 for C. phyllocnistoides and R2 = 0.88 for C. Ingenuus) and oviposition (R2 = 0.89 for C. phyllocnistoides and R2 = 0.83 for C. Ingenuus) of parasitoids (Fig. 4).
Effect of temperature on the percent parasitism (means ± S.E.) of Citrostichus phyllocnistoides and Cirrospilus ingenuus reared on Phyllocnistis citrella, means within the same parasitoid sharing similar letters are not significantly different (α = 0.05; LSD test), ** shows the significance (P < 0.05) among two parasitoids for each temperature regime
Discussion
Knowledge of biological and ecological aspects of a pest and its natural enemies are essentially important for integrated pest management (IPM) (Deguine et al. 2021). It is necessary to conduct biological investigations for the conservation and assessment of the potential of any biocontrol agent. C. phyllocnistoides and C. ingenuus had already been reported from Sargodha (Pakistan) attacking the P. citrella (Arshad et al. 2018b). However, limited literature is available on the effect of temperature on the ovipositional periods, fecundity, longevity and parasitization of both parasitoids.
In this study, temperature showed non-significant effect on the pre-and post-ovipositional periods of C. phyllocnistoides and C. ingenuus. Like some other eulophids (Mafi and Ohbayashi 2010), some of the females of both parasitoids began to lay eggs shortly after being exposed to P. citrella larvae, while others do it in two days. Our findings are also similar to Elekcioğlu (2017) who reported that the females of C. phyllocnistoides started egg-laying immediately after releasing on P. citrella larvae. Similarly, Mafi and Ohbayashi (2010) observed that mated females of Chrysocharis pentheus started oviposition after 1–2 days of emergence and continued egg-laying up to 40 days. The post-ovipositional period of C. phyllocnistoides was noticeably shorter than C. ingenuus, as the females died soon after depositing their last eggs. Mafi and Ohbayashi (2010) reported a shorter post-ovipositional period (5.0 ± 0.74 days) of C. phyllocnistoides than C. pentheus.
A gradual reduction in the ovipositional period of parasitoids by increasing the temperature level was noticed. Thus, temperature could be the main reason influencing the fecundity of insects (Kobori and Hanboosong 2017). The present study also showed a significant effect of temperature on the ovipositional and fecundity of both parasitoids. The temperature on which the oviposition rate of parasitoids was higher, termed as the optimal temperature for oviposition and it was 20°C for both C. phyllocnistoides and C. ingenuus. Whereas, the fecundity of both parasitoids was maximum at 25°C, which significantly reduced when moving away from this temperature. Another study conducted by Urbaneja et al. (2002) reported similar results having maximum oviposition of Cirrospilus sp. near lyncus at 25°C and fecundity at 20°C. The fecundity of Hemiptarsenus varicornis (Eulophidae: Eulophinae), a parasitoid species of Liriomyza sativae significantly affected by a shift in temperature (Ridland et al. 2020). On the other hand, the fecundity of another Palearctic parasitoid Diglyphus isaea (Hymenoptera: Eulophidae) had a higher net reproductive rate on L. trifolii at 15–30°C (Hondo et al. 2006).
Longevity of both C. phyllocnistoides and C. ingenuus had an inverse relationship with the temperature. The life duration of adults of both parasitoids significantly decreased as the temperature increased from 20 to 30°C. C. phyllocnistoides had a higher longevity than C. ingenuus. Several previous studies have supported our results that the longevity of adult parasitoids decreased by the increase in temperature (Elekcioğlu 2017).
It was found that the parasitism rate of both parasitoids was maximum at 25°C, followed by 30°C. Elekcioğlu (2017) reported increased parasitism of C. phyllocnistoides at high temperatures up to 32.5°C that decreased afterward. In Turkey, similar results were recorded in the field conditions where parasitism was higher during summer and autumn than in spring (Elekcioğlu and Uygun 2013). The parasitism of Tetrastichus phyllocnistoides (synonym of C. phyllocnistoides) was recorded 67.6% in the citrus orchards of China (Guangzhou) (Ding et al. 1989).
Conclusions
The findings suggest that parasitoids of P. citrella are better adapted at 20–25°C; both parasitoids can be expected to be an important mortality factor for P. citrella populations at this temperature regime. Data on parasitism rate, host-feeding, and longevity can be used to determine the suitability of the parasitoids and to design an effective strategy for the introduction of parasitoids in citrus orchards. Parasitoid releases may be best suited to the spring flushes to improve overall P. citrella suppression.
Availability of data and materials
Data will not be shared.
Abbreviations
- RH:
-
Relative humidity
- ANOVA:
-
Analysis of variance
- LSD:
-
Least significant difference
References
Amalin DM, Pena JE, Duncan RE, Browning HW, Mcsorley R (2002) Natural mortality factors acting on citrus leafminer, Phyllocnistis citrella, in line orchards in south Florida. Biocontrol 47:327–347
Amiri BB (2007) Efficacy of Bacillus thuringiensis and mineral oil against Phyllocnistis citrella Stainton (Lepidptera: Gracillariidae). Int J Agric Biol 9:893–896
Arshad M, Ullah MI, Qureshi JA, Afzal M (2018a) Physiological effects of citrus leafminer Phyllocnistis citrella (Lepidoptera: Gracillariidae) larval feeding on photosynthetic and gaseous exchange rates in citrus. J Econ Entomol 111:2264–2271. https://doi.org/10.1093/jee/toy150
Arshad M, Ullah MI, Afzal M (2018b) Citrus leafminer and its parasitoids in Sargodha region of Pakistan. Bulletin OEPP/EPPO Bulletin 48:309–313. https://doi.org/10.1111/epp.12479
Arshad M, Ullah MI, Afzal M, Khalid S, Raza ABM, Iftikhar Y (2019) Evaluation of plant extracts for the management of citrus leafminer, Phyllocnistis citrella (Lepidoptera: Gracillariidae). Kuwait J Sci 46:58–67
Beattie A, Hardy S (2004) Citrus leafminer. Agfact H2.AE.4, 4th edition. NSW department of primary industries. Available online: https://www.google.com/search?q=Citrus+leafminer.+Agfact+H2.AE.4%2C+4th+edition&rlz=1C1CHZL_enPK812PK812&oq=Citrus+leafminer.+Agfact+H2.AE.4%2C+4th+edition&aqs=chrome.69i57.594j0j7&sourceid=chrome&ie=UTF-8
Chermiti B, Braham M, Znaidi M, Gahbiche H, Messelmani B, Dali M, Messelmani M (2001) Premiers re´sultats sur lÕ acclimatation dÕ Ageniaspis citricola Logvinovskaya (Hym., Encyrtidae), parasitoide spe´ciÞque de Phyllocnistis citrella Stainton (Lep., Gracillariidae). J Appl Entomol 125:45–52
Clausen CP (1931) Two citrus leaf miners of the Far East. USDA Technical Bulletin 252:1–13
Dahmane M, Chakali G (2022) Population dynamic monitoring of Phyllocnistis citrella (Lepidoptera: Gracillariidae) using immature stages sampling and male moth pheromone-baited traps. Int J Trop Insect Sci 42(4):3107–3113
De Prins W, De Prins J (2005) Gracillariidae (Lepidoptera). In: Landry B (ed) World Catalogue of Insects. Apollo Books, Stenstrup, pp 1–502
Deguine JP, Aubertot JN, Flor RJ, Lescourret F, Wyckhuys KA, Ratnadass A (2021) Integrated pest management: good intentions, hard realities. A Review. Agron Sustain Dev 41(3):1–35
Diez P, Pena J, Fidalgo P (2006) Population dynamics of Phyllocnistis citrella Stainton (Lepidoptera: Gracillaridae) and its parasitoids in Tafi Viejo, Tocuman, Argentina. Fla Entomol 89:328–335
Ding YM, Li M, Huang MD (1989) Studies on biology of two species of parasitoids Tetrastichus phyllocnistoides and Cirrospilus quadristriatus and their parasitization on the citrus leaf-miner Phyllocnistis citrella Stainton. In: Huang MD (ed) Studies on the integrated management of citrus insect pests. Beijing Academic Book and Periodical Press, pp 1063–1113
Elazab DS, Ahmed MAI, El-Mahdy MT, Amro A (2021) Citrus leafminer management: jasmonic acid versus efficient pesticides. J Plant Growth Regul 40:824–830
Elekcioğlu NZ (2017) Effect of different temperatures on the biology of Citrostichus phyllocnistoides (Narayanan) (Hymenoptera: Eulophidae) a parasitoid of Phyllocnistis citrella Stainton (Lepidoptera: Gracillariidae). Pak J Zool 49:685–691. https://doi.org/10.17582/journal.pjz/2017.49.2.685.691
Elekcioğlu NZ, Uygun N (2013) Population fluctuation of citrus leafminer, Phyllocnistis citrella Stainton (Lepidoptera: Gracillariidae) and its parasitoids in the eastern Mediterranean region of Turkey. Pak J Zool 45:1393–1403
Gottwald T, Bassanezi R, Amorim L, Bergamin-Filho A (2007) Spatial pattern analysis of citrus canker-infected plantings in Sao Paulo, Brazil, and augmentation of infection elicited by the Asian leafminer. Phytopathology 97(6):674–683. https://doi.org/10.1094/PHYTO-97-6-0674
Hondo T, Koike A, Sugimoto T (2006) Comparison of thermal tolerance of seven native species of parasitoids (Hymenoptera: Eulophidae) as biological control agents against Liriomyza trifolii (Diptera: Agromyzidae) in Japan. Appl Entomol Zool 41:73–82
Hoy MA, Nguyen R (2003) Parasitoid of the citrus leafminer, Cirrospilus ingenuus Gahan (Insecta: Hymenoptera: Eulophidae). IFAS Extension, University of Florida. Available online: http://entnemdept.ufl.edu/creatures/beneficial/cirrospilus_ingenuus.html
Kalaitzaki A, Perdikis D, Tsagkarakis A, Koufakis I, Lykouressis D (2021) Life table and biological characteristics of the parasitoid Semielacher petiolatus reared on Phyllocnistis citrella. Bull Insectology 74(1):129–137
Kerns D, Wright G (2001) Loghry J (2001) Citrus Leafminer (Phyllocnistis citrella). The University of Arizona, Cooperative Extension
Kobori Y, Hanboosong Y (2017) Effect of temperature on the development and reproduction of the sugarcane white leaf insect vector, Matsumuratettix hiroglyphicus (Matsumura) (Hemiptera: Cicadellidae). J Asia-Pac Entomol 20:281–284
Li J, Wu Y, Zhang Q, Li H, Pan H, Lu W, Wang D, Zhang J, Lu Y (2018) Aphid parasitism and parasitoid diversity in cotton fields in Xinjiang. China Plos One 13(11):e0207034
Mafi S, Ohbayashi N (2010) Biology of Chrysocharis pentheus, an endoparasitoid wasp of the citrus leafminer Phyllocnistis citrella Stainton. J Agric Sci Technol 12:145–154
Mansour D, Braham M (2020) The Citrus Leafminer, Phyllocnistis Citrella, In Tunisia: twenty five years of invasion and pest management. Mun Ent Zool 15(2):526–535
Mustafa I, Arshad M, Ghani A, Ahmad I, Raza ABM, Saddique F, Asif S, Khan MR, Ahmed H (2014a) Population dynamics of citrus leaf miner on different varieties of citrus in correlation with abiotic environmental factors in Sargodha District, Punjab, Pakistan. Phytoparasitica 42:341–348. https://doi.org/10.1007/s12600-013-0371-4
Mustafa I, Aslam M, Arshad M, Saman UMI, Mustaqeem M, Bokhari SA, Asif S, Khan MR, Waqas A, Ahmed H (2014b) Association of citrus leaf miner, Phyllocnistis citrella (Lepidoptera: Gracillariidae: Phyllocnistinae) with leaf biochemical factors (Ca+2, K+ and Mg+2) in kinnow leaves of district Sargodha, Punjab, Pakistan. Pak J Zool 46:953–958
Ridland PM, Umina PA, Pirtle EI, Hoffmann AA (2020) Potential for biological control of the vegetable leafminer, Liriomyza sativae (Diptera: Agromyzidae). Australia with Parasitoid Wasps Austral Entomol 59(1):16–36
Stainton HT (1856) Descriptions of three species of Indian Micro-Lepidoptera. Tran R Entomol Soc Lond 3:301–304
Tsagkarakis AE, Perdikis DC, Lykouressis DP (2013) Introduced and native parasitoids of Phyllocnistis citrella Stainton in Greece: Short-term post-release evaluation. Phytoparasitica 41:417–428. https://doi.org/10.1007/s12600-013-0303-3
Ujiye T (2000) Biology and control of the citrus leafminer, Phyllocnistis citrella Stainton (Lepidoptera: Gracillariidae) in Japan. Jpn Agric Res Q 34:167–173
Urbaneja A, Hinarejos R, Llacer E, Garrido A, Jacas JA (2002) Effect of temperature on the life history of Cirrospilus sp. (Hymenoptera: Eulophidae), an ectoparasitoid of Phyllocnistis citrella (Lepidoptera: Gracillariidae). J Econ Entomol 95:250–255. https://doi.org/10.1603/0022-0493-95.2.250
Vercher R, Costa-Comelles J, Marzal C, García-Marí F (2005) Recruitment of Native Parasitoid Species by the Invading Leafminer Phyllocnistis citrella (Lepidoptera: Gracillariidae) on Citrus in Spain. Environ Entomol 34:1129–1138. https://doi.org/10.1093/ee/34.5.1129
Zhu CD, LaSalle J, Huang DW (2002) A study of Chinese Cirrospilus Westwood (Hymenoptera: Eulophidae). Zool Stud 41:23–46
Acknowledgements
The authors are thankful to the Department of Plant Pathology, University of Sargodha for providing facilities for the experiment.
Funding
There is no funding source to be declared for this study.
Author information
Authors and Affiliations
Contributions
MIU and MA planned and designed the project and experimental layout. SMAZ and NA performed the experiment. SA performed the statistical analysis. The manuscript was prepared by MA and HMA and reviewed by MIU. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ullah, M.I., Arshad, M., Ali, S. et al. Temperature-dependent effects on some biological aspects of two ectoparasitoids of Phyllocnistis citrella (Lepidoptera: Gracillariidae). Egypt J Biol Pest Control 33, 90 (2023). https://doi.org/10.1186/s41938-023-00736-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41938-023-00736-6