Abstract
Parasitoids are insects (usually wasps or flies) that lay eggs within or on other insects (their hosts). Host-feeding parasitoids lay eggs to parasitize the host and feed directly on the host for nourishment. Temperature is the most critical factor affecting insect behavioral responses. Few studies have focused on the impacts of variable temperatures across different life stages on the behaviors of host-feeding parasitoids. This study investigated the effects of temperature experienced during the preadult and adult stages on the life history traits and life table parameters of females of a host-feeding parasitoid, Eretmocerus hayati. Our results show that the temperatures experienced during the preadult and adult stages significantly change life history traits (immature development, adult longevity, host feeding and fecundity). Increasing the preadult temperature resulted in shorter development times for immature stages of the parasitoid, and decreasing the temperature during the adult stage increased reproduction and longevity. Most importantly, we found that host-feeding events changed with temperature rather than life stage. The daily host-feeding ability of the parasitoid increased with increasing temperature at all temperatures except the stress temperature (34 °C). Furthermore, switching temperatures at the immature stage and adult stage can increase the values of life table parameters, with the highest intrinsic rate of increase (r) occurring in the 30/26 °C treatment. This study provides new insight into the mass rearing of parasitic natural enemies.
Similar content being viewed by others
Introduction
Parasitoids are insects (wasps or flies) in which adult females forage for hosts, usually other insects, and deposit their eggs in, on, or near these hosts1,2. Since the foraging behavior of females is directly linked to their reproductive success, parasitoids are interesting biological models for addressing questions in behavioral ecology1. Temperature influences all levels of biological organization3,4,5. With an increase in temperature, the higher kinetic energy of biochemical reactions speeds up the rate of metabolic processes, which subsequently affects the physiology and behavior of individual ectotherms3,4,5. Because parasitoids are typical ectotherms, their behaviors are closely linked to the environmental temperature5,6,7,8. Previous studies have found that temperature can indirectly or directly affect behavioral decisions through its influence on many fitness traits, such as initial egg load9,10, patch residence time9,10,11,12, oviposition rate12, lifetime fecundity9,10,13, and the learning reliability of parasitoids12. So far, a third of parasitoid species (almost 100 000 species) have been found to be host-feeding parasitoids, indicating that they can suppress the populations of their hosts as a result of parasitization and/or by feeding on their hosts when they are in the adult stage14. However, to date, most studies on host-feeding parasitoids have mainly focused on their behavioral and physiological ecology15,16,17, and the impacts of changing temperatures on the varying life stages of long-feeding parasitoids have received comparatively little attention18,19.
Parasitoids are important in the natural control of herbivorous insect populations and are often used as biological control agents in agriculture1. How to maximize their lifetime fitness is a pivotal question, especially in terms of optimizing their reproductive ability to increase the efficacy of biological control20. Many previous studies have compared the life table parameters of parasitoids at different constant temperatures to identify the best intrinsic rate of increase (r)18,19,21,22,23,24,25. The developmental rates of larval parasitoids usually increase monotonically with temperature, showing a monotonic left-skewed pattern, while reproduction/fecundity shows a symmetrical unimodal pattern3,22,23,24,25,26,27,28. This suggests that larval development and adult fecundity respond differently to temperature increases. Furthermore, as one of the most important population parameters in life table studies, r is strongly influenced by the duration of time from birth to the start of reproduction and, to a lesser extent, by survival and reproduction rates22,28,. Hence, it is not reasonable to choose the best r value by directly comparing them among different constant temperatures over an entire generation, as the net effect of temperature on parasitoid fitness should depend on its relative influence on the immature and adult stages22,23,24,25,26,27,28,29. However, surprisingly few studies have considered thermal variation across different life stages9,10.
Eretmocerus hayati Zolnerowich & Rose (Hymenoptera: Aphelinidae) is a host-feeding primary parasitoid of the invasive agricultural pest Bemisia tabaci (commonly known as whitefly, Hemiptera: Aleyrodidae). An adult E. hayati parasitizes and feeds directly on its hosts16,30,31. The main purpose of this study was to explore how variable temperatures experienced during the preadult and adult stages modify the life-history parameters of E. hayati. The immature development time, lifetime feeding, lifetime fecundity, and adult longevity of E. hayati reared on the B. tabaci cryptic species MED were examined at different combinations of temperatures experienced during the preadult and adult stages, and the biological efficacy and life table parameters under the different treatments were analyzed. We speculate that the immature development time and adult longevity decrease with increasing temperature experienced at preadult stages, while lifetime fecundity and host-feeding events are influenced by the temperatures experienced at the preadult and adult stages. Furthermore, we hypothesize that the life table parameters can change in accordance with variable temperature combinations. In other words, increasing the preadult temperature could shorten the developmental time, and variation in the adult temperature could change lifetime fecundity, which could together maximize r.
Results
Immature development time
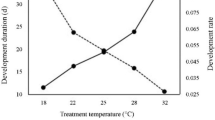
The duration of each immature developmental stage (egg + larva, pupa, and all immature stages) significantly decreased with increasing preadult temperature (egg + larva: F3, 151 = 313.87, P < 0.0001; pupa: F3, 151 = 250.68, P < 0.0001, all immature stages: F3, 151 = 591.59, P < 0.0001, Table 1). Parasitoids showed the fastest rate of development at 34 °C and the slowest at 22 °C.
Female longevity
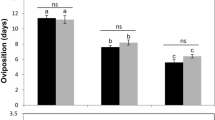
Temperature had a significant effect on female longevity (F3, 232 = 47.98, P < 0.0001, Table 2, Fig. 1) but had no impact on developmental stage (F2, 232 = 0.56, P = 0.5712). There was, however, a significant interaction between female longevity and developmental stage (F6, 232 = 2.75, P = 0.0134). When temperatures were changed at the preadult and adult stages simultaneously, the female longevity at 22 °C and 26 °C was significantly longer than that at 30 °C and 34 °C (all Tukey-Kramer tests: P < 0.05). However, when temperatures were changed at the preadult stage alone or at the adult stage alone, the longest longevity occurred at 22 °C and 26 °C, intermediate longevity occurred at 30 °C, and the shortest longevity occurred at 34 °C (all Tukey-Kramer tests: P < 0.05). When the temperature was fixed, adult longevity did not significantly differ among the developmental stages (all Tukey-Kramer tests: P > 0.05).
The effects of variable temperatures on the life history traits ((a) host feeding, (b) lifetime fecundity, (c) female longevity) of Eretmocerus hayati across different life stages. Bars topped by different capital letters at the same temperature indicate a significant difference among developmental stages; bars topped by different lowercase letters during the same developmental stage indicate a significant difference among temperatures.
Host feeding and lifetime fecundity
Developmental stage and temperature had significant effects on host feeding (developmental stage: F2, 232 = 2.62, P = 0.0754; temperature: F3, 232 = 29.63, P < 0.0001, Table 2, Fig. 1) and lifetime fecundity (developmental stage: F2, 232 = 5.51, P = 0.0046; temperature: F3, 232 = 36.94, P < 0.0001, Table 2, Fig. 1). There was no significant interaction between host feeding and lifetime fecundity. When the developmental stage was fixed, the greatest host feeding and lifetime fecundity values were attained at 26 °C, intermediate values were found at 22 °C and 30 °C, and the smallest values were found at 34 °C (all Tukey-Kramer tests: P < 0.05). Conversely, no significant difference was found in host feeding or lifetime fecundity among the different developmental stages when temperature was fixed (all Tukey-Kramer tests: P > 0.05) with the exception of lifetime fecundity when the temperature was 34 °C (Tukey-Kramer test: P < 0.05).
Daily host feeding and daily fecundity
Developmental stage and temperature had significant effects on daily host feeding (developmental stage: F2, 232 = 5.83, P = 0.0034; temperature: F3, 232 = 52.99, P < 0.0001, Table 2, Fig. 2) and daily fecundity (developmental stage: F2, 232 = 13.01, P < 0.0001; temperature: F3, 232 = 66.76, P < 0.0001, Table 2, Fig. 2), and there were significant interactions between these variables (host feeding: F6, 232 = 5.72, P < 0.0001; fecundity: F6, 232 = 23.81, P < 0.0001).
The effects of variable temperatures on (a) daily host feeding and (b) daily fecundity in Eretmocerus hayati across different stages. Bars topped by different capital letters at the same temperature indicate a significant difference among developmental stages; bars topped by different lowercase letters during the same developmental stage indicate a significant difference among temperatures.
When the developmental stage was fixed, the greatest daily host feeding during three different development stage treatments occurred at 30 °C, intermediate values were found at 22 °C and 26 °C, and the smallest values occurred at 34 °C (all Tukey-Kramer tests: P < 0.05). However, when the temperature was fixed at 22 °C, the most daily host feeding occurred during the preadult stage alone, while the lowest amount occurred during both the preadult and adult stages (all Tukey-Kramer tests: P < 0.05). When the temperature was fixed at 30 °C, the most daily host feeding occurred during both the preadult and adult stages, while the lowest amount occurred during the adult stage alone (all Tukey-Kramer tests: P < 0.05). There were no significant differences among the different development stages when the temperature was fixed at 26 °C and 34 °C (all Tukey-Kramer tests: P > 0.05).
When the developmental stage was fixed at both the preadult and adult stages, the greatest daily fecundity occurred at 26 °C, intermediate daily fecundity occurred at 30 °C and 34 °C, and the lowest daily fecundity occurred at 22 °C (all Tukey-Kramer tests: P < 0.05). Similarly, when the developmental stage was fixed at the adult stage only, the greatest daily fecundity also occurred at 26 °C, but the lowest occurred at 34 °C (all Tukey-Kramer tests: P < 0.05). When the developmental stage was fixed at the preadult stage only, the daily fecundities at 26 °C, 30 °C, and 34 °C were significantly greater than those at 22 °C (all Tukey-Kramer tests: P < 0.05), and there were no significant differences among the effects of 26 °C, 30 °C, and 34 °C (all Tukey-Kramer tests: P > 0.05). When the temperature was fixed at 30 °C or 34 °C, the greatest effect on daily fecundity was found at the preadult stage only, and the smallest effect on daily fecundity occurred during the adult stage only (all Tukey-Kramer tests: P < 0.05). There were no significant differences when the temperature was fixed at 22 °C or 26 °C (all Tukey-Kramer tests: P > 0.05).
Life table and population parameters
When the temperature was altered at both the preadult and adult stages, r and finite rate of increase (λ) were at their highest at 30 °C/30 °C, intermediate at 26 °C/26 °C, and lowest at 34 °C/34 °C (all P < 0.05). However, the net reproductive rate (R0), mean generation time (T), and gross reproductive rate (GRR) decreased significantly with increasing temperature (all P < 0.05) in all treatments except the 22 °C/22 °C treatment (Table 3).
When the temperature was changed at the preadult stage only, r and λ were also highest at 30 °C/26 °C, intermediate at 34 °C/26 °C, and lowest at 22 °C/26 °C (all P < 0.05; Table 3). However, R0, T, and GGR decreased significantly with increasing temperature in all cases except in the 22 °C/26 °C treatment (all others: P < 0.05; Table 3).
When the temperature was altered at the adult stage only, r and λ were also the highest at 26 °C/26 °C, intermediate at 26 °C/30 °C, and lowest at 26 °C/34 °C (all P < 0.05; Table 3). However, R0, T, and GGR decreased significantly with increasing temperature in all cases except the 26 °C/22 °C treatment (all P < 0.05; Table 3).
When the temperature was switched between 22 °C and 26 °C, r and λ changed significantly (all P < 0.05, Table 3), and optimal values were found for the two parameters at 26 °C/22 °C. When the temperature was switched between 30 °C and 26 °C, r and λ changed significantly (all P < 0.05, Table 3), and optimal values were found for the two parameters at 30 °C/26 °C. When the temperature was switched between 34 °C and 26 °C, r and λ changed significantly (all P < 0.05, Table 3), and optimal values were found for the two parameters at 34 °C/26 °C.
Discussion
All immature stages of E. hayati exhibited the same temperature-dependent trend in duration: shorter developmental stages at higher preadult temperatures. This is consistent with other temperature-dependent studies on parasitoids, such as Encarsia citrina Craw33, Microplitis manilae Ashmead23, Trichogramma pretiosum Riley32 and Campoletis chlorideae Uchida22. It is likely a common rule that the development rate of parasitoids, typically ectothermic insects, increases with temperature because the impacts of increased temperature on insect performance depend on the ambient temperature and its difference from the insects’ optimal temperature. If the ambient temperature is below the optimal temperature, a temperature increase to close to its optimal temperature will improve an insect’s performance. However, if the ambient temperature is close to the optimal temperature, the temperature increase may decrease the insect’s performance.
Similarly, we found that temperature significantly affected the longevity of female adults regardless of the stage of development. When temperature varied at the preadult stage only, our results demonstrated that females that developed at low temperatures and were then exposed to 26 °C had a longer lifespan than those that developed at higher temperatures. This is consistent with the results found for the parasitoid species Aphidius colemani13 and Aphidius ervi10 but differed from the findings of Le Lann et al.9, who found that when females of the wasp Aphidius rhopalosiphi developed at low temperatures and were then exposed to 20 °C, they had a higher metabolic rate and subsequently a lower longevity. These contrasting results could be due to differences in their study species, such as the climatic origin of the populations9,13 or their reproductive modes34,35.
As a typical host-feeding parasitoid, E. hayati feeds directly on its host, not only obtaining nutritional benefits but also killing the host30. We found that host feeding was mainly affected by temperature rather than developmental stage. The parasitoids underwent the most lifetime host-feeding events at 26 °C and the least at 34 °C. However, the largest amount of daily host feeding occurred at 30 °C, while the smallest amount occurred at 22 °C. In other words, as the temperature increased, the parasitoids became more active at every temperature except 34 °C. There are two possible explanations for this result: (1) The metabolic rate could increase, with resources for energy being utilized at a higher rate. In this situation, parasitoids need to replenish their energy or nutrients by feeding on the host, which could result in an increase in daily host feeding at higher temperatures. (2) As the temperature increases, the use of nutrient substances, especially that of the limited lipid reserves, for body maintenance could also increase. Therefore, the nutrient levels of female parasitoids would decrease, indirectly increasing their tendency to feed on their hosts. Our results explored the host-feeding behavioral response of a host-feeding parasitoid facing thermal variance. However, we did not take the real-time metabolic rate or morphological and physiological traits into consideration, so further research should be systematically conducted in the future.
In the present study, we found that the lifetime fecundity of E. hayati depended on thermal variance and showed a concave thermal reaction norm shape. The greatest effect on lifetime fecundity occurred at 26 °C while the smallest occurred at 34 °C regardless of the developmental stage. This implies that 26 °C, as an intermediate temperature, is the optimal temperature for E. hayati and that 34 °C would be a stressful temperature for development. When the temperature varied only at the preadult stage, the different temperatures significantly affected the fecundity of E. hayati. These results were consistent with those of previous studies on other parasitoids, such as Leptopilina heterotoma36 and Aphidius colemani13. Fecundity in parasitoids generally depends on the egg load, and oogenesis usually commences during the pupal stage37. Different preadult temperatures could not only change the initial egg load but also affect the capital resources, further resulting in different behavioral responses13,36,. However, the results contrasted with those found in Aphidius rhopalosiphi9, showing that when A. rhopalosiphi individuals developed at low temperatures (10 °C) and were then exposed to 20 °C, they laid more eggs per patch than those at higher temperatures. Thus far, the reasons for these contradictory results are not clear.
When the temperature was altered in the adult stage only, the effect of temperature on fecundity was similar to that in the treatments mentioned above. This means that variation in temperature during the adult stage significantly altered the fecundity of E. hayati. Amat et al.34 identified two reproductive modes (thelytokous and arrhenotokous) in Venturia canescens females, in which fecundity differed in response to variable thermal conditions experienced during the adult stage. The thelytokous mode was not significantly altered by changes in temperature, but fecundity in arrhenotokous individuals significantly decreased with increasing temperature. The differences in the response to variable temperature between reproductive modes may be accounted for by differences in selective pressure in the preferred habitats of the wasps using these two modes34.
When temperature was changed at both the preadult and adult stages, the effects on fecundity were similar to those found in many previous studies, such as those found for Campoletis chlorideae22, Eretmocerus mundus18, Encarsia acaudaleyrodis19, Microplitis manilae23, and Encarsia inaron24. These results were similar to those found when constant temperatures were varied across the entire generation. Most of these parasitoids were nonhost-feeding parasitoids, in which almost the entire egg load matures at the pupal stage or before emergence. In host-feeding parasitoids, however, the lifetime egg load includes both the initial egg load that matures during the pupal stage and the eggs that mature during the adult stage38,39. Host-feeding parasitoids can replenish nutrients for enhanced fecundity and prolonged longevity during the adult stage through host feeding15,16,17. Although E. mundus is a host-feeding parasitoid40, Zandi-Sohani and Shishehbor19 did not focus on its host-feeding behavior. To the best of our knowledge, our study could be the first to investigate the behavioral responses (oviposition and host feeding) of a host-feeding parasitoid in relation to temperature. However, because it is difficult to estimate the life history traits of a host-feeding parasitoid at different temperatures when host-feeding behavior is obstructed, we could not further analyze the effects on the costs and benefits to life history traits by host feeding under different thermal conditions.
All the fitness traits (lifetime fecundity, daily fecundity, host feeding, and daily host feeding) of the parasitoids sharply decreased at 34 °C. This means that 34 °C is a stressful temperature for E. hayati. There are two possible explanations for this phenomenon: (1) higher metabolic rates at higher temperatures cost more in terms of energetic substance requirements, and (2) stressful temperatures may alter the patch exploitation behavior of parasitoids, such that they spend more time resting or grooming on the patch and less time on host feeding along with reduced investment in reproduction. Although we did not record these behaviors, they were frequently observed during the study. Similar results have been previously reported. Le Lann et al.9 stated that females of the parasitic wasp A. rhopalosiphi reared at 25 °C spent considerably more time resting and grooming on a patch than females reared at lower temperatures. This again suggests that 25 °C is a stressful temperature for development. Sentis et al.26 found that the relationship between the host-handling rate of Coleomegilla maculata lengi Timberlake (Coleoptera: Coccinellidae) and temperature was hump shaped, suggesting that their handling activity was reduced at extreme temperatures. This has also been confirmed in other studies41,42. We proposed that when facing a stressful thermal environment, parasitoids will not only directly consume more energy resources for body maintenance but also reduce activity to preserve their energy resources.
The population parameters, such as r and λ, showed an almost symmetrical, unimodal pattern as λ = e r. The greatest r and λ values were found in the females from the 30 °C/30 °C treatment when temperature was changed at both the preadult and adult stages, while when temperature was only changed at the preadult stage, the greatest r and λ values were found in the females from the 30 °C/26 °C treatment. Meanwhile, we found that increasing the preadult temperatures (30 °C) shortened the immature developmental times but that the same preadult temperatures (26 °C) prolonged adult longevity and increased fecundity compared to the effects of the 30 °C/30 °C treatment. In other words, by varying the thermal conditions across the different life stages, we decreased the developmental time from birth to reproductive maturity and improved the net fecundity rate, which further affected the reproductive and host suppression efficacy (Fig. 1S). This suggests that switching temperatures across the preadult stages and the adult stage can accelerate the immature developmental rate, prolong adult longevity and enhance fecundity, further achieving better reproductive and host suppression efficacy. These results also reveal that the net effect of temperature on parasitoid fitness should depend on its relative influence on the preadult and adult stages, so it would be unreasonable to identify the optimal r value by directly comparing them from different constant temperatures during the entire generation21,22,23,24,25. These results may also have implications for the use of parasitic biological control agents. Switching temperatures across different life stages could improve rearing efficiency during the mass production of biological control agents. For example, immature parasitoids may be allowed to develop at a relatively high temperature to shorten the time from egg to emerging adult. Female parasitoids can then be transferred to an environment with a relatively cooler temperature to increase lifetime fecundity and prolong longevity. Naturally, the improvement in the performance of adult parasitoids would have to be weighed against the cost of decreasing the immature developmental time.
In summary, our study demonstrates that the temperatures experienced during the preadult and adult stages in a host-feeding parasitoid significantly change its life history traits (immature development, adult longevity, host feeding and fecundity). Increasing the preadult temperature resulted in shorter development times for immature stages of the parasitoid, and decreasing the temperature in the adult stage promoted reproduction and longevity. Most importantly, we found that host feeding changed with temperature rather than life stage. The daily host-feeding ability of parasitoids increased with increasing temperature at all temperatures except the stress temperature (34 °C). Furthermore, switching temperatures at the immature stage and adult stage can improve the values of life table parameters. This study provides new insight into the mass rearing of parasitic natural enemies.
Methods
Whitefly and parasitoid cultures
Both whiteflies and their parasitoids were collected from cotton fields during the summer of 2012 in the Ha-Mi region of Xinjiang Uygur Autonomous Region, northwestern China (E89°8′, N42°53′, 12 m a.s.l). Then, colonies of these insects were constructed at the Department of Biological Invasions (DBI), Institute of Plant Protection, Chinese Academy of Agricultural Sciences, Beijing, China. All plants and insects were maintained in a climate-controlled chamber at 26 ± 1 °C and 70–80% relative humidity (RH) and under a 14 h:10 h L:D photoperiod.
Colonies of B. tabaci MED were isolated and maintained on young cotton plants (Gossypium hirsutum L.) in a plastic box (20 × 30 × 20 cm). The cotton plants were grown in black turf soil and were used to culture the whitefly colonies when they had four fully opened leaves. The identity of the whitefly species in the colony was checked each month by sequencing the cytochrome oxidase I mtDNA from the whiteflies and then comparing it with the available sequences in the NCBI database.
To generate a laboratory colony of the parasitoid, E. hayati was cultured on 2nd–3rd instar nymphs of B. tabaci collected from the same site as the parasitoids and maintained on young cotton plants in the greenhouse of the DBI.
Experimental setup
The temperatures applied in the study were divided into preadult temperatures for preadult parasitoids and adult temperatures for adult parasitoids. The temperature regimen consisted of four levels: 22 °C, 26 °C, 30 °C, and 34 °C, reflecting the temperature gradient the whitefly and parasite had experienced in the field based on five years of observations. To prevent potential maternal effects, all females were reared for several generations (50 generations) at the same temperature (26 °C). To explore the effects of variable temperatures across different life stages on the life history parameters, we used variable temperatures in the preadult and adult stages (preadult temperature/adult temperature: 22 °C/22 °C, 30 °C/30 °C, 26 °C/26 °C and 34 °C/34 °C), variable temperatures in the preadult stage only (22 °C/26 °C, 26 °C/26 °C, 30 °C/26 °C and 34 °C/26 °C), and variable temperatures in the adult stage only (26 °C/22 °C, 26 °C/26 °C, 26 °C/30 °C and 26 °C/34 °C). All experiments were conducted in climate-controlled growth chambers (75 ± 5% RH, 14 h:10 h L:D photoperiod).
Development of immature parasitoids at different rearing temperatures
For the development experiments, clean cotton plants with four fully open leaves were transferred into a cage (20 × 20 × 20 cm) and covered with fine gauze. We then transferred 100–120 adult whiteflies into this cage. The whiteflies were allowed to lay eggs for 24 h before being removed. The cotton leaves and eggs were incubated at 26 °C for 10 d and then checked daily with a binocular microscope until the whiteflies reached the appropriate developmental stage for parasitization. According to previous experiments, the late 2nd to early 3rd instar stage of the host nymph is the appropriate stage31. We found that this stage was reached after 15–17 d.
The cotton plants infested with suitable nymphs were moved into a cage (20 × 20 × 20 cm). Five newly emerged and mated E. hayati females were then released into the cage and allowed to lay eggs for 2 h. A total of 12 cotton plants were treated in this manner. These plants were randomly divided among the four preadult temperatures (22 °C, 26 °C, 30 °C, and 34 °C).
The parasitized nymphs were randomly collected and numbered to facilitate the precise monitoring of their larval and pupal development times. As the E. hayati eggs were laid between the nymph host and cotton plant30, it was too difficult to observe the process of egg hatching or to record the egg stage. Therefore, we considered the egg and larval stages together and named it the “egg + larval” stage.
Life history of adult parasitoids at different oviposition temperatures
For each experimental oviposition temperature, 20 pairs of newly emerged (less than 6 h old) adult parasitoids were isolated on a cotton plant with four leaves and infested with 2nd–3rd instar nymphs (40–50 nymphs per plant). The cotton plants were refreshed daily at 18:00 until all the parasitoids had died. The cotton plants with parasitized whiteflies were transferred to gauze cages (20 × 20 × 20 cm) and kept at their respective oviposition temperatures in climate-controlled growth chambers. If a host was parasitized, mycetoma displacement was visible though the cuticle under a microscope. If a host was directly fed upon, the body became flat and desiccated30. Fecundity, host feeding, and longevity were recorded after 8 d. Because adult males and females were kept as pairs and only female parasitoids have host suppression capacity (lifetime fecundity and host feeding), fecundity and host feeding records were assigned to the females, and daily host feeding and daily fecundity were further calculated.
Life table analysis
To predict the effects of variable temperatures across different stages on the population viability of a host-feeding parasitoid, we collected raw data on the survivorship, longevity, and daily fecundity of individual females. We analyzed the life table traits (such as the intrinsic growth rate28) according to the age-stage, two-sex life table method using the computer program TWOSEX-MSChart43,44,45. Following Chi and Liu (1985)43, we calculated the age-stage-specific survival rate (sxj), where x is age and j is the stage, as well as the age-specific survival rate (lx), the age-stage-specific fecundity (fxj), the age-specific fecundity (mx), R0, r, λ, and T. R0 was calculated as follows:
r was estimated using the iterative bisection method and the Euler-Lotka equation with the age indexed from 046:
λ and T were calculated as follows:
Statistical analysis
The immature development times (including those of the larva, pupa and all immature stages) for E. hayati at the four different preadult temperatures were compared with one-way ANOVA complemented with Tukey’s honestly significant difference (HSD) test, as the raw data met the assumptions of normality and homoscedasticity.
A general linear model (GLM, two-way ANOVA) was used to test for differences in life history traits (lifetime fecundity, lifetime host feeding, adult longevity, daily fecundity, and daily host feeding). Temperature (22 °C, 26 °C, 30 °C, and 34 °C) and developmental stage (preadult only, adult only, and preadult + adult) were used as the two factors in the model, as all life history traits met the assumptions of normality and homoscedasticity. Tukey multiple comparisons among least-square means were conducted when any of the factors in the models were found to be significant. All analyses were carried out with SAS software version 9.2.
Bootstrapping47 was used to estimate the means, variances, and standard errors of the population parameters. Because bootstrapping uses random resampling, a small number of replicates can generate variable means and standard errors. To generate less variable results, we used 10,000 replicates. We used the Tukey-Kramer procedure48 in the TWOSEX-MSChart computer program to identify differences among treatments involving variable temperatures in both the preadult and adult stages, adult stage only, and preadult stage only.
Informed consent
Informed consent was obtained from all authors included in the study.
Research involving human participants and/or animals
This article does not contain any studies involving human participants or animals performed by any of the authors.
References
Van Alphen, J. J. M., Bernstein, C. & Driessen, G. Information acquisition and time allocation in insect parasitoids. Trends Ecol. Evol. 18(2), 81–88 (2003).
Heimpel, G. E., Neuhauser, C. & Hoogendoorn, M. Effects of parasitoid fecundity and host resistance on indirect interactions among hosts sharing a parasitoid. Ecol. Lett. 6, 556–566 (2003).
Gillooly, J. F., Charnov, E. L., West, G. B., Savage, V. M. & Brown, J. H. Effects of size and temperature on developmental time. Science. 293, 2248–2251 (2002).
Angilletta, M. J. Thermal adaptation: a theoretical and empirical synthesis. Oxford University Press. Oxford, UK (2009).
Abram, P. K., Boivin, G., Moiroux, J. & Brodeur, J. Behavioural effects of temperature on ectothermic animals: unifying thermal physiology and behavioural plasticity. Biol. Rev. 92(4), 1859–1876 (2017).
Atlihan, R. & Chi, H. Temperature-dependent development and demography of Scymnus subvillosus (Coleoptera: Coccinellidae) reared on Hyalopterus pruni (Homoptera: Aphididae). J. Econ. Entomol. 101(2), 325–333 (2008).
Dixon, A. Relationship between the minimum and maximum temperature thresholds for development in insects. Func. Ecol. 23, 256–264 (2009).
Colinet, H., Sinclair, B. J., Vernon, P. & Renault, D. Insects in fluctuating thermal environments. Ann. Rev. Entomol. 60, 123–140 (2015).
Le Lann, C., Wardziak, C., van Baaren, J. & van Alphen, J. J. Thermal plasticity of metabolic rates linked to life history traits and foraging behaviour in a parasitic wasp. Func. Ecol. 25, 641–651 (2011).
Moiroux, J., Boivin, G. & Borodeur, J. Temperature influences host instar selection in an aphid parasitoid: support for the relative fitness rule. Biol. J. Linn. Soc. 115, 792–801 (2015).
Rosenheim, J. A. & Rosen, D. Foraging and oviposition decisions in the parasitoid Aphytis lingnanensis: distinguishing the influences of egg load and experience. J. Anim. Ecol. 60, 873–893 (1991).
Abram, P. K. et al. Thermal stress affects patch time allocation by preventing forgetting in a parasitoid wasp. Behav. Ecol. 26, 1326–1334 (2015).
Colinet, H., Boivin, G. & Hance, T. Manipulation of parasitoid size using the temperature-size rule: fitness consequences. Oecologia. 152, 425–433 (2007).
Kidd, N. A. C. & Jervis, M. A. Host-feeding and oviposition strategies of parasitoids in relation to host stage. Res. Popul. Ecol. 33, 13–28 (1991).
Zhang, Y. B., Liu, W. X., Wang, W., Wan, F. H. & Li, Q. Lifetime gains and patterns of accumulation and mobilization of nutrients in females of the synovigenic parasitoid, Diglyphus isaea Walker (Hymenoptera: Eulophidae), as a function of diet. J. Insect Physiol. 57, 1045–1052 (2011).
Zhang, Y. B., Zhang, H. L., Yang, N. W., Wang, J. J. & Wan, F. H. Income resources and reproductive opportunities change life history traits and the egg/time limitation trade-off in a synovigenic parasitoid. Ecol. Entomol. 39, 723–731 (2014).
Heimpel, G. E., Rosenheim, J. A. & Kattari, D. Adult feeding and lifetime reproductive success in the parasitoid Aphytis melinus. Entomol. Exp. App. 83, 305–315 (1997).
Zandi-Sohani, N., Shishehbor, P. & Kocheili, F. Parasitism of cotton whitefly, Bemisia tabaci on cucumber by Eretmocerus mundus: bionomics in relation to temperature. Crop Protection. 28, 963–967 (2009).
Zandi-Sohani, N. & Shishehbor, P. Temperature effects on the development and fecundity of Encarsia acaudaleyrodis (Hymenoptera: Aphelinidae), a parasitoid of Bemisia tabaci (Homoptera: Aleyrodidae) on cucumber. Biocontrol. 56, 257–263 (2011).
Krebs, J. R. & Davis, N. B. Behavioural ecology: an evolutionary approach. Oxford: Wiley-Blackwell (2009).
Krugner, R., Daane, K. M., Lawson, A. B. & Yokota, G. Y. Temperature-dependent development of Macrocentrus iridescens (Hymenoptera: Braconidae) as a parasitoid of the obliquebanded leafroller (Lepidoptera: Tortricidae): Implications for field synchrony of parasitoid and host. Biol. control. 42, 110–118 (2007).
Pandey, A. K. & Tripathi, C. P. M. Effect of temperature on the development, fecundity, progeny sex ratio and life-table of Campoletis chlorideae, an endolarval parasitoid of the pod borer, Helicoverpa armigera. BioControl. 53, 461–471 (2008).
Qiu, B., Zhou, Z. S., Luo, S. P. & Xu, Z. F. Effect of temperature on development, survival, and fecundity of Microplitis manila (Hymenoptera: Braconidae). Environ. Entomol. 41(3), 657–664 (2012).
Malekmohammadi, A., Shishehbor, P. & Kocheili, F. Influence of constant temperatures on development, reproduction and life table parameters of Encarsia inaron (Hymenoptera: Aphelinidae) parasitizing Neomaskellia andropogonis (Hemiptera: Aleyrodidae). Crop Protect. 34, 1–5 (2012).
Yu, J. Z., Chi, H. & Chen, B. H. Comparison of the life tables and predation rates of Harmonia dimidiate (F.) (Coleoptera: Coccinellidae) fed on Aphis gossypii Glover (Hemiptera: Aphididae) at different temperatures. Biol. Control. 64, 1–9 (2013).
Sentis, A., Hemptinne, J. L. & Brodeur, J. Parsing handling time into its components: implications for responses to a temperature gradient. Ecology. 94, 1675–1680 (2013).
Amarasekare, P. & Savage, V. A framework for elucidating the temperature dependence of fitness. Am. Natl. 179, 178–191 (2012).
Amarasekare, P. & Coutinho, R. M. The intrinsic growth rate as a predictor of population viability under climate warming. J. Animal Ecol. 82, 1240–1253 (2013).
Wu, G. M. et al. Temperature influences the handling efficiency of an aphid parasitoid through body size-mediated effects. Environ. Entomol. 40, 737–742 (2011).
Zhang, Y. B., Yang, N. W., Sun, L. Y. & Wan, F. H. Host instar suitability in two invasive whiteflies for the naturally occurring parasitoid Eretmocerus hayati in China. J. Pest Sci. 88(2), 1612–1618 (2015).
Zolnerowich, G. & Rose, M. The genus Eretmocerus. In: Gould J, Hoelmer K, Goolsby J (Eds.). Classical biological control of Bemisia tabaci in the United States-A review of interagency research and implementation, Springer Netherlands, Amsterdam. 4, 89–109 (2008).
Pratissoli, D. & Parra, J. R. P. Fertility life table of Trichogramma pretiosum (Hym., Trichogrammatidae) in eggs of Tuta absoluta and Phthorimaea operculella (Lep., Gelechiidae) at different temperatures. J. Appl. Entomol. 124, 339–342 (2000).
Matadha, D., Hamilton, G. C. & Lashomb, J. H. Effect of Temperature on Development, Fecundity, and Life Table Parameters of Encarsia citrina Craw (Hymenoptera: Aphelinidae), a Parasitoid of Euonymus Scale, Unaspis euonymi (Comstock), and Quadraspidiotus perniciosus (Comstock) (Homoptera: Diaspididae). Environ. Entomol. 33(5), 1185–1191 (2004).
Amat, I., Castelo, M., Desouhant, E. & Bernstein, C. The influence of temperature and host availability on the host exploitation strategies of sexual and asexual parasitic wasps of the same species. Oecologia 148, 153–161 (2006).
Foray, V., Desouhant, E. & Gibert, P. The impact of thermal fluctuations on reaction norms in specialist and generalist parasitic wasp. Func. Ecol. 28, 411–423 (2014).
Ris, N., Allemand, R., Fouillet, P. & Fleury, F. The joint effect of temperature and host species induce complex geotype-by-environment interactions in the larval parasitoid of Drosophila, Leptopilina heterotoma (Hymenoptera: Figitidae). Oikos. 106, 451–456 (2004).
Volkoff, A. N. & Daumal, J. Ovarian cycle in immature and adult stages of Trichogramma cacoeciae and T. brassicae (Hym. Trichogrammatidae). Entomophaga. 39, 303–312 (1994).
Jervis, M. A. & Kidd, N. A. C. Host-feeding strategies in hymenopteran parasitoids. Biol. Rev. 61, 395–434 (1986).
Heimpel, G. E. & Collier, T. R. The evolution of host-feeding behavior in insect parasitoids. Biol. Rev. 71, 373–400 (1996).
Gerling, D. & Fried, R. Biological studies with Eretmocerus mundus Mercet (Hymenoptera: Aphelinidae) in Israel. OILB/SROP Bull. 23, 117–123 (2000).
Englund, G., Ohlund, G., Hein, C. L. & Diehl, S. Temperature dependence of the functional response. Ecol. Lett. 14, 914–921 (2011).
Rall, B. C. et al. Universal temperature and body-mass scaling of feeding rates. Phil. Trans. Roy. Soc. B. 367, 2923–2934 (2012).
Chi, H. & Liu, H. Two new methods for the study of insect population ecology. Bulletin of the Institute of Zoology. Academia Sinica. 24, 225–240 (1985).
Chi, H. Life-table analysis incorporating both sexes and variable development rates among individuals. Environ. Entomol. 17, 26–34 (1988).
Chi, H. TWOSEX-MSChart: computer program for age stage, two-sex life table analysis. Available from: http://140.120.197.173/ecology/ (2012).
Goodman, D. Optimal life histories, optimal notation, and the value of reproductive value. Am. Natl. 119, 803–823 (1982).
Efron, B. & Tibshirani, R. J. An Introduction to the Bootstrap. Chapman and Hall, New York (1993).
Dunnett, C. W. Pairwise multiple comparisons in the homogeneous variance, unequal sample size case. J. Am. Statist. Assoc. 75, 789–795 (1980).
Acknowledgements
We would like to express our deep appreciations and thanks to Prof. Gongyin Ye and two anonymous reviewers for giving so many valuable comments to improve our manuscript. This research was supported by the National Natural Science Foundation of China (31701856), National Key R&D Project of China (2017YFC1200603, 2016YFC1202105).
Author information
Authors and Affiliations
Contributions
Z.Y.B., Z.G.F., L.W.X. and W.F.H. designed the experiment and wrote the paper. Z.Y.B. performed the experiment. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhang, YB., Zhang, GF., Liu, WX. et al. Variable temperatures across different stages have novel effects on behavioral response and population viability in a host-feeding parasitoid. Sci Rep 9, 2202 (2019). https://doi.org/10.1038/s41598-018-38087-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-38087-0
- Springer Nature Limited