Abstract
Background
Avocado “tristeza,” a disease caused by Phytophthora cinnamomi, is one of the main limiting factors of avocado production in the Caribbean region. To control the pathogen, the application of agrochemicals is required, but this has caused environmental problems. Trichoderma spp. present properties in promoting plant growth and controlling phytopathogens, being proposed as an alternative to replace chemical fertilizers. The objective of the study was to evaluate the antagonistic activity of Trichoderma spp. against P. cinnamomi and its possible potential for promoting plant growth in vitro. Soil samples were taken from avocado cultivars from the municipalities of Ovejas (Sucre-Colombia) and Chalán (Sucre-Colombia).
Results
Serial dilutions were carried out for the isolation of Trichoderma spp. Once the strains were purified, the antagonism test against P. cinnamomi was carried out in PDA culture medium. For growth promotion, SRS medium was used for phosphate solubilization and CAS medium for siderophore production. DNA extraction and identification of the isolates were performed using the tef1 gene. Trichoderma harzianum and T. asperellum presented a 93.4% inhibition against the pathogen, followed by T. viride with an inhibition of 83.5% and finally T. longibrachiatum with 78.4% inhibition, showing significant differences in the control of the growth of the pathogen (p < 0.05) and promoted plant growth. These species release enzymes that can degrade the cell wall of the pathogen causing its death or inhibit its growth through the production of secondary metabolites.
Conclusions
The results showed that the application of Trichoderma spp. in crops confers protection against pathogens and stimulates the growth of plants to obtain a high yield.
Similar content being viewed by others
Background
Avocado is considered one of the main crops of major importance in tropical climates (Rodríguez et al. 2017), providing a basic food source for millions of people in the world (Caro et al. 2020). Likewise, the fruit is used in traditional medicine because its pulp and seed are rich in antioxidants that have the ability to reduce the risk of cardiovascular diseases (Vivero et al. 2019). According to the Ministry of Agriculture and Rural Development, in Colombia, 86% of the total area planted with avocado in the country.
There are different constraints that affect the production and marketing of avocado, among which is the disease called avocado “tristeza” caused by the Oomycete Phytophthora cinnamomi. The symptomatology caused by this disease causes root rot, where necrotic areas appear. The plant begins to show chlorotic leaves due to reduced water and nutrient absorption (Marais et al. 2002). In the field, the trees present growth problems, withered leaves and small fruit size, which limits their commercialization (Belisle et al. 2019). For the control of avocado “tristeza,” chemical fungicides are applied, but these have caused environmental problems and their excessive use generated fungicide-resistant strains (Hardham and Blackman 2018).
Biological control has become an alternative to replace agrochemicals. The use of fungi and bacteria capable of inhibiting the growth of P. cinnamomi has been proposed for the control of cause of root wilt. Among these microorganisms is the fungus Trichoderma spp. which are capable of controlling pathogens that cause phytosanitary problems through the production of volatile and nonvolatile secondary metabolites, mycoparasitism and the ability to compete for space and nutrients in their habitat (Zin and Badaluddin 2020). In recent years Trichoderma spp. fungi have been applied in crops of economic interest such as grapevine, tomato and Broccoli (De Britto and Jogaiah 2022). Likewise, the fungus has the ability to be used in bioremediation processes (Guoweia et al. 2011). It can be easily captured and replicated in the laboratory due to its rapid growth. Thanks to its potential biocontrol and plant growth promoter are currently marketed as biofertilizer, its inoculation in plants allows the uptake of macronutrients and micronutrients allowing in crop yield and soil quality (Sandheep et al. 2013). For this reason, the objective of this study was to evaluate the antagonistic activity of Trichoderma spp. against P. cinnamomi and its plant growth-promoting potential in vitro.
Methods
Study area
The study was carried out in avocado plantations in the municipalities of Ovejas and Chalán, subregion Montes de María, department of Sucre, Colombia. It corresponds to a tropical dry forest zone and its characteristic landscape is mountainous. It presents an average temperature of 27 °C and with a rainfall that can vary between 1000 and 1200 ml per year (Hernández Díaz 2013).
Soil sampling
With the help of a previously sterilized auger, a soil sample was taken at the base of the avocado tree. This sample was deposited in zip lock bags and taken to the Microbiological Research Laboratory of the University of Sucre for processing.
Isolation of Trichoderma spp.
The soil samples were deposited in an Erlenmeyer that contained 90 ml of sterile water, this was left in constant agitation for its homogenization. After the time elapsed, with the help of a micropipette, 1 ml of the homogenate was taken and inoculated in test tubes containing 9 ml of saline solution (Camargo and Ávila 2014). From this solution, serial dilutions were performed in triplicate. From each of these dilutions, 10 µl were taken to be plated on PDA medium and placed in incubation for 72 h at a temperature of 32 °C (Rivera et al. 2016).
Purification and identification of isolates
Petri dishes that presented growth of microorganisms with cultural characteristics to those belonging to the genus Trichoderma were purified in PDA culture medium. The strains were incubated for 10 days at a temperature of 32 °C for optimal development. Once the fungus showed growth in the culture medium, the morphological structures such as conidia, conidiophores and phialides were observed under the microscope using the paper tape technique. Taxonomic identification at the genus level was carried out using the keys proposed by Barnett and Hunter (1998). In the case of P. cinnamomi, it was taken from the microorganism bank of the Agricultural Bioprospecting group of the University of Sucre and activated in PDA culture medium.
The rDNA extraction was performed using the DNeasy Plant Mini® kit, following the manufacturer's protocol (Gamarra et al. 2017). The extracted DNA was subjected to polymerase chain reaction (PCR) to amplify the tef1 gene using primers EF1-728F 5′-CATCGAGAAGTTCGAGAAGAAGG-3′ Tef1-Llevrev 5′-AACTTGCAGGCAGGCAATGTGG-3′, following the methodology proposed by Druzhinina (2009) and Maniscalco and Dorta (2015). The amplified products were sent for sequencing to Macrogen. The nucleotide sequence entities obtained were compared with those stored in GenBank. The MEGA X program was used to perform sequence alignment and phylogenetic inferences applying the Neighbor Joining method based on the kimura-2-parameter model with bootstrap test 1,000 replicates.
In vitro antagonism test of Trichoderma spp. against: P. cinnamomi
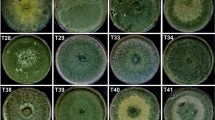
With the help of a punch, a mycelial block of both pathogen and antagonist was taken and placed at a distance of 6 cm from the PDA culture medium (Fig. 1). The Petri dishes were incubated for 3 days at a temperature of 30 °C. The positive result of antagonistic activity is evidenced by the growth of the antagonist in the culture medium.
To evaluate the antagonistic capacity, the following formula proposed by Rivera et al. (2016) was applied:
where R1 is the radius of the control pathogen and R2 is the radial growth of the phytopathogen exposed to the antagonist.
Siderophore production
Qualitative siderophore production was determined using the chrome azurol-S (CAS) medium proposed by Schwyn and Neilands (1987).
Phosphate solubilization
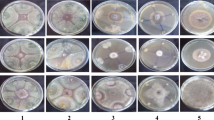
The phosphate solubilizing ability of each strain was determined using SRS culture medium. Color change from purple to yellow in the medium is considered positive for phosphate solubilization (Sundara and Sinha 1963). Strains showing both inhibition and plant growth-promoting activity are candidates for the molecular identification process.
Statistical analysis
A completely randomized design (CRD) was applied for the antifungal activity of Trichoderma spp. against P. cinnamomi. Likewise, the Duncan rank multiple test was applied to establish significant statistical differences (p < 0.05) in terms of the percentage of inhibition. The arcsine transformation was applied, since it is the suggested transformation for this type of data. The student version of the InfoStat program was used for data analysis. The tests were performed in triplicate.
Results
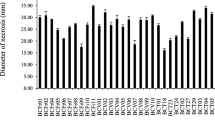
A total of 16 strains of Trichoderma spp. were isolated, of which 6 belonged to the municipality of Ovejas-Colombia and 10 to the municipality of Chalán-Colombia. Likewise, 3 strains from Chalán (C1CHLIM, C7CHLIM and C3CHLIM) and 1 strain from Ovejas (C9OVLIM) showed an in vitro antagonistic effect against the pathogen. The Duncan rank multiple test yielded significant statistical differences (p < 0.05) in the percentage of inhibition of each of the Trichoderma spp. strains. In addition, strain C1CHLIM and C9OVLIM presented the highest percentages of inhibition and did not present significant statistical differences against the pathogen (p > 0.05) (Figs. 1, 2).
The results of molecular analysis showed that strain C1CHLIM was identified as (Trichoderma harzianum), C7CHLIM (T. viride) C3CHLIM (T. longibrachiatum) and C9OVLIM as (T. asperellum). In turn, they showed in vitro plant growth-promoting activity (Figs. 3, 4). These results should be further supported by other tests including siderophore types and their evaluation in the plant.
Discussion
Trichoderma spp. are characterized by rapid growth which allows them to compete for space and nutrients in its environment. In addition, they have the ability to release enzymes such as chitinases and glucanases that cause damage to the cell wall of the pathogen. Once the cell wall has been degraded, the hyphae of Trichoderma spp. can completely colonize the pathogen causing its death by necrotrophic mycoparasitism (Vargas and Gilchrist 2015).
Studies by Tchameni et al. (2017) demonstrated that Trichoderma asperellum inhibited the in vitro growth of Phytophthora megakarya by necrotrophic mycoparasitism destroying the cells present in the hyphae and generating coiling in the hyphae of the pathogen. Likewise, Troian et al. (2014) demonstrated that T. harzianum inhibited the growth of Sclerotinia sclerotiorum by mycoparasitism in which the fungus releases enzymes such as glucanases and peptidases capable of degrading the cell wall of some pathogens. Once the cell wall was degraded the fungus multiplied forming a dense mycelium that penetrated the hyphae of the pathogen. Das et al. (2019) isolated Trichoderma spp. strains from the rhizosphere of ginger cultivars grown in different regions of Palakkad and Idukki districts, India. These isolates were identified as Trichoderma asperellum, strain AFP, T. asperellum, strain MC1, T. brevicompactum MF1 and T. harzianum, strain CH1 which showed in vitro antagonistic activity against several soil-borne plant pathogens such as Fusarium oxysporum, Rhizoctonia solani and Phytophthora capsici indicating that Trichoderma spp. isolates can be used as effective microbial biological control agents.
Trichoderma species have shown great benefits on the growth and yield of crops of economic interest through the production of siderophores, phosphate solubilization and Indole Acetic Acid (IAA) (Hermosa et al. 2013). For example, in the study conducted by Qi and Zhao (2013) evaluated the plant growth promotion of T. asperellum on cucumber seedlings under salt stress conditions. The results obtained were that the fungus promoted plant growth and improved plant conditions induced by salt stress. Likewise, the production of siderophores allowed the plant to show signs of recovery from the effects of salinity and available iron deficiency Zhang et al. (2016) reported that the application of T. longibrachiatum produced a high growth in maize seedlings that were under salt stress. In addition, the application of this strain increased the water content in roots, leaves and decreased the stress in the plant thanks to the production of siderophores. In turn, Ghosh et al. (2017) showed that T. harzianum species produced hydroxamate- and carboxylate-type siderophores (85%), while T. viride (65%), T. asperellum (60.27%) and T. longibrachiatum (45.5%) recorded lower production of hydroxamates and carboxylates as confirmed by the color intensity in CAS medium. The production of siderophores in Trichoderma species fulfills the function of trapping iron in order to avoid the activation of the enzymes of the pathogen and not to cause damage inside the plant. The production of siderophores is one of the mechanisms that induce the promotion of plant growth and defense against pathogens (Ghosh et al. 2020).
Moreover, phosphate solubilization by microorganisms is considered one of the most important benefits for agriculture, which are being used as bio-fertilizers. Several studies have demonstrated the application of Trichoderma spp. fungi for their ability to solubilize phosphates, including in areas contaminated with heavy metals (Rawat and Tewari 2011).
Li et al. (2015), demonstrated that T. harzianum species applied on tomato plants under hydroponic conditions significantly improved biomass and nutrient uptake when these were grown in nutrient-deprived soil. In addition, the strain was able to solubilize poorly soluble minerals such as phytate by releasing organic acids such as citric, lactic and succinic acids, which were detected by HPLC. Subsequently, the contribution given by Saravanakumar et al. (2013) demonstrated that Trichoderma spp. isolated from Avicennia marina (Forssk.) Vierh. (Acanthaceae) solubilized phosphate in vitro using NBRIP liquid medium, which contains tricalcium phosphate Ca3(PO4)2 as insoluble phosphate.
This study demonstrated that phosphate solubilization by Trichoderma spp. can be used to improve agricultural soils and mangrove growth Busato et al. (2021) determined that when applying the combination of T. asperellum and T. virens in vermicompost, the content of soluble P increased through the release of citric acid compared to noninoculated vermicompost. These results give indications to continue studying the possibility of using Trichoderma species in compost production, since it could favor the absorption of soluble phosphate by plants and improve crop production.
Conclusion
The results of this study demonstrate that Trichoderma species can be applied in crops of economic interest for biological control against pathogens and stimulate plant growth in order to obtain a good yield. In addition, it allows reducing the costs in the application of chemical fertilizers.
Availability of data and materials
Not applicable.
Abbreviations
- PDA:
-
Potato dextrose agar
- DNA:
-
Deoxyribonucleic acid
- CAS :
-
Chrome Azurol-S
References
Barnett HL, Hunter BB (1998) Illustrated genera of imperfect fungi (1.era ed.) https://www.academia.edu/35499449/Illustrated_genera_of_imperfect
Belisle RJ, McKee B, Hao W, Crowley M, Arpaia ML, Miles TD, Manosalva P (2019) Phenotypic characterization of genetically distinct Phytophthora cinnamomi isolates from avocado. Phytopathology 109(3):384–394
Busato JG, Ferrari LH, Chagas Junior AF, da Silva DB, dos Santos PT, de Paula AM (2021) Trichoderma strains accelerate maturation and increase available phosphorus during vermicomposting enriched with rock phosphate. J Appl Microbiol 130(4):1208–1216
Camargo DF, Ávila ER (2014) Efectos del Trichoderma sp. sobre el crecimiento y desarrollo de la arveja (Pisum sativum L.). Cienc Agric 11(1):91–100
Caro D, Alessandrini A, Sporchia F, Borghesi S (2020) Global virtual water trade of avocado. J Clean Prod 285:124–917
Das MM, Haridas M, Sabu A (2019) Biological control of black pepper and ginger pathogens, Fusarium oxysporum, Rhizoctonia solani and Phytophthora capsici, using Trichoderma spp. Biocatal Agric Biotechnol 17:177–183
De Britto S, Jogaiah S (2022) Priming with fungal elicitor elicits early signaling defense against leaf spot of broccoli underlying cellular, biochemical and gene expression. Microbiol Res. https://doi.org/10.1016/j.micres.2022.127143
Druzhinina I (2009) PCR protocols for amplification of Trichoderma phylogenetic markers. https://www.isth.info/methods/method.php?methodid=10
Gamarra MAF, Ojeda MM, Maldonado GAE (2017) Identificación molecular y tasa de crecimiento de cepas nativas de Trichoderma spp. aisladas de la Región Norte del Paraguay. Invest Agrar 19(2):127–132
Ghosh SK, Banerjee S, Sengupta C (2017) Bioassay, characterization and estimation of siderophores from some important antagonistic Fungi. J Biopest 10(2):105–112
Ghosh SK, Bera T, Chakrabarty AM (2020) Microbial siderophore—a boon to agricultural sciences. Biol Control 1(44):104–214
Guoweia S, Man H, Shikai W, He C (2011) Effect of some factors on production of cellulase by Trichoderma reesei HY07. Procedia Environ Sci 8:357–361
Hardham AR, Blackman LM (2018) Phytophthora Cinnamomi. Mol Plant Pathol 19(2):260–285
Hermosa R, Rubio MB, Cardoza RE, Nicolás C, Monte E, Gutiérrez S (2013) The contribution of Trichoderma to balancing the costs of plant growth and defense. Int Microbiol 16(2):69–80
Hernández Díaz W (2013) Efectos en las condiciones socioeconómicas de la población generado por el hongo Phythophthora spp, que afecta los cultivos de aguacate del municipio de el Carmen de Bolívar [Tesis de maestría, Universidad de Manizales, Colombia]. https://ridum.umanizales.edu.co/xmlui/handle/20.500.12746/1251
Li RX, Cai F, Pang G, Shen QR, Li R, Chen W (2015) Solubilization of phosphate and micronutrients by Trichoderma harzianum and its relationship with the promotion of tomato plant growth. PLoS ONE 10(6):1–16
Maniscalco DP, Dorta B (2015) Diversidad del hongo Trichoderma spp. en plantaciones de maíz de Venezuela. Interciencia 40(1):23–31
Marais LJ, Menge JA, Bender GS, Faber B (2002) Phytophthora root rot. AvoRes Calif Avocado Commun 2:3–6
Qi W, Zhao L (2013) Study of the siderophore-producing Trichoderma asperellum Q1 on cucumber growth promotion under salt stress. J Basic Microbiol 53(4):355–364
Rawat R, Tewari L (2011) Effect of abiotic stress on phosphate solubilization by biocontrol fungus Trichoderma sp. Curr Microbiol 62(5):1521–1526. https://doi.org/10.1007/s00284-011-9888-2
Rivera W, Meneses-Montero K, Zúniga-Vega C, Brenes-Madriz JA (2016) Antagonismo de Trichoderma sp ante el patógeno Stromatinia cepivora en el cultivo de cebolla. Rev Tecnol Marcha 2(9):22–30
Rodríguez CE, Hernández-Brenes C, Treviño V, De La Garza RID (2017) Avocado fruit maturation and ripening: dynamics of aliphatic acetogenins and lipidomic profiles from mesocarp, idioblasts and seed. BMC Plant Biol 17(1):1–23
Sandheep AR, Asok AK, Jisha MS (2013) Combined inoculation of Pseudomonas fluorescens and Trichoderma harzianum for enhancing plant growth of vanilla (Vanilla planifolia). Pak J Biol Sci 16(12):580–584
Saravanakumar K, Shanmuga Arasu V, Kathiresan K (2013) Effect of Trichoderma on soil phosphate solubilization and growth improvement of Avicennia marina F. (Acanthaceae). Aquat Bot 104:101–105
Schwyn B, Neilands JB (1987) Universal chemical assay for the detection and determination of siderophores. Anal Biochem 160(1):47–55
Sundara R, Sinha M (1963) Organisms phosphate solubilizers in soil. Indian J Agric Sci 3(3):272–278
Tchameni SN, Sameza ML, O’donovan A, Fokom R, Mangaptche Ngonkeu EL, Wakam Nana L, Nwaga D (2017) Antagonism of Trichoderma asperellum against Phytophthora megakarya and its potential to promote cacao growth and induce biochemical defense. Mycology 8(2):84–92
Troian R, Steindorff A, Ramada MHS, Arruda W, Ulhoa CJ (2014) Mycoparasitism studies of Trichoderma harzianum against Sclerotinia sclerotiorum: evaluation of antagonism and expression of cell wall-degrading enzymes genes. Biotech Lett 36(10):2095–2101
Vargas H, Gilchrist E (2015) Producción de enzimas hidrolíticas y actividad antagónica de Trichoderma asperellum sobre dos cepas de Fusarium aisladas de cultivos de tomate (Solanum lycopersicum L.). Rev Mex Micol 42:9–16
Vivero A, Valenzuela R, Valenzuela A, Morales G (2019) Palta: compuestos bioactivos y sus potenciales beneficios en salud. Rev Chil Nutr 46(4):491–498. https://doi.org/10.4067/S0717-75182019000400491
Zhang S, Gan Y, Xu B (2016) Application of plant-growth-promoting fungi Trichoderma longibrachiatum T6 enhances tolerance of wheat to salt stress through improvement of antioxidative defense system and gene expression. Front Plant Sci 7:1405. https://doi.org/10.3389/fpls.2016.01405
Zin NA, Badaluddin NA (2020) Biological functions of Trichoderma spp. for agriculture applications. Ann Agric Sci 65(2):168–178. https://doi.org/10.1016/j.aoas.2020.09.003
Acknowledgements
The authors acknowledge the Colombia Scientific Program “Reconstruction of the social fabric in post-conflict areas in Colombia” PGIS Code: 57579 with the research project “Entrepreneurial and innovation competencies for economic development and productive inclusion of regions affected by the Colombian conflict” SIGP Code: 58907.
Funding
The present research work was funded under the call Colombia Scientific, Contract No. FP44842-213-2018, through Grant modality II.
Author information
Authors and Affiliations
Contributions
ABG and APC conducted the experiments and analyzed the data. DMV and APC contributed to conceptualization and writing—review and editing. All authors have read and approved the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Montes Vergara, D.E., Barboza-García, A. & Pérez-Cordero, A. Antifungal and growth activity of strains of Trichoderma spp. against the Avocado “tristeza” disease, Phytophthora cinnamomi. Egypt J Biol Pest Control 32, 115 (2022). https://doi.org/10.1186/s41938-022-00613-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41938-022-00613-8