Abstract
Background
The plant pathogenic fungi, Alternaria solani and Fusarium oxysporum, are considered among the fungal pathogens which cause severe damages to tomato plants. The application of chemicals fungicides reduced fungal infection and entails great risks to human health and to the environment. Using biological control agents is considered one of the most effective techniques which suppress fungal pathogens and preserve the environment. The beneficial bacterium, Lysobacter enzymogenes, is applied as a biocontrol agent against different plant pathogens.
Results
The present work was carried out, under laboratory conditions, to investigate the efficacy of L. enzymogenes different concentrations of the strain ch3B10 on controlling the linear growth of A. solani and F. oxysporum compared to using the fungicide Benlate®. Treatment with Benlate® and the highest concentration (2 × 108 CFU/ml) of L. enzymogenes strain ch3B10 showed the highest inhibition of 70.6–94.0% on linear growth of all the tested fungi. Also, treatments with two concentrations (2 × 106 and 2 × 107 CFU/ml) of L. enzymogenes strain ch3B10 inhibited linear growth of all tested fungi by means of 47.1–69.7%. The low concentration of 2 × 103 CFU/ml of the strain ch3B10 resulted in the lowest linear growth inhibition 19.8–28.1% in all tested than the check treatment.
Conclusion
Further experiments under both greenhouse and field conditions are needed to approve the efficacy of the strain ch3B10 as an effective bioagent and ecologically safer approach than chemical treatments.
Similar content being viewed by others
Background
Tomato is a widespread crop cultivated all over the world. Tomato cultivation has been attacked by many pathogens including fungi, bacteria, viruses and nematodes (Jones et al. 2000). Tomato plants are affected by several or many fungal pathogens, Alternaria solani and Fusarium oxysporum, which caused diseases including early blight and Fusarium wilt, respectively. These diseases resulted in a severe yield loss worldwide and considered very difficult to be controlled (Dun-chun et al. 2016).
In spite of their negative influence on the environment and human health and its high cost, chemical fungicides have been used extensively for the management of many fungal diseases (Terna et al. 2016). Lately, demand is increasing for applying eco-friendly management methods (Odhiambo et al. 2017).
The potential of the genus Lysobacter as a biological control agent for controlling plant pathogenic diseases has been described since 1978 because of their antagonistic relationships with other microorganisms (Odhiambo 2021). Lysobacter from the family Xanthomonadaceae in Gammaproteobacteria is Gram-negative bacteria with gliding motility characterized by a high G + C content, and antimicrobial lytic activity against different microorganisms (Reichenbach 2006). Over 30 Lysobacter spp. have been identified and used as biocontrol agents against various plant pathogens, e.g. bacteria, fungi and nematodes (Singh et al. 2015). Recently, Lysobacter has been applied as a biological control agent against different fungal pathogens (Zhang et al. 2011).
The most important common features of all Lysobacter species are secreting different extracellular enzymes, including the production of chitinases, glucanases, proteases and lipases (Vasilyeva et al. 2014). Different studies have revealed that chitinases have the most important role in biocontrol (Ko et al. 2009).
Thus, the objective of this research was to evaluate the antagonistic effects of L. enzymogenes strain ch3B10 on A. solani and F. oxysporum under laboratory conditions.
Methods
Lysobacter enzymogenes strain ch3B10 isolation, identification and growth conditions.
Lysobacter enzymogenes strain ch3B10, a bioagent (Ba) isolated from mango soil samples collected from Jazan area southwest KSA (Alharbi 2017). Isolated strain was purified, identified and recorded in Gene bank under the accession No. LT601528. The strain ch3B10 was stored in sterilized 20% (vol/vol) glycerol at − 82 °C until use (Kobayashi et al. 2005).
In all experiments, the strain ch3B10 was cultured at 28 ± 2 °C with shaking overnight (200 rpm) on 10% tryptic soy agar medium. The bacterial suspension was centrifuged at 3000 g and concentrated up to 2 × 108 CFU/ml by measuring absorbance at optical density of 590 nm (OD590) (Palumbo et al. 2003).
Source of pathogenic fungi
Two isolates of each of the pathogenic fungus, Alternaria solani (Sorauer) and Fusarium oxysporum (Synder & Hans), were obtained from the culture collection of the Biology Department, Science College, Jazan University KSA, which isolated from infected tomato plants.
All fungal isolates were cultured on Petri plates filled with a Czapek Dox agar (CDA) medium at 28 ± 2 °C. Pure cultures grown on CDA plates were saved for further work.
In vitro assay for L. enzymogenes strain ch3B10 and the fungicide Benlate® was developed on growth diameters of A. solani and F. oxysporum isolates.
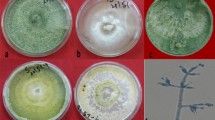
Under laboratory conditions, two experiments were conducted to study the efficiency of the bioagent, L. enzymogenes strain ch3B10, using four concentrations of 2 × 103, 2 × 106, 2 × 107 and 2 × 108 colony-forming unit (CFU)/ml compared to one concentration 50 μg/ml of the fungicide Benlate®. The inhibition % of linear growth diameters of A. solani and F. oxysporum isolates was determined. Petri plates were filled with CDA medium (20 ml/each) and inoculated at the centre with 5-mm fungal disc from a week-old culture of each isolate of A. solani and F. oxysporum by using cork borer.
Data of fungal growth diameter inhibition percentage/each treatment were determined 3 and 5 days later. Two hundred and forty Petri plates (120 plates/each experiment) were used. Plates were incubated at 28 ± 2 °C. Treatments were replicated to 10 times. Ten plates of each fungal isolate untreated with the bioagent were used as a check treatment.
Statistical analysis
Data obtained were statistically analysed using ANOVA procedure (SAS 1997). Comparison among means was made via the least significant difference test (LSD) at ≤ 5% level of probability.
Results
Data presented in Tables 1 and 2 showed the effects of L. enzymogenes strain ch3B10 and the fungicide Benlate® on linear growth inhibition % of the tested fungi after 3 and 5 days of incubation. Treatments with the fungicide, Benlate® and the highest concentration of L. enzymogenes strain ch3B10 (2 × 108 CFU/ml) showed great inhibitions of 70.6–94.0% on linear growth of A. solani and F. oxysporum isolates followed by treatments of the two concentrations (2 × 106 and 2 × 107 CFU/ml) of L. enzymogenes which resulted in 47.1–69.7% inhibition on linear growth of all tested fungal isolates. Meanwhile, treatment with the lowest concentration (2 × 103 CFU/ml) of L. enzymogenes revealed that the lowest inhibition % on linear growth of A. solani and F. oxysporum ranged 17.9–30.3% than the linear growth of check treatment.
Discussion
The present data indicated that treatments with different tested concentrations of L. enzymogenes strain ch3B10 resulted in a significant inhibition on linear growth of all tested species of F. oxysporum and A. solani under laboratory conditions. Many investigations reported that all known strains of L. enzymogenes have been considered bioagents against several microorganisms through their ability of produced different extracellular degradation enzymes (Pidot et al. 2014).
The observations provide persuasive evidence that strain ch3B10 could have enzymatic activity like that produced by other Lysobacter strains against a variety of plant fungal pathogens. The present results are in harmony with those of Odhiambo et al. (2017).
Previous work indicated that culture filtrates of L. enzymogenes strains (3.1T8 and SB-K88) caused inhibition on fungal spore germination (Islam et al. 2005). Jochum et al. (2006) reported that L. enzymogenes strain C3 was very effective as a biological control agent against Fusarium graminearum.
Also, the studies of Postma et al. (2008) indicated the suppressive effect of several Lysobacter species on Rhizoctonia solani. Zhao et al. (2017) reported that L. enzymogenes strain OH11 could attach, penetrate and lyse the hyphae of Aphanomyces cochlioides and Pythium aphanidermatum.
Conclusions
Present observations made in this study refer to the efficacy of L. enzymogenes strain ch3B10 on inhibiting growth of F. oxysporum and A. solani under laboratory conditions. These inhibition activities may be attributed to the inhibition effect against mycelium growth or degradation of fungal structures. Insertion of strain ch3B10 as a biocontrol agent in integrated pest management systems for controlling plant pathogens needs further studies under greenhouse and field conditions.
Abbreviations
- CFU:
-
Colony-forming unit
- I%:
-
Inhibition %
- CDA:
-
Czapek Dox agar
- Ba:
-
Lysobacter enzymogenes Strain ch3B10 as a bioagent
References
Alharbi AA (2017) Molecular characterization using 16S rRNA gene of isolated bacterial genera associated with mango cultivation in Jazan province, South West KSA. Glob Adv Res J Agric Sci 6(9):285–292
Dun-chun HE, Zhan J, Xie L (2016) Problems, challenges and future of plant disease management: from an ecological point of view. J Integr Agric 15(4):705–715
Islam MT, Hashidoko Y, Deora A, Ito T, Tahara S (2005) Suppression of damping-off disease in host plants by the rhizoplane bacterium Lysobacter sp. strain SB-K88 is linked to plant colonization and antibiosis against soil borne Peronosporomycetes. Appl Environ Microbiol J 71:3786–3796
Jochum CC, Osborne LE, Yuen GY (2006) Fusarium head blight biological control with Lysobacter enzymogenes strain C3. Biol Control 39:336–344
Jones JB, Bouzar H, Stall RE, Almira EC, Roberts PD, Bowen BW, Sudberry J, Strickler PM, Chun J (2000) Systematic analysis of Xanthomonads (Xanthomonas spp.) associated with pepper and tomato lesions. Int J Syst Evol Microbiol 50:1211–1219
Ko HS, Jin RD, Krishnan HB, Lee SB, Kim KY (2009) Biocontrol ability of Lysobacter antibioticus hs1 24 against Phytophthora blight is mediated by the production of 4-hydroxy phenyl acetic acid and several lytic enzymes. Curr Microbiol 59:608–615
Kobayashi DY, Reedy RM, Palumbo JD, Yuen GY (2005) A clp gene homologue belonging to the Crp gene family globally regulates lytic enzyme production, antimicrobial activity, and biological control activity by Lysobacter enzymogenes strain C3. Appl Environ Microbiol 71:261–269
Odhiambo BO (2021) Antimicrobial compounds biosynthesis and biocontrol mechanisms of Lysobacter enzymogenes. Pan Africa Sci J 2(1):85–102
Odhiambo BO, Xu G, Qian G, Liu F (2017) Evidence of an unidentified extracellular Heat-Stable Factor Produced by Lysobacter enzymogenes (OH11) that Degrade Fusarium graminearum PH1 hyphae. Curr Microbiol 74(4):437–448
Palumbo JD, Sullivan RF, Kobayashi DY (2003) Molecular characterization and expression in Escherichia coli of three-1,3-glucanase genes from Lysobacter enzymogenes strain N4–7. J Bacteriol 185:4362–4370
Pidot SJ, Coyne S, Kloss F, Hertweck C (2014) Antibiotics from neglected bacterial sources. Int J Med Microbiol 304:14–22
Postma J, Schilder MT, Bloem J, Van Leeuwen-Haagsma WK (2008) Soil suppressiveness and functional diversity of the soil microflora inorganic farming systems. Soil Biol Biochem 40:2394–2406
Reichenbach H (2006) The genus Lysobacter. In: Dworkin M, Falkow S, Rosenberg E, Schleifer KH, Stackebrandt E (eds) The Prokaryotes. Springer, New York
SAS Institute (1997) SAS/STAT user's guide. 6th Edition. SAS Institute Inc. North Carolina, Cury. Inc
Singh H, Du J, Ngo HT, Won K, Yang JE, Kim KY, Yi TH (2015) Lysobacter fragariae sp. nov. and Lysobacter rhizosphaerae sp. nov. isolated from rhizosphere of strawberry plant. Antonie Van Leeuwenhoek J 107:1437–1444
Terna TP, Okoro JK, Bem AA, Okogbaa JI, Waya JI (2016) Incidence and severity of diseases associated with rain-fed tomatoes in Benue State, Nigeria. J Agric Vet Sci 9(4):59–65
Vasilyeva NV, Shishkova NA, Marinin LI, Ledova LA, Tsfasman IM, Muranova TA, Stepnaya OA, Kulaev IS (2014) Lytic peptidase L5 of lysobacter sp. XL1 with broad antimicrobial spectrum. J Mol Microbiol Biotechnol 24(1):59–66
Zhang W, Li Y, Qian YW, Chen H, Li Y, Liu F, Shen Y, Du L (2011) Identification and characterization of the anti-methicillin-resistant Staphylococcus aureus WAP-8294A2 biosynthetic gene cluster from Lysobacter enzymogenes OH11. Antimicrob Agents Chemother 55(12):5581–5589
Zhao Y, Qian G, Chen Y, Du L, Liu F (2017) Transcriptional and antagonistic responses of biocontrol strain Lysobacter enzymogenes OH11 to the plant pathogenic oomycete Pythium aphanidermaAtum. Front Microbiol 8:1–13
Acknowledgements
The author would like to thank Prof. Dr. Asmaa Abdelhamid Mokbel, Professor of Plant Nematology, Faculty of Agriculture, Plant Pathology Department, Alexandria University, Egypt, for suggestions and review of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Alharbi, A.A. Efficacy of the bacterium Lysobacter enzymogenes strain ch3B10 as a new biocontrol agent on the pathogenic fungi Alternaria solani and Fusarium oxysporum under laboratory conditions. Egypt J Biol Pest Control 32, 70 (2022). https://doi.org/10.1186/s41938-022-00569-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41938-022-00569-9