Abstract
Background
Cabbage butterfly, Pieris brassicae Linnaeus (Lepidoptera: Pieridae), is a major insect pest affecting cole crops worldwide. Excessive applications of chemical-based insecticides have a devastating impression over the organisms and environment.
Results
In this study, entomopathogenic nematode (EPN) Heterorhabditis bacteriophora Poinar strain EUPT-S26 (local isolate) was evaluated for Pieris brassicae control under polyhouse and field conditions. Under the polyhouse conditions, the highest insect mortality 91.6 and 94.0% was observed in the plots treated with the nematodes suspension 1500 IJs/ml and 2000 IJs/ml, respectively. Based on the highest cabbage plant protection under polyhouse conditions, H. bacteriophora EUPT-S26 was also applied for field assays in the course of the crop’s productive phase. Data demonstrated from the field treatments signify the highest concentration (2000 IJs/ml) showed the maximum larval mortality and least damage percentage 45 ± 1.07% that remained constant until harvesting; this resulted in the highest productivity in polyhouse and under field conditions.
Conclusion
According to assessed field conditions, it was suggested to perform 3 applications of EPNs during the vegetative phase and at the time of head formation to increase productivity and to reduce damage. The results approved that EPNs are an effective alternative of chemical-based insecticides to control the cabbage butterfly.
Similar content being viewed by others
Background
Pieris brassicae Linnaeus of family Pieridae is the key insect pests of cabbage. It generates significant losses as compared to costs of synthetic chemical insecticides for its control (Kasi et al. 2021). Cabbage butterfly is dispersed all over the world where brassicaceae are planted. Winter generations showed a longer life stages than those through the rainy and summer seasons. Temperature range (15.2–30 °C) was found ideal for its multiplication. Other different abiotic factors did not affect the life stages of insect (Thakur and Deka 1997). The gregariously feeding insect larvae consumed the whole leaves, except veins of the cauliflower, cabbage and flower buds of broccoli (Boczek and Lewandowski 2016). Over 50% yield losses have been observed due to the infestation by these insect pests in cabbage annually (Abbas et al. 2021). Sood (2007) reported that cabbage butterfly caused extensive damage to the cabbage crop in the month of April (last week) and May in Himachal Pradesh, India.
Although the chemical synthetic insecticides has been proven most trustworthy for the control of these voraciously feeding larvae, the repercussions of the persistent use of these synthetic insecticides result in various environmental hazards, especially the chemical residues also affect the soil fertility, affects the wildlife, contaminate resources of ground water, developed resistance amongst insects along with re-emergence of huge insect population along with minor insect invasion (Thakur et al. 2021). At present, a huge amount of micro-biome-based biopesticides were developed from bacteria, fungi, protozoan and nematodes and are used against insect pests worldwide (Kumar and Singh 2015). EPNs application against cabbage butterfly is a biological management strategy (Abbas et al. 2021).
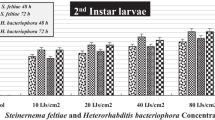
Earlier a laboratory experiment showed mortality 67–72% triggered by Steinernema feltiae and H. bacteriophora in 2nd larval instar of P. brassicae (Kumar et al. 2021). In field, mortality rates up to 100% have been achieved by using H. bacteriophora together with the Steinernema glaseri (Rhabditida: Heterorhabditidae) (Abbas et al. 2021). These nematodes were recognized as very successful biopesticides against several insect pests’ species belonging to the order; Diptera, Isoptera, Lepidoptera, Hemiptera, Coleoptera and Hymenoptera (Labaude and Griffin 2018). EPNs are also compatible (tank-mixed) with other chemical fungicides, herbicides and insecticides (also with fungal or bacterial products) (Gupta et al. 2009). Nematodes biocontrol agents (BCAs) are also compatible with Azadirachta indica (Neem) products (Mahmoud et al. 2007) at lesser doses and at short-term exposures. Application of entomopathogens also encourages sustainable agriculture (Singh et al. 2020). The foliar application of these BCAs in field do not show any detrimental impact on the applicant and consumer (Damalas and Koutroubas 2018). To evaluate the role of EPNs in managing the population of insect pests, the present study on the application of H. bacteriophora EUPT-S26 was evaluated against P. brassicae in cabbage at polyhouse and under field conditions.
Methods
Biological material
The nematodes (H. bacteriophora EUPT-S26) were procured from rhizospheric soil of fruit orchards of district Sirmaur of Himachal Pradesh, India, via soil baiting technique. The isolated nematodes were identified as H. bacteriophora EUPT-S26 on the basis of morphological observations and identification keys. To attain pathogenic and viable infective juveniles (IJs), they were replicated through in vivo using last instar of Galleria mellonella L. (Lepidoptera: Pyralidae) via larvae contagion (Kaya and Stock 1997). Infective juveniles (IJs) were kept at 15 °C in sterilized sponge foams. The seeds of Brassica oleraceae (Brassicales: Brassicaceae) were purchased from local market and grown at polyhouse to establish breeding stock at the University biological control polyhouse (average temperature: 17–20 °C; average relative humidity: 75–81%; light regime: 12 h light/12 h dark). Cabbage plants after 27 days of post-germination were transplanted into the plastic pot (20 × 20 × 14 cm), filled with rice husk and soil. Watering was done daily and plants were fertilized with Agrobium's® (NPK-8:25:25, 2 g/plant). Initiation of breeding stock of P. brassicae was maintained by transferring the P. brassicae larvae on cabbage plant (28 days from seedling stage) 5 larvae/plant, which were then placed in entomological cages (40 cm3) for confinement. The P. brassicae life cycle lasted approximately 27 days on average under polyhouse conditions.
Polyhouse phase
Heterorhabditis bacteriophora EUPT-S26 application
Pieris brassicae susceptibility to H. bacteriophora EUPT-S26 infection was determined by conducting an experiment on cabbage plants at different vegetative stages. The experiment was conducted under the polyhouse (44 × 36 m) where the area of the experiment was conducted in area of 22 × 11 m, further divided into 2 blocks containing 13 plots on each side (2 m2). The cabbage plants were transplanted at 12 plants per plot with a planting distance of 16 cm. To this laboratory reared, 4th instar larvae were stationed in each plant (5 larvae/plant) along with 260 P. brassicae adults, 8 h before the test (experiment). Different treatments containing IJs were suspended in the distilled water with the 0.3% Polysorbate 20. These suspensions with varied juvenile concentrations were sprayed via pressure sprayer over the cabbage leaves (abaxial and adaxial). The result variables were the leaf damage percentage and larvae mortality percentage were assessed at every 24 h for the nine days. Plant damage level was mainly evaluated through graphical method by using scale with 4 levels, namely 1, 2, 3 and 4 with differences in degrees of infestation ranging from 1 to 10%: level 1, 10–40%: 2, 40–70%: 3 and 70–100%: level 4 (Carballo et al. 1989).
Insect pest mortality was determined by yellow-ochre cadavers when placed into the white traps up to 9 days to authenticate the presence of IJs (Kaya and Stock 1997). A completely randomized experimental design was implemented, where nematodes dose factor had 5 levels, IJs (0, 500, 1000, 1500 and 2000 IJs/ml of water). For statistical analyses of damage progress, area under the patches was observed. Results’ variable such as damage percentage of plants and mortality percentage in larvae was calculated. Statistical differences were determined by ANOVA using OPSTAT software. The experiment was repeated twice for the 2 year during the cropping season along with 5 replicates.
Field phase
Heterorhabditis bacteriophora EUPT-S26 treatment
Field phase was carried out in the agricultural farm, located at (30.7537° N, 77.2965° E, 1900 m altitude), having mean temperature between 15 and 20 °C and a RH between 50 to 64%. For planting a 100 m2 area was prefabricated first, soil was allowed to recover strength and then plowing was done. The planting area was fertilized with Agrobium's NPK (8:25:25). The experimental area was then partitioned into 2 equal blocks (50 m2). Each block was distributed into the 13 plots (3 m2). In the each plot, 26-day post-germinated cabbage plants (16 plants/plot) were transplanted with a planting distance of 18 cm. After 3–4 weeks of cabbage transplantation, 780 pupae and 260 P. brassicae adults were released in the entire field, homogeneously spreading 390 pupae and 130 adults per block. Treatments of nematode juvenile mixed with 0.3% Polysorbate 20 were applied in each plot along with complete control.
Throughout the crop growth or development, 3 applications of EPNs were used, according to the variation in the population dynamic of P. brassicae and cabbage plant’s phenological stage. The 1st application was applied 1 week of post-inoculation of insects containing 5 larvae/ plant at vegetative stage. The 2nd application was sprayed 1 week after the first application (4 larvae/ plant) also at vegetative stage. At the end, the 3rd application was applied 1 week after the 2nd application (during reproductive stage at the completion of head formation). These applications were performed throughout the crop cycle up to harvesting according to P. brassicae population fluctuation.
A completely randomized block experiment was carried out where the result variables, i.e., productivity and damage percentage, were calculated. Percentage of damage was determined according to formula (Carballo et al. 1989) scales. Data were obtained weekly from the time of pest released; the damaged area was also calculated to examine progress and consequently performed statistical analyses. Insect mortality and cabbage productivity was also noted.
Statistical analyses
For statistical analyses, the used treatments were congregated to perform one-way ANOVA. Each 5 treatments included absolute controls. The treatments correspond to the 4 applications of H. bacteriophora EUPT-S26 along with absolute control. Pearson’s correlation analysis among damage percentage and productivity were also calculated.
Results
Polyhouse phase
Heterorhabditis bacteriophora EUPT-S26 application
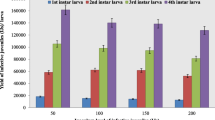
Cabbage plants treated with H. bacteriophora EUPT-S26 IJs revealed a significant decline in the damage percentage than the non-treated plants (F = 3.4, DF = 4, P < 0.05). The minimum damage (injury) percentage was noticed at 1500 and 2000 IJs/ml concentrations (Figs. 1 and 5). The damage percentage were also high (58.4 and 48.4%, respectively, at 5-day post-application) after 1st spray, which was reduced after 2nd spray and infestation level was very low after 3rd spray. A significant control result was detected at infestation evaluated level 5 larvae/plant, beyond economic threshold level established for P. brassicae (1 larva/plant). Larvae mortality was also demonstrated a considerable difference amongst the IJs applied concentrations (F = 131.21, DF = 4, P < 0.05) with the mortality percentage 38.0, 76.4, 91.6 and 94.0% at 5-day post-nematodes application after the 3rd spray (Fig. 2). In this first period of evaluation under the polyhouse conditions, it was determined that H. bacteriophora 2000 IJs/ml concentrations produced the highest protection to cabbage plants. The larval mortality by H. bacteriophora EUPT-S26 was confirmed by collecting the dead cadavers from the polyhouse and kept into the laboratory using the white trap. The emergence of nematode juvenile was observed.
Field phase
Application of H. bacteriophora EUPT-S26
Application of H. bacteriophora EUPT-S26 presented significant variances in the percent damage (F = 3.2, DF = 4, P < 0.05 (Figs. 3 and 5). Latterly treatments detected damage percentage maximum in control (100%). In the lowest concentrations of EPNs, damage percentage ranged from 84 ± 1.07% in the treatment concentrations of 500 IJs/ml and 70 ± 1.07% in concentrations of 1000 IJs/ml after 3rd application of nematodes. Although mortality data were recorded after each spray, the mortality percentage was quite low in the initial application. Maximum larval control was observed after 3rd application of EPNs. At the highest concentration, the damage percentage was 45 ± 1.07% retained constant until harvesting, established the biological pest control by EPNs. For the confirmation of larval mortality by H. bacteriophora EUPT-S26, the dead cadavers were brought to the laboratory and kept over the white trap. The emergence of nematode juvenile was recorded.
Regarding the yield response variable, characterized by cabbage head weight was observed the highest in H. bacteriophora EUPT-S26 (2000 IJs/ml) with high productivity values in treatments with the 3 applications (Fig. 4). The data of productivity in different concentrations (2000 IJs/ml) showed a cabbage head weight between 320 and 410 g (Fig. 4). Regarding damage percentage at harvesting time, it was revealed that the lowermost damage percentage (52.7–44%) correspond to the treatment concentrations of (1500 IJs/ml and 2000 IJs/ml), which were significantly differ than control (100%). Additionally, a negative correlation was also observed among the damage percentage and productivity. In this investigation correlation coefficient was reported high but were statistical significant (Pearson correlation, r = 0.98 for productivity and r = 0.92 for damage) that suggested foliage consumption by P. brassicae showed effect on the head formation (Fig. 5).
Application of Heterorhabditis bacteriophora against Pieris brassicae in the laboratory and field condition a H. bacteriophora population; b Mass multiplication in the laboratory; c Multiplied nematodes; d, e Stored nematodes; f P. brassicae larvae; g, h P. brassicae larvae infesting cabbage; i P. brassicae pupae; j Polyhouse trial; k Field trial; l application of H. bacteriophora; m, n Dead cadavers of P. brassicae collected from the polyhouse and field
Discussion
Cruciferous or Brassicaceous crops like cabbage are extensively dispersed in India, signifying an economically important source to the farmers. Quality and productivity limitations are mostly due to P. brassicae cabbage butterfly insect pest. P. brassicae resistant to several chemical insecticides, predominant in all regions, where cabbage is planted. The absence of the other biocontrol agents of cabbage crops allows cabbage butterfly for promptly establishment in the producing regions. Moreover, cabbage butterfly migrates to the new areas that increased its reproductive potential. Due to the great threat P. brassicae represents in India’s cruciferous crops; curiosity has developed on adopting friendly alternative for the control of insect pest. In biocontrol agents by employing the natural enemies, including EPNs, whose application resulted in the reduction in larvae plant damage and reflected in the better productivity per hectare.
Treatment time required by H. bacteriophora EUPT-S26 to generate mortality in cabbage butterfly larvae, reducing cabbage damage percentage may be because of its foliar applications. In the present study, it is probably IJs mobility and viability was abridged due to drying of leaves, temperature fluctuations and low relative humidity (Bueno-Pallero et al. 2018). This data showed the damage percentage reduced with concentration acceleration and the mortality percentage not resulted in any significant differences as the concentration increase. The possibility of searching a host increased as the contact of IJs over leaf surface increases (Correa-Cuadros et al. 2014). Similarly, IJs were applied in the 5:00–5:30 pm that allowed greater time to establish them over leaf surface and contributed in maximum larvae infection. In contrast, at morning or noon, leaves dry out. Furthermore, adjuvant usage favors the IJs mobility, also increasing area of contact and its adherence to the leaf surface (Mahmoud 2014). The highest P. brassicae mortality percentage was noticed by H. bacteriophora at 2000 IJs/ml concentration (Abbas et al. 2021). At this time, used surfactant prevents the evaporation and facilitating IJs to penetrate into insect cuticle. The above-mentioned is the acceptable clarification how at low concentration it can generate a high mortality percentage in cabbage butterfly under polyhouse conditions.
Pest management using H. bacteriophora EUPT-S26 was done with the infestation level (5 larvae/plant) that surpasses economic threshold reported earlier (1 larva/plant) (Londoño 2001). Cabbage butterfly control would begin from vegetative phage onward to elude the infestation levels 5 larvae/plant during primordium formation (flower) and starting of head formation, as foliage damage on these times indirectly disturbs the head formation and development (Mpumi et al. 2020). Schroer and Ehlers (2005) recorded that the highest attack by P. brassicae was done between 1 and 5 h of post-spray. Gupta et al. (2009) assessed the effectiveness of H. indica, S. carpocapsae strain PDBC and strain JMU against P. brassicae in field conditions. They reported that S. carpocapsae (JMU) @ 2 billion IJs/ha resulted in the highest mortality. The recorded larval mortality was 7.5% after first day of application that further increased and reached to 41.8% after 13 days of post-application. Leaf damage by pests alters the plant photosynthetic rate that initiating an imbalance between plant respiration and photosynthesis, reduced photosynthetic synthesis compulsory for the new organ formation plus development as head (Martínez-Jaime et al. 2016). Cabbage butterfly controlled by EPNs’ applications permitted complementarily between the biocontrol agents at the field environments to cause infections, where diverse the theory can be concluded. Nematode’s IJs penetrations to the insect larvae suppressed the immune system of insect and liberated their bacterial symbiont. Immune response by host might not be distinguished the bacterial infection and speedily leading to the septicaemia (Askary and Ahmad 2020).
Under field conditions, nematodes IJs application enhanced pest mortality resulted in lightened percent damage than the control, as short-term use of EPNs resulted in a rapid action against pest control. The utilization of EPNs, as a biological control agent, functioned in a complimentary and successful manner and they had the capability of potentiating the larval mortality to decrease plant damages (Gozel and Gozel 2016). Wang and Li (1987) also assessed the Steinernema spp. in the conditions against cabbage butterfly and recorded 90% larval mortality after15 hrs of nematode application. Abbas et al. (2022) applied H. bacteriophora, S. glaseri, combination of H. bacteriophora + S. glaseri, at the rate of 1500 infective juvenile/ ml, Xenorhabdus spp., and Photorhabdus spp. at the rate of 4 × 1012 CFU, against cabbage butterfly in the greenhouse and field conditions. They recorded the maximum 90.43% larval mortality in the combined treatment of EPNs after 7 days of application.
Conclusions
The present study included foliar applications of locally isolated bioagent, EPNs strain (H. bacteriophora EUPT-S26) under the polyhouse and field conditions against P. brassicae larvae. The results demonstrated that the application of H. bacteriophora EUPT-S26 resulted in less damage percentage throughout the cabbage’s phenological phases and the most crop productivity was obtained. This suggested nematodes as an effective bio-insecticide against P. brassicae. Therefore, to decrease the damage percentage and to enhance the productivity in assessed field environments, it is better to perform the applications of nematode (EPNs 2000IJs/ml). The nematode applications at the developmental stages of cabbage are an efficient alternative to chemical insecticides, in order to obtain significant cabbage butterfly control. By this way, farmers should have an effective and eco-friendly strategy that may be incorporated in cabbage integrated pest control management also.
Abbreviations
- H. bacteriophora :
-
Heterorhabditis bacteriophora
- P. brassicae :
-
Pieris brassicae
- IJs:
-
Infective juveniles
- EPNs:
-
Entomopathogenic nematodes
- BCAs:
-
Biocontrol agents
- %:
-
Percent
References
Abbas W, Javed N, Haq IU, Ahmed S (2021) Pathogenicity of entomopathogenic nematodes against cabbage butterfly (Pieris brassicae) Linnaeus (Lepidoptera: Pieridae) in laboratory conditions. Int J Trop Insect Sci 41:525–531
Abbas W, Javed N, Haq IU, Ahmed S (2022) Virulence potential of two entomopathogenic nematodes, their associated bacteria, and its metabolites to larvae of Pieris brassicae L. (Lepidoptera, Pieridae) in cabbage under greenhouse and field bioassays. Int J Trop Insect Sci 42:557–563
Askary TH, Ahmad MJ (2020) Efficacy of entomopathogenic nematodes against the cabbage butterfly (Pieris brassicae (L.) (Lepidoptera: Pieridae) infesting cabbage under field conditions. Egypt J Biol Pest Co 30:1–7
Bueno-Pallero FÁ, Blanco-Pérez R, Dionísio L, Campos-Herrera R (2018) Simultaneous exposure of nematophagous fungi, entomopathogenic nematodes and entomopathogenic fungi can modulate belowground insect pest control. J Invertebr Pathol 154:85–94
Correa-Cuadros J, Rodríguez-Bocanegra M, Sáenz-Aponte A (2014) Susceptibility of Plutella xylostella (Lepidoptera: Plutellidae; Linnaeus 1758) to Beauveria bassiana Bb9205, Metarhizium anisopliae Ma9236 and Heterorhabditis bacteriophora HNI0100. Univ Sci 19(2):277–285
Damalas CA, Koutroubas SD (2018) Current status and recent developments in biopesticide use. Agriculture 8(1):1–13
Gupta S, Kaul V, Shankar U, Sharma D, Ahmad H (2009) Field efficacy of steinernematid and heterorhabditid nematodes against Pieris brassicae (L.) on cauliflower. Ann Plant Sci 17:181–184
Kasi IK, Singh M, Waiba KM, Monika S, Waseem M, Archie D, Gilhotra H (2021) Bio-efficacy of entomopathogenic nematodes, Steinernema feltiae and Heterorhabditis bacteriophora against the Cabbage butterfly (Pieris brassicae [L.]) under laboratory conditions. Egypt J Biol Pest Co 31:1–7
Kumar S, Singh A (2015) Biopesticides: present status and the future prospects. J Fertil Pestic 6:100–129
Kumar P, Akhter T, Bhardwaj P, Kumar R, Bhardwaj U, Mazumdar-Leighton S (2021) Consequences of ‘no-choice, fixed time’reciprocal host plant switches on nutrition and gut serine protease gene expression in Pieris brassicae L. (Lepidoptera: Pieridae). PLOS ONE 16:1–23
Labaude S, Griffin CT (2018) Transmission success of entomopathogenic nematodes used in pest control. Insects 9:72
Mahmoud M (2014) Efficacy of entomopathogenic nematodes to certain insect pests infesting oilseed rape in the laboratory and greenhouse. Egypt J Biol Pest Co 24:387–391
Mahmoud M, Mandour N, Pomazkov Y (2007) Efficacy of the entomopathogenic nematode Steinernema feltiae Cross N 33 against larvae and pupae of four fly species in the laboratory. Nematol Medit 35:221–226
Martínez-Jaime OA, Salas-Araiza MD, JA D-G, (2016) Estimación del número de adultos de Plutella xylostella (Linnaeus, 1758)(Lepidoptera: Plutellidae) en función de temperatura y precipitación en brócoli (Brassica oleracea var. itálica) en Irapuato, Guanajuato. México Entomol Mex 3:375–381
Mpumi N, Machunda RS, Mtei KM, Ndakidemi PA (2020) Selected insect pests of economic importance to Brassica oleracea, their control strategies and the potential threat to environmental pollution in Africa. Sustainability 12(3824):1–22
Schroer S, Ehlers R-U (2005) Foliar application of the entomopathogenic nematode Steinernema carpocapsae for biological control of diamondback moth larvae (Plutella xylostella). Biol Control 33(1):81–86
Sood P (2007) Effect of transplanting dates on the incidence of Pieris brassicae Linn. and extent of losses in cabbage under dry temperate conditions of Himachal Pradesh. Legume Res An Int l J 30:297–300
Thakur N, Deka T (1997) Ecological studies on the cabbage butterfly, Pieris brassicae (Linnaeus) in north eastern India. Pest Manag Hort Ecosyst 3:13–15
Wang J, Li L (1987) Entomogenous nematode research in China. Revue De Nématologie 10(4):483–489
Boczek J, Lewandowski M (2016) Nauka o szkodnikach roĹ› lin uprawnych. Wydawnictwo SGGW, pp 1–412
Carballo M, Hernández M and Quezada JR (1989) Efecto de los insecticidas y de las malezas sobre Plutella xylostella (L) y su parasitoide Diadegma insulare (Cress) en el cultivo de repollo. In: Manejo Integrado de Plagas (CATIE) no 11, pp 1–20
Gozel U, Gozel C (2016) Entomopatogenic mematodes in pest management. In: Integrated pest management (IPM): environmentally sound pest management. Intech, Croatia, pp 55–72
Kaya HK, Stock SP (1997) Techniques in insect nematology. In: Manual of techniques in insect pathology. Elsevier, pp 281–324
Londoño Z (2001) Lepidopteros asociados a la formación de cabeza o florete en criciferas. Hortalizas Plagas y Enfermedades. Corpoica-Socolen. RíoNegro Antioquia, Colombia, pp. 63–71.
Singh A, Kumari R, Yadav AN, Mishra S, Sachan A and Sachan SG (2020) Tiny microbes, big yields: Microorganisms for enhancing food crop production for sustainable development. In: New and future developments in microbial biotechnology and bioengineering. Elsevier, pp 1–15
Thakur N, Tomar P, Kaur S, Jhamta S, Thakur R, Yadav AN (2021) Entomopathogenic soil microbes for sustainable crop protection. In: Soil microbiomes for sustainable agriculture. Springer, pp 529–571
Acknowledgements
The study was carried out in the Zoology laboratory at Eternal University Baru Sahib, Himachal Pradesh. Authors acknowledge the financial assistance provided by Department of Science and Technology, Govt. of India (SP/YO/506/2018-G). The authors are also thankful to Vice Chancellor, Eternal University, Baru Sahib, for providing necessary laboratory facilities.
Funding
This work belongs to the project that has been funded by Department of Science and Technology, Govt. of India (SP/YO/506/2018-G).
Author information
Authors and Affiliations
Contributions
NT gave the concept. PT performed the experiment, wrote the manuscript and did the statistical analysis. AS looked into the language part mainly grammar section of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Informed consent was obtained from all individual participants included in the study.
Competing interest
The authors declare that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tomar, P., Thakur, N. & Sharma, A. Infectivity of entomopathogenic nematode against the cabbage butterfly (Pieris brassicae L.) in polyhouse and in field condition. Egypt J Biol Pest Control 32, 38 (2022). https://doi.org/10.1186/s41938-022-00535-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41938-022-00535-5