Abstract
Background
Entomopathogenic nematodes (EPNs) are evidently a useful nematode group for the biocontrol of insect pests. It is well known that efficacy of different EPN strains, even belonging to the same species, can be significantly varied in different localities. Therefore, exploring EPNs and testing their efficacy in various ecological regions is of crucial importance to find out more efficient EPN strains. On the other hand, this practice is also needed to enhance the knowledge on diversity and distribution model of EPNs over the world.
Results
In this study, a species belonging to the genus Steinernema, S. surkhetense, has been characterized for the first time in Vietnam based on morphological and molecular characterizations. Morphological characterizations of infective juveniles, the first and second-generation adults, and molecular characterization of D2-D3 expansion segment of 28S rRNA region were given. Molecular phylogeny of the genus Steinernema was also provided. In addition, the study showed that the lethal efficacy of this local strain to larvae of Galleria mellonella L. was relatively higher than other reported EPN strains in Vietnam.
Conclusions
The Vietnamese EPN population found in this study was determined to be conspecific with S. surkhetense, revealed its new distribution in Vietnam. Besides, detailed morphological and molecular characterizations of it was provided with small variations compared to other populations in the world, and its relatively high lethal efficacy on larvae of G. mellonella implied that this strain can be potentially a useful strain for biological control of insect pests.
Similar content being viewed by others
Background
Entomopathogenic nematodes (EPNs) are obligate parasites and have significant potential in the biological control of insects. They are capable of parasitizing and causing death to hundreds of insect pests without having any clear adverse effect on humans and other vertebrate animals (Nguyen and Hunt 2007). Although most EPN species can control a wide range of insect pests, they are also known that different EPN strains belonging to the same species having different efficacy to control the same host insect (Laznik et al. 2010). Therefore, the works such as isolating, identifying, and testing the efficacy of EPNs strains from different localities are of crucial importance in providing excellent EPN strains for producing biological pesticides (Laznik and Trdan 2011). Besides, the above works also help to provide the information about diversity, distribution, and host range of EPNs over the world, which is necessary to better understand the evolution of life.
Currently, more than 121 valid EPNs species have been described in the world (Didiza et al. 2021). Among them, Steinernema surkhetense Khatri-Chhetri, Waeyenberge, Spiridonov, Manadhar and Moens 2011 belongs to carpocapsae-group (presence of short juveniles). This species has been reported from several regions of Nepal, India, and China (Bhat et al. 2020). In Vietnam, 13 EPN species belonging to the genera Steinernema and Heterorhabditis have been found (Phan et al. 2014). The present study provides the first morphological and molecular characterizations of the EPN S. surkhetense from Vietnam, and molecular phylogeny of the genus Steinernema. Besides, the pathogenic potential of this strain to the larvae of great wax moth, G. mellonella, was also tested.
Methods
Nematode isolation and rearing
Fifty soil samples were collected randomly from Lam Dong and Ninh Thuan, Vietnam. Nematodes were recovered from soil samples using the modified Galleria bait method (Bedding and Akhurst 1975). Positive samples were found at the following coordinates: 11°45′ 37″ N, 109°35′ 47″ E and 11°45′ 55″ N, 109°11′ 01″ E. Subsequently, they were cultured on the larvae of G. mellonella using the method described by Nguyen (2008). The first and second-generation adults were obtained by dissecting infected cadavers after 3 and 5 days, respectively. Infective juveniles (IJs) were obtained when they emerged from the cadavers after 8–10 days.
Morphological characterization
Heat-killed nematodes were fixed using TAF (Formalin 40%: 7 ml, Triethanolamine: 2 ml, distilled-water: 91 ml) for 3–4 days and subsequently transferred to glycerin to make permanent slides, following Seinhorst (1959). Measurements and pictures of all nematode stages were taken from permanent slides using Carl Zeiss Axio Lab. A1 light microscope equipped with a Zeiss Axiocam ERc5s digital camera.
Molecular characterization
The D2-D3 region of 28S rRNA was amplified using D2A (5′-ACAAGTACCGTGAGGGAAAGTTG-3′) and D3B (3′-TCCTCGGAAGGAACCAGCTACTA-5′) primers (De Ley et al. 1999). The obtained sequence was analyzed following Nguyen et al. (2020). Blast search was used to search for closely related species on GenBank (Altschul et al. 1997). Forward and reverse sequences were assembled and analysed using Geneious R11. The best fit model was chosen using Mega 7, following Nguyen et al. (2020). Phylogenetic trees were created using MrBayes 3.2.6 Add-in in Geneious R11 under GTR + G + I model (Markov chains were set with 1 × 106 generations, four runs, 20% burn-in, and subsampling frequency of 500 generations) following Nguyen et al. (2020).
Virulence of S. surkhetens from Vietnam
In this study, an experiment on the lethal efficacy of S. surkhetense to larvae of G. mellonella was done, following Nguyen (2008). There were 10 treatments with the concentration of 10, 20, 30, 40, 50, 60, 70, 80, 90, and 100 IJs per larva of G. mellonella. Each treatment was repeated 3 times, 15 larvae were used for a replication resulted in a total of 45 larvae per treatment. In the control treatment, IJs were replaced by distilled-water. The experiment was checked after 48 h. at 28 °C. Cadavers of larvae were used for a white trap to confirm the infection of IJs (Nguyen 2008).
Statistical analysis
Results were analyzed using SPSS version 25 to determine LC50, to detect significant differences and correlation (IBM Corp 2017).
Results
Measurements
Data of different measurements are presented in Table 1.
Morphological characterization
Female (first generation)
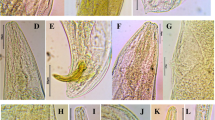
Body was spiral-shaped. Cuticle was smooth under LM (Fig. 1A). Lip region was rounded and slightly offset to body contour. Mouth was funnel-shaped. Cheilorhabdions and cardia were prominent. Secretory-excretory pores were prominent and located anterior to nerve ring (Fig. 1B). Vulva was slit-like with protruding lips, located at mid-body with short epiptygmata (Fig. 1C). Genital tract was amphidelphic-didelphic with reflexed ovaries. Tail was conical with short mucron (Fig. 1D).
LM pics of Steinernema surkhetense from Vietnam. A–D first-generation female. A entire body; B pharyngeal region; C vulva region; D tail region. E–F second-generation female. E entire body; F tail region. G–I first-generation male. G pharyngeal region; H entire body; I tail region. J entire body of second-generation male. K–N juvenile. K entire body; L pharyngeal region; M lateral field; N tail region
Female (second generation)
Similar to the first-generation females. Body size was smaller and slightly curved ventrally. Tail was elongate and tapering to a pointed tail end. (Fig. 1E and F).
Male (first generation)
Body was J-shaped. Head region was similar to female. Testis was reflexed ventrally (Fig. 1H). Spicules were paired, symmetrical, and moderately curved. Gubernaculum was boat-shaped (Fig. 1I). A single precloacal papilla and 11 pairs of genital papillae were arranged in normal position for Steinernema (i.e. 6 or 7 pairs precloacal subventral, one pair adcloacal, one pair lateral, 2 pairs subterminal, and one pair subdorsal).
Male (second generation)
Similar to first-generation of males, but body size was smaller (Fig. 1J).
Infective juvenile
Body was short, slender, slightly curved ventrally, and tapering to both two ends (Fig. 1K). Lip region was rounded and continuous to body contour (Fig. 1L). Lateral field was with 9 lines at mid-body (Fig. 1M). Amphids was slit-like. Hemizonid was distinct and located at beginning of basal bulb. Secretory-excretory pore was prominent and located at anterior third of pharyngeal region. Hyaline region was occupying half of tail length. Tail was tapering to pointed tail end (Fig. 1N). Phasmid was pore-like and located at mid-tail.
Molecular characterization
Obtained D2-D3 region of 28S rRNA sequence of S. surkhetense from Vietnam was 695 bp long (assession number: MW703809). It was 100% similar to other sequences of S. surkhetense from GenBank (accession number: MF621001, HQ190043). The phylogenetic tree showed that the D2-D3 region of 28S rRNA sequence of S. surkhetense from Vietnam had a sister relationship to the sequences of S. surkhetense, S. nepalense Khatri-Chhetri et al. (2011), S. siamkayai, S. huense, and S. carpocapsae with a maximal support (1 PP). (Fig. 2).
Virulence of S. surkhetense from Vietnam
Probit analysis in SPSS version 25 showed that LC50 (nematode inoculation concentration kill 50% larvae of G. mellonella) of S. surkhetense was 14 IJs/larva. Besides, a highly significant correlation between nematode inoculation concentration and the mortality rate of larvae was found based on the Pearson correlation test (R = 0.752, p < 0.01). The One-way ANOVA analysis showed that the lethal efficacies of all treatments are significantly different than the control (Table 2).
Discussion
Generally, the morphology of S. surkhetense from Vietnam is highly in agreement with the description of Khatri-Chhetri et al. (2011). Variations in some measurements between these populations were found, including larger L, EP, ES, body diam., T, a, b, c, E% in the first-generation females; longer body length L, EP, ES, T, smaller c and E values in the first-generation males; longer body length and smaller H% value in juveniles (Table 1). However, according to Hunt and Nguyen (2016), large variations in measurements of adult Steinernema spp. are known to be present since their body sizes are much larger than juveniles. Besides, other populations of S. surkhetense from different localities in the world also showed variations in measurements.
Khatri-Chhetri et al. (2011) differentiated S. surkhetense from S. nepalense using a combination of morphological and molecular characterizations. Especially, ITS rDNA sequence of S. surkhetense was significantly different from that of S. nepalense. However, obtained result showed that D2-D3 expansion segment of 28S rRNA sequence of S. surkhetense in this study was very similar (only 4 nucleotides difference) to that of S. nepalense (accession number: HQ190045). On the other hand, Nguyen and Hunt (2007) reported that cross-hybridization study can be applied to separate closely related species of Steinernema spp., therefore, such studies can be done in the laboratories where populations of both S. surkhetense and S. nepalense present to support species status of these species in future.
The study of De Doucet et al. (1999) suggested that larvae of G. mellonella can be used as a standard host for testing and comparing virulence (based on LC50) of EPNs. Their experiments on larvae of G. mellonella showed that LC50 of Steinernema rarum = 6 IJs/larva, Steinernema feltiae = 9 IJs/larva, and Heterorhabditis bacteriophora = 3 IJs/larva. In Vietnam, Nguyen (2008) reported that LC50 of 2 potential strains of EPNs (Steinernema S-TK10 and Heterorhabditis H-TN3) on larvae of Spodoptera litura (Fab.), Omiodes indicate (Fab.), Agrotis ipsilon (Huf.), Spodoptera exigua (Hub.), Pieris rapae (L.), and Plutella xylostella (Linn.) were 13–95 IJs/larva. In this study, LC50 of S. surkhetense from Vietnam was 14 IJs/larva, indicating that this is a relatively potential strain of EPNs to be used in the biocontrol of insect pests. However, it is also important to test the virulence of this strain on other insect pests to evaluate its potential in biocontrol exactly.
Conclusions
Results conclude that S. surkhetense was present in Vietnam and this is a new distribution of the species. There exist small variations in measurements of S. surkhetense from different population, for example, variations in L, EP, ES, body diam., T, a, b, c, and E% in the first-generation females; body length, EP value, ES value, T value, c value, and E% value in the first-generation males; body length and H% value in juveniles. The new strain of S. surkhetense found in this study can be a potential strain for biological control.
Abbreviations
- EPNs:
-
Entomopathogenic nematodes
References
Altschul SF, Madden TL, Schäffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ (1997) Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res 25:3389–3402. https://doi.org/10.1093/nar/25.17.3389
Bedding RA, Akhurst RJ (1975) A simple technique for the detection of insect parasitic rhabditid nematodes in soil. Nematologica 21:109–116
Bhat AH, Sharma L, Chaubey AK (2020) Characterisation of Steinernema surkhetense and its symbiont Xenrorhabdus stockiae and a note on its geographical distribution. Egypt Acad J Biol Sci A Entomol 13:105–122
De Doucet MMA, Bertolotti MA, Giayetto AL, Miranda MB (1999) Host range, specificity, and virulence of Steinernema feltiae, Steinernema rarum, and Heterorhabditis bacteriophora (Steinernematidae and Heterorhabditidae) from Argentina. J Invertebr Pathol 73:237–242
De Ley P, Felix MA, Frisse L, Nadler S, Sternberg P, Thomas WK (1999) Molecular and morphological characterisation of two reproductively isolated species with mirror-image anatomy (Nematoda: Cephalobidae). Nematology 1:591–612
Didiza, L, Lephoto, TE & Gray, VM (2021) Morphological and molecular phylogenetic description of Steinernema batswanae n. sp. (Rhabditida: Steinernematidae): a new species of an entomopathogenic nematode from South Africa. Arch Phytopathol Plant Prot. https://doi.org/10.1080/03235408.2021.1931648.
Hunt DJ, Nguyen KB (2016) Advances in entomopathogenic nematode taxonomy and phylogeny. Brill, Leiden, The Netherlands
Ibm Corp (2017) IBM SPSS Statistics for Windows, Version 25.0. IBM Corp, Armonk, NY
Khatri-Chhetri HB, Waeyenberge L, Spiridonov S, Manandhar HK, Moens M (2011) Two new species of Steinernema Travassos, 1927 with short infective juveniles from Nepal. Russ J Nematol 19:53–74
Laznik Ž, Tóth T, Lakatos T, Vidrih M, Trdan S (2010) Control of the Colorado potato beetle (Leptinotarsa decemlineata [Say]) on potato under field conditions: a comparison of the efficacy of foliar application of two strains of Steinernema feltiae (Filipjev) and spraying with thiametoxam. J Plant Dis Prot 117:129–135
Laznik Z, Trdan S (2011) Entomopathogenic nematodes (Nematoda: Rhabditida) in Slovenia: from tabula rasa to implementation into crop production systems. In: Perveen F (ed) Insecticides - advances in integrated pest management. InTech, Rijeka, pp 627–656
Nguyen HT, Nguyen TD, Le TML, Trinh QP (2020) First report of Xiphinema hunaniense Wang & Wu, 1992 (Nematoda: Longidoridae) in Vietnam. J Nematol 52:e2020–e2078. https://doi.org/10.21307/jofnem-2020-078
Nguyen KB, Hunt DJ (2007) Entomopathogenic nematodes: systematics, phylogeny and bacterial symbionts. Brill, Leiden, The Netherlands
Nguyen NC (2008) Entomopathogenic nematodes in Vietnam (in Vietnamese). Publishing House for Science and Technology, Hanoi, Vietnam
Phan KL, Mráček Z, Půža V, Nermut J, Jarošová A (2014) Steinernema huense sp. n., a new entomopathogenic nematode (Nematoda: Steinernematidae) from Vietnam. Nematology 16:761–775
Seinhorst JW (1959) A rapid method for the transfer of nematodes from fixative to anhydrous glycerin. Nematologica 4:67–69. https://doi.org/10.1163/187529259x00381
Acknowledgements
Not applicable.
Funding
This research was funded by the Institute of Ecology and Biological Resources, Vietnam Academy of Sciences and Technology (project code: VAST04.04/21-22).
Author information
Authors and Affiliations
Contributions
PQT was the supervisor of the project. DTN prepared the data. LTML was responsible for molecular data. THN prepared the manuscript. All authors contributed to writing and editing the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and materials
All data generated or analysed during this study are included in this published article.
Competing interests
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Trinh, P.Q., Nguyen, D.T., Le, L.T.M. et al. First report of entomopathogenic nematode Steinernema surkhetense and its pathogenic potential to larvae of the Greater Wax Moth (Galleria mellonella L.) in Vietnam. Egypt J Biol Pest Control 31, 147 (2021). https://doi.org/10.1186/s41938-021-00496-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41938-021-00496-1