Abstract
Background
Outbreak of the fall armyworm Spodoptera frugiperda (J.E. Smith, 1797) (Lepidoptera: Noctuidae) occurred in Mizoram, North-Eastern India. The infestation spread in the entire state covering a total area of around 2840 hectares of maize cultivated land. Entomopathogenic nematodes (EPNs) represent potential candidates for the biological control of S. frugiperda. In the study, the susceptibility of the pest against 4 locally isolated EPN species Heterorhabditis indica, H. baujardi, Steinernema sangi and S. surkhetense was evaluated.
Results
The results indicated that all the isolated EPN species showed a high rate of larvicidal and pupicidal activities against the pest. Mortality between 43.75–100.00 and 25.00–100.00% of 3rd and 5th larval instars, respectively (at concentrations 10–800 IJs/larva), and 37.50–68.75% mortality of pupae (at concentrations 200–1600 IJs /pupa) were found after exposure to the EPN species. The mortality rate of the pest showed significant variations with life stages of the host insect, nematode concentrations and incubation time. Based on the median lethal concentration (LC50), H. indica was the most pathogenic species, followed by S. sangi, H. baujardi and S. surkhetense. The LC50 values of H. indica at 72 h post-incubation were 20.26 and 62.07 IJs/larva for the 3rd and 5th larval instars, respectively, and 913.34 IJs/pupa. The penetration assay showed that H. indica had the highest penetration rate into the hosts, 27.24, 21.30 and 20.00% in the 3rd, 5th larval instars and pupae, respectively. Furthermore, all the EPN isolates were capable of successful multiplication inside the cadaver of S. frugiperda that showed significant differences with the EPN isolates and life stages of the pest. Among the isolates, H. indica showed the highest multiplication rates, 17,692.25 ± 2103.59, 8345.63 ± 785.34 and 79,146.38 ± 5943.73 IJs per 3rd instar larva, 5th instar larva and pupa, respectively.
Conclusions
The study revealed that the 4 species of EPNs showed a high potency against S. frugiperda, thereby having the potential to be developed as a biological control agent against the pest. Moreover, the isolated EPN species could potentially serve as alternatives for chemical insecticides and could further be incorporated into the Integrated Pest Management (IPM).
Similar content being viewed by others
Background
Maize (Zea mays L.) is one of the most important cultivated cereal crops in the world and the third most important agricultural crop in India after rice and wheat (Joshi et al. 2005). In India, a total loss of 13.20% of the crop has been estimated due to insect pests and diseases, thereby adversely affecting the production of maize in the country (Kumar et al. 2014).
Fall armyworm (FAW), Spodoptera frugiperda (J.E. Smith, 1797) (Family: Noctuidae) is an invasive lepidopteran pest native to America (Todd and Poole 1980). It is a polyphagous pest of more than 350 species of plants, causing serious damage to economically important cultivated crops such as maize, rice, sorghum, sugarcane, cotton and other vegetable crops (CABI 2020). Subsequently, FAW was reported to invade Central and Western Africa in the year 2016 (Goergen et al. 2016), the Indian subcontinent in Asia in the year 2018 (Sharanabasappa et al. 2018) and currently with worldwide distribution (CABI 2020). In India, FAW was firstly reported in maize fields in Shivamogga, Karnataka, in May 2018 (Sharanabasappa et al. 2018) with subsequent scientific reports from other regions of the country (Repalle et al. 2020).
The use of chemical insecticides is the most effective approach for the control of FAW (Belay et al. 2012). However, the pest was reported to develop resistance against major classes of commonly used insecticides (Zhu et al. 2015) and Cry proteins of Bacillus thuringiensis Berliner (Bacillaceae) as well (Murúa et al. 2019); therefore, safer, eco-friendly control strategies need to be developed and further implemented. Biological control offers a promising strategy against a wide range of insect pests, and biopesticides being environmentally safer are potential alternatives to chemical pesticides. Like other biological control agents, entomopathogenic nematodes (EPNs) are potential and promising agents for controlling insect pests (Lacey and Georgis 2012). EPNs of the family Steinernematidae and Heterorhabditidae are parasitic nematodes of insects with natural occurrence in the soil. Many researchers have reported the application of indigenous EPN strains with better adaptation to the prevailing local climatic conditions in comparison with exotic strains (Bedding 1990). The study on the susceptibility of S. frugiperda to EPNs has been conducted by some workers (Viteri et al. 2018). However, to date, no studies have been conducted on the pathogenicity of the EPN isolates, Heterorhabditis baujardi, Steinernema sangi and S. surkhetense against S. frugiperda.
The present study aimed to evaluate the susceptibility of S. frugiperda to locally isolated EPN species.
Methods
Collection of S. frugiperda
Larvae of FAW were collected from infested maize plants and reared in the laboratory using their natural diet at 26 ± 2 °C. Different life stages were obtained for morphological and molecular identification of the pest. In addition, 3rd and 5th larval instars and pupal stage were selected for the evaluation of their susceptibility against the locally isolated EPN species.
Morphological and molecular identification
Morphological characteristics of different life stages of S. frugiperda, including female genitalia were examined and compared to previous studies of the pest (Ganiger et al. 2018). Adult females were dissected under an Olympus CX41 microscope and the female genitalia was studied for further identification.
Molecular characterization of the pest involved extraction of DNA from adult insects by using QIAamp DNA mini kit (Qiagen). The mitochondrial gene, cytochrome C oxidase subunit I (COI) was amplified by using a ProFlex™ 3 × 32-Well PCR System (Applied Biosystems). The COI (M1–M6 partition) primers, LCO 1490 (5′-GGTCAACAAATCATAAAGATATTGG-3′) forward and HCO 2198 (5′-TAAACTTCAGGGTGACCAAAAAATCA-3′) reverse (Folmer et al. 1994) were used for amplification of the gene. PCR conditions were: 1 cycle of 94 °C for 1 min followed by 35 cycles at 94 °C for 60 s; 55 °C for 60 s; and 72 °C for 2 min. The last step was 1 cycle at 72 °C for 10 min. The PCR product was directly sequenced in both directions at AgriGenome Labs Private Limited, Kakkanad, Kerala, India. The sequence was edited using FinchTV 1.4.0 software packages (Geospiza, Inc.; Seattle, WA, USA; http://www.geospiza.com), aligned using MEGA version X (Kumar et al. 2018) and submitted to NCBI GenBank. Nucleotide sequences of Spodoptera along with one out-group sequence (Helicoverpa armigera) were retrieved from GenBank. The phylogenetic tree was constructed using maximum likelihood tree (ML), in MEGA X with a total of 1000 bootstrap data sets.
Rearing of Galleria mellonella (L.)
Larvae of greater wax moth, Galleria mellonella L. were collected from local beekeepers and reared in the laboratory by using their natural diet.
Nematode sources
Four species of locally isolated EPNs, H. indica, H. baujardi, S. sangi and S. surkhetense, were used for the study. The 2 species, S. sangi and H. baujardi, were reported by previous studies of Vanlalhlimpuia et al. (2018). Meanwhile, the study represents the first report of H. indica and S. surkhetense from Mizoram, India. The nematodes were isolated from soil samples by baiting methods, following Bedding and Akhurst (1975). A survey was done in different areas of Mizoram, NE India, covering undisturbed and disturbed forest areas. From each collection site, a total of 400–500 g of soil sample was collected from a depth of 10–15 cm. The collected soil samples were transferred to the laboratory and immediately baited with the last instar larvae of G. mellonella in a container and incubated at room temperature. For 10 consecutive days, larval mortality was checked every 24 h of time and dead larvae were transferred to a modified white trapped as per Kaya and Stock (1997). The nematodes that emerged from the dead cadaver were used for re-infection of fresh host for confirmation. The emerged IJs from the re-infection plate were collected and stored with aerated water in an incubator at 12–15 °C. For the experiment, only freshly emerged IJs from dead cadaver were used.
Susceptibility test
Four species of EPN, Heterorhabditis indica, H. baujardi, Steinernema sangi and S. surkhetense, were used against S. frugiperda to assess the difference in virulency among the nematode isolates. Meanwhile, 3rd, 5th larval instars and pupae of the pest were selected to assess whether susceptibility varies with advancement in life stages of the host.
Larval mortality assay
The experiment was performed using Petri dish assay, following Kaya and Stock (1997). Different concentrations of nematodes (10, 25, 50, 100, 200, 400 and 800 IJs/larva) were selected, and healthy larvae (3rd and 5th instars) of S. frugiperda were used for this assay. A Petri dish (35 × 10 mm) was lined by double-layer Whatman filter paper No. 1., and 0.5 ml of distilled water containing each concentration of nematode was introduced into an individual Petri dish and incubated for 30 min. A single larva was introduced into individual Petri plate pre-inoculated with nematodes. For both larval stages, 8 replicates were set for each concentration of nematodes and EPN isolates, and the experiment was repeated thrice. A Petri dish lined with filter paper wetted only with distilled water was set for each concentration of nematode to serve as a control plate.
Pupal mortality assay
Different concentrations of nematodes (200, 400, 800 and 1600 IJs/pupa) were used to examine the susceptibility of pupa against the EPN isolates. The assay was performed by heating sandy loamy soil to dry for 24 h and the moisture content was then adjusted at 15–20% (v/w) by adding distilled water (including pipetted water in nematode suspension). For all the EPN isolates, each of the nematode concentrations was added to an individual Petri dish (35 × 10 mm) filled with pre-wetted soil sample containing burrowed one-day-old pupa of S. frugiperda. Control plates and replicates were set as in the larval assay and the experiment was repeated thrice.
For both the assays, the experiments were carried out in an incubator at 28 ± 2 °C and mortality rate was check at 24 h intervals time for 120 h (5 days). Colour change, no movement and the smell emanating from the dead body of larvae and pupae were used for primary confirmation of death due to EPNs. After 48 h of mortality, the dead insects were rinsed with distilled water and individually dissected under an Olympus CX41 microscope for further confirmation.
Host penetration
After 48 h of mortality, dead larvae and pupae from mortality assays were rinsed by distilled water, individually dissected in Ringer’s solution and the total number of adult nematodes in the dead cadaver was counted and recorded.
Nematode multiplication in host
A concentration of 200 IJs/insect was used for examining the reproductive potential of the EPN isolates in S. frugiperda. The experiment was set up as in mortality assay. Dead larvae (3rd and 5th instar larvae) and pupae were placed individually on a modified white trap for multiplication. The experiment was maintained at 28 ± 2 °C in an incubator. The date of emergence of IJs from a dead cadaver was recorded, and a total emerged IJs were counted at 15–30 days post-emergence from individual plates to record the multiplication rate of the EPN isolates.
Statistical analysis
For all the experimental data, statistical data analysis was performed. One-way analysis of variance (ANOVA) was conducted to determine significant differences (at the level of p ≤ 0.05) in the parameters of the experiment. To determine a correlation among different experimental parameters, regression analysis was performed. Values of both median lethal concentration (LC50) and median lethal time (LT50) were calculated by using Probit regression analysis (SPSS 20.0 software).
Results
Morphological and molecular identification of S. frugiperda
The analysis of morphological characters along with that of female genitalia strongly suggests that the pest belongs to the species S. frugiperda (Ganiger et al. 2018). The generated partial sequence of the mitochondrial COI gene consisting of 677 bp long was deposited in GenBank under accession number MT677868. The BLAST search result of the sequence showed 100% similarity with sequences (MN640599, MK790611, MK318297, MT180097, MT881755 and MN630563) available for S. frugiperda in NCBI GenBank. The developed and database sequences of S. frugiperda showed an intraspecific (K2P) distance of 0.00%. However, based on the K2P distance, the developed sequence and sequence of closely related species studied: S. litura (KX863232), S. littoralis (KJ634300.1) and S. picta (HQ950412) exhibit interspecific distance of 4–6%. The phylogenetic relationship of the developed sequence and sequences acquired from GenBank given in Fig. 1 revealed that upon comparison, the insect was identified as S. frugiperda.
Phylogenetic relationship of Spodoptera frugiperda with other Spodoptera spp. based on distance analysis of COI DNA regions. Maximum likelihood tree was constructed by using General Time Reversal model. Numbers at the nodes indicate bootstrap values (> 50%, 1000 replicates). GenBank Accession numbers are given after each species
Susceptibility of S. frugiperda
The study revealed that larvae and pupae of S. frugiperda were highly susceptible to the EPN isolates, H. indica, H. baujardi, S. sangi and S. surkhetense (Figs. 2, 3, 4). The overall mortality rate showed significant differences with life stages of the host insect (F = 14.85, df = 2, 69, p < 0.001), nematode concentrations (F = 4.79, df = 6, 61, p < 0.001) and incubation time (F = 4.002, df = 4, 135, p < 0.001). However, non-significant differences in S. frugiperda mortality rates were observed among the studied EPN isolates (p > 0.05). Overall, the mortality rates of S. frugiperda ranged from 43.75 to 100.00% in 3rd instar larvae, 25.00–100.00% in 5th instar larvae and 37.50–68.75% in the pupal stage. Also, the application of nematode concentrations showed a positive correlation with host mortality for all the 4 EPN isolates (values are 0.91, 0.96 and 1.0 in H. indica; 0.94, 0.92 and 0.98 in H. baujardi; 0.95, 0.91 and 0.99 in S. sangi; 0.93, 0.89 and 0.98 in S. surkhetense, for 3rd and 5th larval instars and pupa, respectively).
At a concentration of 10 IJs/larva, all the studied EPN species could cause larval mortality after 24 h of incubation (Figs. 2, 3). A further increase in nematode concentration and incubation period resulted in high mortality of the pest. At a concentration of 100 IJs/larva and incubation periods (24–120 h), mortality rate of the 3rd instar ranged between 62.50–100.00%, 68.75–93.75%, 56–100.00% and 56.25–87.50%, respectively, for H. indica, H. baujardi, S. sangi and S. surkhetense. For the same conditions, mortality rate of the 5th larval instar ranged between 50.00–100.00% and 43.75–75.00%, respectively, for H. indica and H. baujardi; 43.75–81.25% for both S. sangi and S. surkhetense. Meanwhile, for the same nematode concentration, the isolates H. indica and S. sangi caused 100 percent mortality for 3rd instar larvae at 96 and 120 h post-incubation, respectively, while H. baujardi and S. surkhetense caused comparatively low mortality of 87.50 and 93.75%, respectively, at 120 h post-incubation. A 100 percent mortality of the 5th larval instar was initially observed at 100 IJs/larva after 120 h of incubation for H. indica. However, for the isolates of H. baujardi, S. sangi and S. surkhetense, the same mortality rate was observed at 200 IJs/larva after 96 h of incubation.
Against the pupal stage, similar mortality patterns were observed with a comparatively low mortality rate as given in the Fig. 4. The highest mortality rate (68.75%) was observed with S. sangi at a concentration of 1600 IJs/pupa after 96 h of incubation. With the exposure of 400 IJs/pupa and 72 h post-incubation, the three EPN isolates of H. indica, H. baujardi and S. surkhetense caused 37.50% mortality while S. sangi caused low mortality of 31.25%.
LC50 and LT50
Based on the calculated LC50 values, H. indica was the most pathogenic among the EPN isolates with minimum values (Table 1). At 72 h post-incubation, H. indica showed LC50 values of 20.26 and 62.07 IJs/larva for the 3rd and 5th larval instars, respectively, and 913.34 IJs/pupa. For the same incubation period, S. surkhetense was the least virulence that caused the highest LC50 values of 35.08 and 80.50 IJs/larva for the 3rd and 5th larval instars, respectively, and 1111.75 IJs/pupa. Meanwhile, at 72 h post-incubation, the LC50 values of S. sangi were lower (22.64 and 70.07 IJs/larva, respectively) compared to that of H. baujardi (30.62 and 96.44 IJs/larva) for the 3rd and 5th larval instars, respectively. However, for the same incubation period, the LC50 values of H. baujardi (963.44 IJs/pupa) in the pupae was lower than that of S. sangi (1106.72.75 IJs/pupa).
The calculated LT50 values are given in Table 2. At a concentration of 100 IJs, the LT50 value of H. indica, H. baujardi, S. sangi and S. surkhetense in the 3rd larval instar was 15.99, 16.12, 14.33 and 17.64 h, respectively. For the same nematode concentration, in the 5th larval instar, a comparatively high LT50 value of 21.74, 28.98, 28.90 and 32.39 h was recorded for H. indica, H. baujardi, S. sangi and S. surkhetense, respectively. In the case of pupal stage, at a concentration of 400 IJs, LT50 value for H. indica, H. baujardi, S. sangi and S. surkhetense was 112.00, 114.52, 114.69 and 105.00 h, respectively.
Host penetration
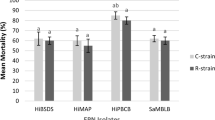
Host penetration (Fig. 5) showed significant differences among the studied EPN isolates (F = 19.16, df = 3, 68, p = 0.00) and non-significant among life stages of the host insect (p > 0.05). Furthermore, the study recorded positive correlation between mean penetration rates and nematode concentrations for all EPN isolates tested (r = 0.987 in H. indica, r = 0.97 in H. baujardi, r = 0.951 in S. sangi and r = 0.97 in S. surkhetense). Among the 4 studied EPN isolates, the total mean penetration of the 2 heterorhabditids was 27.24, 21.30 and 20.00% for H. indica and 25.25, 20.60 and 18.00% for H. baujardi in 3rd, 5th instars and pupae, respectively. It showed significantly higher values than the steinernematids, S. sangi (17.74, 15.93 and 13.00%) and S. surkhetense (19.17, 17.45 and 14.00%) in 3rd, 5th larval instars and pupae, respectively.
Nematode multiplication in host
The isolated EPNs successfully reproduced inside cadaver of S. frugiperda (Fig. 6). Progeny production showed significant variations with life stages of the host (F = 228.628, df = 2, 189, p = 0.00) and among the EPN isolates (F = 3.432, df = 2, 189, p = 0.018). However, progeny production in host insect did not show significant difference within the same EPNs family (Heterorhabditidae and Steinernematidae) (p > 0.05). Among the studied EPNs, the highest progeny production was recorded for H. indica, followed by H. baujardi, S. surkhetense and S. sangi. Against 3rd instar larvae, a total of 17,692.25 ± 2103.59, 16,375.31 ± 1688.37, 9862.68 ± 1031.45 and 12,218.63 ± 1501.93 IJs/larva was recorded for H. indica, H. baujardi, S. sangi and S. surkhetense, respectively. Furthermore, a correspondent total progeny production of 8345.63 ± 785.34, 7357.44 ± 832.56, 5506 ± 608.56 and 4827.38 ± 707.91 IJs/5th instar larva was recorded. In case of pupal stage, comparatively high multiplication rate was recorded as 79,146.38 ± 5943.73, 77,999 ± 7432.20, 42,747.44 ± 3934.22 and 52,383.63 ± 4331.35 IJs/pupa H. indica, H. baujardi, S. sangi and S. surkhetense, respectively.
Discussions
The study involved evaluation on the susceptibility of S. frugiperda to 4 species of EPNs isolated from Mizoram, North-Eastern India. A susceptibility test was performed by using different concentrations of each EPN isolates against the pest. Mortality of host insect was evaluated based on nematode concentration and incubation period; nematode penetration rate of host insect was evaluated based on nematode concentration. Several workers have conducted scientific studies to evaluate the susceptibility of S. frugiperda to different EPN species under laboratory and field conditions. It is not surprising that the susceptibility varied among the different species of the nematodes. Andaló et al. (2010) reported that at a dosage of 200 IJs/5th instar larva of S. frugiperda, 96.07 and 100.00% mortality rates were recorded when treated with Heterorhabditis sp. and Steinernema arenarium, respectively. At 48 h post-incubation, H. indica and S. sangi caused 75.00% mortality, while H. baujardi and S. surkhetense caused 68.75% host mortality at a concentration of 200 IJs/5th instar larva. Caccia et al. (2014) reported that concentrations of 50 and 100 IJs of S. diaprepesi caused 93.00 and 100.00% mortality rates of last instar larvae of S. frugiperda at 144 h post-incubation. However, at the concentration of 50 IJs/5th instar larva, the present study recorded 68.78% host mortality due to H. indica, while S. sangi, H. baujardi and S. surkhetense caused 75.00% mortality at 120 h post-incubation. At 100 IJs/5th instar larva, both S. sangi and S. surkhetense caused 81.25% mortality, while H. indica and H. baujardi caused 100.00 and 75.00% mortality, respectively. In another study, Garcia et al. (2008) reported that against 3rd instar larvae of S. frugiperda, Steinernema sp. (280 IJs/larva) and H. indica (400 IJs/larva) caused 100.00 and 75.00% mortality rates at 48 h post-incubation. In the present study, at the concentration of 400 IJs/3rd instar larva, S. sangi and H. indica caused 100.00% mortality; S. surkhetense and H. baujardi caused 93.75 and 87.50%, respectively. The slight variation in the mortality rate with other studies may be attributed to the difference in the nematode species and life stage of the pest. The present study showed that H. indica was found to be the most pathogenic in terms of LC50 and LT50 values, rate of host penetration and reproduction in host cadaver. This may be attributed, in part, to the fact that H. indica showed generally the highest prevalence among the locally isolated EPN isolates, thereby predicting its high adaptability in the prevailing climatic conditions.
Host penetration is one of the important factors related to the pathogenicity of EPNs (Kaya and Gaugler 1993). The potency of host invasion and penetration ability of EPN species may be attributed to variations in their virulence against insect pests (Glazer et al. 2001). The observed host invasion rates of the studied EPN isolates are within the range as per reports of other scientific investigations (Phan et al. 2005). A high penetration rate may be correlated with high production of toxins (Akhurst and Boemare 1990) that will ultimately result in rapid killing of the insect. Therefore, high mortality caused by H. indica and H. baujardi in the present study may be correlated with a high rate of penetration in heterorhabditids. However, the study recorded that S. sangi with a comparatively low penetration rate showed a similar level of pathogenicity with heterorhabditids. It is thus clear that, even though many studies reported the dependent relationship of nematode virulence and penetration rate (Glazer et al. 2001), the determination of virulence varied with the species since some workers still reported the lack of relationship between penetration and mortality rate (Ricci et al. 1996).
Reproductive efficacy inside the host insect plays an important role in the effectiveness of EPNs as a biological control agent. The nematode upon infecting the host undergoes growth and reproduction inside the cadaver for multiple generations after which the IJs emerged and seek new hosts. The progeny production data of the present study showed a successful host infection and reproduction of the EPN isolates, thereby revealing their potency as effective biopesticides. Caccia et al. (2014) evaluated the capacity of S. diaprepesi on the larvae of S. frugiperda and reported that the nematode produced 11,329 and 27,155 IJs at a concentration of 50 and 100 IJs/larva, respectively. Obtained findings on progeny production by EPN isolates showed comparatively significant differences among the 4 species, which agree with the results of Rahoo et al. (2018), where reproductive rate significantly varied among EPN isolates. Besides, a high rate of multiplication in the pupal stage as per obtained observation may be correlated with the mass and nutrient content of the host (Loya and Hower 2003). Moreover, the 2 heterorhabditids, H. indica and H. baujardi, showed comparatively higher production of progeny than the 2 steinernematids, S. sangi and S. surkhetense. Hermaphroditic reproduction of the first generation in heterorhabditids (Glazer et al. 1994) may be attributed to the higher reproductive rate in comparison with steinernematids that reproduce sexually in the first generation (Kondo and Ishibashi 1987) as this may strongly correlate with an initial establishment in the host.
Conclusions
The EPN isolates evaluated in the present study showed a high pathogenicity against S. frugiperda with successful reproductions, thereby indicating potential candidates to control the pest. This is the first scientific study on the evaluation of the susceptibility of S. frugiperda against the locally isolated EPNs.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- EPNs:
-
Entomopathogenic nematodes
- IJs:
-
Infective juveniles
- LC:
-
Lethal concentration
- LT:
-
Lethal time
- NE:
-
North-Eastern
- hr:
-
Hour
References
Akhurst RJ, Boemare NE (1990) Biology and taxonomy of Xenorhabdus. In: Gaugler R, Kaya HK (eds) Entomopathogenic nematodes in biological control. CRC Press, Boca Raton, pp 75–90
Andaló V, Santos VA, Moreira GF, Moreira CC, Junior AM (2010) Evaluation of entomopathogenic nematodes under laboratory and greenhouses conditions for the control of Spodoptera frugiperda. Ciênc Rural 40(9):1860–1866. https://doi.org/10.1590/S0103-84782010005000151
Bedding RA (1990) Logistics and strategies for introducing entomopathogenic nematode technology in developing countries. In: Gaugler R, Kaya HK (eds) Entomopathogenic nematodes in biological control. CRC Press, Boca Raton, pp 233–248
Bedding RA, Akhurst RJ (1975) A simple technique for the detection of insect parasitic rhabditid nematodes in soil. Nematologica 21:109–110
Belay DK, Huckaba RM, Foster JE (2012) Susceptibility of the fall armyworm, Spodoptera frugiperda (Lepidoptera: Noctuidae), at Santa Isabel, Puerto Rico, to different insecticides. Fla Entomol 95:476–478. https://doi.org/10.1653/024.095.0232
CABI (2020) Invasive Species Compendium, Wallingford. https://www.cabi.org/isc. Accessed 26 Oct 2020.
Caccia MG, Del Valle E, Doucet ME, Lax P (2014) Susceptibility of Spodoptera frugiperda and Helicoverpa gelotopoeon (Lepidoptera: Noctuidae) to the entomopathogenic nematode Steinernema diaprepesi (Rhabditida: Steinernematidae) under laboratory conditions. Chil J Agric Res 74(1):123–126. https://doi.org/10.4067/S0718-58392014000100019
Folmer O, Black M, Hoeh W, Lutz R, Vrijenhoek R (1994) DNA primers for amplification of mitochondrial Cytochrome C oxidase subunit I from diverse metazoan invertebrates. Mol Mar Biol Biotechnol 3:294–299
Ganiger PC, Yeshwanth HM, Muralimohan K, Vinay N, Kumar ARV, Chandrasekhar K (2018) Occurrence of new invasive pest, fall armyworm, Spodoptera frugiperda (J.E. Smith) (Lepidoptera Noctuidae), in the maize field of Karnataka, India. Curr Sci 115:621–623
Garcia LC, Raetano CG, Leite LG (2008) Application technology for the entomopathogenic nematodes Heterorhabditis indica and Steinernema sp. (Rhabditida: Heterorhabditidae and Steinernematidae) to control Spodoptera frugiperda (Smith) (Lepidoptera: Noctuidae) in corn. Neotrop Entomol 37(3):305–311. https://doi.org/10.1590/s1519-566x2008000300010
Glazer I, Alekseev E, Samish M (2001) Factors affecting the virulence of entomopathogenic nematodes to engorged female Boophilus annulatus ticks. J Parasitol 87:808–812. https://doi.org/10.1645/0022-3395(2001)087[0808:fatvoe]2.0.co;2
Glazer I, Koltai H, Zioni, CNS, Segal D (1994) Life cycle and reproduction in Heterorhabditis. In: Burnell AM, Ehlers RU, Masson JP (eds) Genetics of entomopathogenic nematodes-bacterium complex. European Commission, Luxembourg
Goergen G, Kumar PL, Sankung SB, Togola A, Tamò M (2016) First report of outbreaks of the fall armyworm Spodoptera frugiperda (J.E. Smith) (Lepidoptera, Noctuidae), a new alien invasive pest in West and Central Africa. PLoS ONE 11(10):e0165632. https://doi.org/10.1371/journal.pone.0165632
Joshi PK, Singh NP, Singh NN, Gerpacio RV, Pingali PL (2005) Maize in India: production systems, constraints, and research priorities. CIMMYT, Mexico
Kaya HK, Gaugler R (1993) Entomopathogenic nematodes. Annu Rev Entomol 38:181–206. https://doi.org/10.1146/annurev.en.38.010193.001145
Kaya HK, Stock SP (1997) Techniques in insect nematology. In: Lacey LA (ed) Manual of techniques in insect pathology. Academic Press, San Diego, pp 281–324. https://doi.org/10.1016/B978-012432555-5/50016-6
Kondo E, Ishibashi N (1987) Comparative infectivity and development of the entomopathogenic nematodes Steinernema spp. on the lepidopterous insect larvae, Spodoptera litura (Noctuidae) and Galleria mellonella (Galleridae). Jpn J Nematol 17:35–41. https://doi.org/10.14855/jjn1972.17.35
Kumar S, Stecher G, Li M (2018) MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol Biol Evol 35:1547–1549
Kumar S, Kumar P, Bana JK, Shekhar M, Sushil SN, Sinha AK, Asre R, Kapoor KS, Sharma OP, Bhagat S, Sehgal M, Boopathi T, Amaresan N, Chattopadhyay C, Satyagopal K, Jeyakumar P (2014) Director, Integrated Pest Management Package, New Delhi
Lacey LA, Georgis R (2012) Entomopathogenic nematodes for control of insect pests above and below ground with comments on commercial production. J Nematol 44:218–225
Loya LJ, Hower AA Jr (2003) Infectivity and reproductive potential of the Oswego strain of Heterorhabditis bacteriophora associated with life stages of the clover root curculio, Sitona hispidulus. J Invertebr Pathol 83:63–72. https://doi.org/10.1016/s0022-2011(03)00044-2
Murúa MG, Vera MA, Michel A, Casmuz AS, Fatoretto J, Gastaminza G (2019) Performance of field-collected Spodoptera frugiperda (Lepidoptera: Noctuidae) strains exposed to different transgenic and refuge maize hybrids in Argentina. J Insect Sci 19:21. https://doi.org/10.1093/jisesa/iez110
Phan KL, Tirry L, Mones M (2005) Pathogenic potential of six isolates of entomopathogenic nematodes (Rhabditidia: Steinernematidae) from Vietnam. Biocontrol 50:477–491
Rahoo M, Mukhtar T, Abro SI, Bughio BA, Rahoo RK (2018) Comparing the productivity of five entomopathogenic nematodes in Galleria melonella. Pak J Zool 50(2):679–684. https://doi.org/10.17582/journal.pjz/2018.50.2.679.684
Repalle N, Jethva DM, Bhut JB, Wadaskar PS, Kachot A (2020) Present status of new invasive pest fall armyworm, Spodoptera frugiperda in India: a review. J Entomol Zool Stud 8(2):150–156. https://doi.org/10.22271/j.ento
Ricci M, Glazer I, Campbell JF, Gaugler R (1996) Comparison of bioassays to measure virulence of different entomopathogenic nematodes. Biocontrol Sci Technol 6(2):235–246. https://doi.org/10.1080/09583159650039421
Sharanabasappa KCM, Asokan R, Mahadeva Swamv HM, Marutid MS, Pavithra HB, Kavita H, Shivaray N, Prabhu ST, Georg G (2018) First report of the fall armyworm, Spodoptera frugiperda (J E Smith) (Lepidoptera: Noctuidae), an alien invasive pest on maize in India. Pest Manag Hort Ecosyst 24:23–29
Todd EL, Poole RW (1980) Keys and illustrations for the armyworm moths of the noctuid genus Spodoptera Guenée from the Western Hemisphere. Ann Entomol Soc Am 73(6):722–738.
Vanlalhlimpuia L, Lalramnghaki HC, Vanramliana, (2018) Morphological and molecular characterization of entomopathogenic nematode, Heterorhabditis baujardi (Rhabditida, Heterorhabditidae) from Mizoram, northeastern India. J Parasit Dis 42(3):341–349
Viteri D, Linares A, Flores L (2018) Use of the entomopathogenic nematode Steinernema carpocapsae in combination with low-toxicity insecticides to control fall armyworm (Lepidoptera: Noctuidae) larvae. Fla Entomol ss101(2):327–329. https://doi.org/10.1653/024.101.0228
Zhu YC, Blanco CA, Portilla M, Adamczyk J, Luttrell R, Huang F (2015) Evidence of multiple/cross resistance to Bt and organophosphate insecticides in Puerto Rico population of the fall armyworm, Spodoptera frugiperda. Pestic Biochem Phys 122:15–21. https://doi.org/10.1016/j.pestbp.2015.01.007
Acknowledgements
The authors are grateful to the Principal, Pachhunga University College, Head, Department of Zoology, Pachhunga University College, for providing required facilities for conducting the research work and Department of Agriculture, Government of Mizoram, Lunglei District, for their support and cooperation. The first author is a recipient of UGC-CSIR JRF Fellowship funded by Council of Scientific and Industrial Research (CSIR), India.
Funding
Department of Biotechnology, Government of India, funded all the field sampling, laboratory analysis and gene sequencing charges through Advance Level Biotech Hub (BT/22/ NE/2011, Dated: July 19, 2017).
Author information
Authors and Affiliations
Contributions
HCL, VLH and MLR did the field survey, collection and identifications of the pest. HCL, LRL and HTL involved in the isolation and identification of the EPN isolates. HCL, LRL and VRL designed the research experiment, data analysis and manuscript writings. The final manuscript was read and accepted by all the authors.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lalramnghaki, H.C., Lalramliana, Lalremsanga, H.T. et al. Susceptibility of the fall armyworm, Spodoptera frugiperda (J.E. Smith, 1797) (Lepidoptera: Noctuidae), to four species of entomopathogenic nematodes (Steinernematidae and Heterorhabditidae) from Mizoram, North-Eastern India. Egypt J Biol Pest Control 31, 110 (2021). https://doi.org/10.1186/s41938-021-00453-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41938-021-00453-y