Abstract
Biopesticides based on the entomopathogenic bacterium, Bacillus thuringiensis toxins, have shown high ability to control several pests belonging to order Lepidoptera. Egyptian cotton leaf worm, Spodoptera littoralis (Boisd.) (Lepidoptera:Noctuidae), is a major pest that attacks various crops in Egypt, and Cry1C toxin-based formulations are heavily used to its control. A laboratory investigation was conducted to study the resistance development in S. littoralis against Cry1C toxin. Thus, a field strain of S. littoralis was selected and maintained under laboratory conditions for 12 generations. The selection pressure resulted in 32.12-fold of resistance ratio after 12 generations. The hydrolysis of Cry1C toxin by the midgut extracts of the susceptible line, and the resistant line was compared. Results revealed that Cry1C toxin was hydrolyzed more rapidly in the resistant line than the susceptible one. The Cry1C toxin was processed till 1 hour after incubation in the susceptible line; but in case of resistant line, the Cry1C toxin was degraded in 1 hour after incubation.
Similar content being viewed by others
Background
The Egyptian cotton leaf worm, Spodoptera littoralis (Boisd.) (Lepidoptera:Noctuidae), is an important polyphagous and destructive pest that attacks various crops in Egypt and many other countries all over the world. Continuous and repetitive use of pesticides caused environmental contamination, and the pests raised insecticidal resistance (Koul, 1982). Therefore, the use of biological control for management of the insect pests (as a safe alternative control method) became an urgent requirement to preserve the environment and natural enemies (Bale et al.,2007).
The biopesticides based on insecticidal crystal protein of B. thuringiensis (Bt) toxin had been widely used in insect control. In the midgut of sensitive pests, the Bt protoxin is activated by the gut proteases into active toxin, which binds to specific receptors like cadherin and aminopeptidase-N or alkaline phosphates in the peritrophic membrane, forming pores in the midgut epithelial cells (Fortier et al. 2007, Abdullah et al.2009, and Talaei-Hassanloui et al. 2014).
Currently, Bt formulations that are promoted for lepidopteran control contain the Bt subsp. aizawai. This subspecies produces various Cry1 toxins including Cry1C, which has been shown to be highly toxic to S. littoralis (Chaufaux et al. 1997).
The first report of resistance to Bt toxins was by Mc-Gaughey (1985) in Poldia interpunctata (Hubner), and later, many other cases were reported either in field or after laboratory selection, e.g., S. littoralis (Müller-Cohn et al. 1996), Helicoverpa zea (Luttrell et al. 2004 and Tabashnik et al. 2008), Spodoptera frugiperda (Kruger et al. 2009), and Busseola fusca (Strydom et al. 2019).
Recently, dependence on Bt-pesticides to control cotton leaf worm in Egypt has been increased, and due to the economic importance of the cotton crop, these compounds are applied repeatedly for several times per season in the cotton fields. However, very little information is available on the status of Bt resistance in S. littoralis in Egypt.
This research therefore reports studies on the resistance development to Bt Cry1C toxin in the Egyptian cotton leaf worm and its processing pattern.
Materials and methods
Insect culture
Larvae of the cotton leaf worm, S. littoralis, originally collected from cotton fields located in Kafer Elsheikh Governorate, Egypt, were reared on the artificial diet described by Kranthi (2005) at 27 ± 1 °C, 70% RH, and a photoperiod of 14:10 (L:D) h. After pupation, the pupae were collected and kept in glass jars until adult emergence. The adults were allowed to feed on 10% sugar solution and lay eggs in the same jars. The eggs were collected on tissue paper and kept in small jars along with a wetted cotton piece as a source of moist for hatching.
Preparation of B. thuringiensis Cry1C toxin
Cry1C toxin was cultured and purified as described by Abdullah et al. (2009) and modified by Moussa (2009). The bacterial cells were inoculated in a 5-ml culture tube for overnight. The overnight culture was then subcultured in a 1-L flask to grow further for 3–5 days in T3 medium (3.0 g Trypton, 2.0 g Tryptose, 1.5 g yeast extract, 0.0005 g MnCl2, 8.9 g NaH2PO4). The growth was harvested at 5200 rpm for 10 min at 4 °C. The pellets were collected and washed in (50 mM EDTA) buffer for 4–6 times/each with 10,000 rpm for 10 min at 4 °C. The obtained pellet was re-suspended in 5 ml of (50 mM Tris, HCl, 5 mM EDTA, pH 7.0) buffer. These inclusion bodies were examined by 10% SDS-PAGE gel electrophoresis. The protein concentration was determined using the Bradford method according to Bradford (1976). The inclusion bodies were then aliquoted in 1.5 ml Eppendroff tubes and stored at − 20 °C.
Bioassay of Cry1C toxin
Bioassay Cry1C toxin was conducted using diet incorporation method. Five different concentrations of Cry1C toxin in water were prepared, and 4 replicates for each concentration were represented. Moreover, the control replicates were treated by dH2O instead of Cry1C toxin. Twenty newly hatched neonates were placed onto the surface of the diet in each replicate, using a thin brush and then kept under laboratory conditions. The larval mortality was recorded 7 days after treatment, and the median lethal concentration LC50 was calculated according to Finney (1971).
Selection pressure
Selection of S. littoralis neonates for resistance was initiated by transferring of 500 neonates onto the surface of artificial diet incorporating Cry1C toxin for 4 subsequent days with care always taken to obtain about 75% mortality or higher. The survived larvae were then transferred to feed on toxin-free diet until pupation. The emerged adults were allowed to feed on 10% sugar solution. The laid eggs were collected and kept in plastic cups along with wetted cotton piece until hatching. The above selection procedure was repeated on the newly hatched neonates of the second generation, and this regular work was performed at every generation until 12 generations. The bioassay was conducted at F1, F3, F6, F9, and F12 in order to calculate the LC50. Resistance ratios were calculated by dividing the LC50 of selected generation by the LC50 of F1.
Preparation of gut extract
Gut extract was performed referring to the method described by Moussa (2009). Ten 4th instar larvae were dissected on ice, and their guts were pooled in 300 μl dH2O in microcentrifuge tubes. An amount of 1.5 phenyl methane sulfonyl fluriode (PMSF) was added to inhibit proteinase enzymes. The guts were grinded gently then centrifuged at 14,000 rpm for 15 min at 4 °C. The supernatant was carefully transferred into sterilized Eppendorf tubes and kept at − 20 °C for further use.
Digestion and processing of Cry1C toxin
In order to compare the processing of Cry1C toxin in the midgut of susceptible (F1) and resistant line (F12) of the cotton leaf worm, an amount of 50 μl gut extract sample was mixed with 10 μg of Cry1C toxin in microcentrifuge tube, and the volume was completed to 25 μl, using universal buffer (11.5 mM boric acid, 7.8 nM citric acid, 18.7 mM Na2HPO4, and 68.9 mM NaOH, PH 9.75) (Frugoni 1957). Samples were incubated at room temperature at different time intervals, viz., 5, 15, 30 min, and 1 h with Cry1C toxin. Toxin processing was terminated by heating the sample at 100 °C for 5 min. The samples were cooled down at room temperature, and sample buffer was added. The samples were again boiled for 5 min for protein digestion (Moussa 2009). Finally, the samples were analyzed using SDS-PAGE (4% stalking gel and 10% separating gel).
Results and discussion
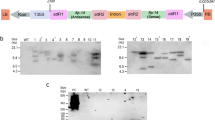
Cry1C toxin-based insecticide is being repetitively used to control S. littoralis in Egypt. In 1996, a strain of S. littoralis was selected with Cry1C toxin that developed 500-fold resistance (Muller-Cohn et al. 1996). Another case of Cry1C toxin resistance was reported by Chaufaux et al. (1997) as after selection that the 12th generation of S. littoralis showed more than 500-folds of resistance than the control. In the present experiment, neonates of S. littoralis were selected against Cry1C toxin. Cry1Ctoxin was purified from Bt culture, and the concentration of Cry1C toxin stock solution was 0.5 μg/μl. The stock solution of Cry1C toxin was checked on SDS-PAGE gel and its band appeared at 65 kDa molecular weight (Fig. 1).
Development of resistance
Before selection, the larvae of S. littoralis (F1) were sensitive to Cry1C toxin with LC50 of 0.17 μg/g diet (Table 1). LC50 of Cry1C toxin increased to 2.40 μg/g diet in F3 than F1 with resistance ratio of 14.12 folds. μg/g diet. The resistance ratio increased from generation to another until it reached 32.12 folds in F12. The present data cleared that S. littoralis had the ability to develop the resistance to Bt toxins, while exposed to diet mixed with the toxin for subsequent generations, as other lepidopteran pests (Tabashnik et al., 1997 and Moussa and Gujar, 2005).
The earlier studies proved that laboratory strain of the cotton leaf worm had evolved moderate resistance in 8 subsequent generations of selection pressure against inclusion bodies of Cry1C toxin. The resistance ratio reached 5.8 and 3.4-folds for the Egyptian local Bt isolates of DI29 and entomocidus, respectively. Additionally, when the cotton leaf worm was selected against commercialized Bt formulations of Agerin and Diple 2x, the LC50 increased to 20.5 and 16.3-folds, respectively (Mohammed 1997). The spore/crystal mixture used in the previous study might contain associated particles that may delay resistance development in cotton leaf worm strain compared to purified toxins in the present investigation.
Processing of Cry1C by S. littoralis midgut extract
In the present study, the hydrolysis of Cry1C toxin by the midgut extracts in the susceptible line of S. littoralis (F1) and the resistant line (F12) was studied to determine if there were differences in protoxin activation in both lines. The Cry1C partially purified toxin was incubated with their gut extracts at different time intervals, viz., 5, 15, 30 min, and 1 h. Then, the samples were separated on SDS-PAGE. Results revealed that at 5 min after incubation, one fragment of 65 KDa appeared in susceptible line, while 3 fragments were observed between 62 and 70 KDa in resistant line (Fig. 2).
After 15 min of incubation, 3 fragments between 65 and 70 kDa appeared in the susceptible line. On the other hand, after 30 min of incubation with gut extract of resistant line, one more lowering band at 65 kDa appeared but did not appear in the susceptible line. When Cry1C was incubated by gut extract for 1 h, the upper band at 70 kDa position in resistant line was totally disappeared, and the lowest band became thicker; however, the same band in susceptible line was not totally degraded despite the lowest band was increased. The above observation revealed that the degradation of the Cry1C fragment in resistant line was faster than in the susceptible line. The processing of Cry1C toxin with susceptible and resistant lines varied at different time intervals. In susceptible line, the Cry1C toxin was processed till 1 h after incubation. But in case of resistant line, the Cry1C toxin was degraded in 1 h after incubation. This data is in agreement with that reported by Moussa (2009) who mentioned that Cry1Ac toxin was digested and processed in Helicoverpa armigera (H.) within an hour after treatment.
Conclusions
The present study proved that the Egyptian cotton leaf worm, S. littoralis, has the ability to build up the resistance gradually, while exposure to Bt toxin for subsequent generations. Additionally, the Bt toxin was hydrolyzed more rapidly in the resistant line than in the susceptible one.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- Bt:
-
Bacillus thuringiensis
References
Abdullah, M. A. F.; Moussa, S.; Taylor, M. D. and Adang, M. J. (2009) Manduca sexta (Lepidoptera: Sphingidae) cadherin fragments function as synergists for Cry1A and Cry1C Bacillus thuringiensis toxins against noctuid moths Helicoverpa zea, Agrotis ipsilon and Spodoptera exigua. Pest Manag Sci., 65: (10) 1097-1103.
Bale JS, van Lenteren JC, Bigler F (2007) Biological control and sustainable food production. Philosophical transactions of the Royal Society of London. Series B, Biological sciences 363(1492):761–776
Bradford M (1976) A rapid and sensitive method for the quantitative of microgram quantities utilizing the principle of protein-dye binding. Anal Biochem. 72:248–254
Chaufaux, J., Muller-Cohn, J., Buisson, C., Sanchix, V., Lereclus, D. and Pasteur, N., (1997) Inheritance of resistance to the Bacillus thuringiensis Cry1 C toxin in Spodoptera littoralis (Lepidoptera: Noctuidae), J Econom Entomol. 90, 873-878.
Finney, D.J. (1971) Probit Analysis, third ed., Cambridge University Press, Cambridge, UK.
Fortier M, Vachon V, Frutos R, Schwartz JL, Laprade R (2007) Effect of insect larval midgut proteases on the activity of Bacillus thuringiensis Cry toxins. Appl. Environ. Microbiol. 73:6208–6213. https://doi.org/10.1128/AEM.01188-07
Frugoni, J. A. C. (1957) Tampone universale di Britton e Ronbinson a for Za ionica constante. (Gazz. Chim. Ital.) 87: 403-407.Koul O., (1982) Insect feeding deterrents in plants. Indian Rev. Life Sci. 2:97-125.
Kranthi KR (2005) Insecticide resistance -monitoring, mechanisms and management manual'. Published by CICR, Nagpur, India and ICAC, Washington
Kruger MJ, van Rensburg JBJ, Van den Berg J (2009) Perspective on the development of stem borer resistance to Bt maize and refuge compliance at the Vaalharts irrigation scheme in South Africa. Crop Protection 28:684–689
Luttrell, R.G., Ali, I., Allen, K.C., Young, S. Y., Szalanski, A., Williams, K. and Lorenz G., (2004) Resistance to Bt in Arkansas populations of cotton bollworm, pp. 1373–1383. In D. A. Richter (ed.), Proceedings, Belt wide Cotton Conferences, 5-9 January 2004, pp. 5–9. National Cotton Council of America, Memphis.
Mc-Gaughey HW (1985) Insect resistance to the biological insecticide Bacillus thuringiensis. Science 229:193–195
Mohammed AM (1997) Development and characterization of resistance to some biocides in cotton leaf worm Spodoptera littoralis. Ain Shams University, Faculty of Science, M.Sc. thesis, 102 pages
Moussa MS (2009) Processing analysis of Bacillus thuringiensis Cry1Ac in susceptible and resistant selected strains of Helicoverpa armigera. Bull. ent. Soc. Egypt 35:91–101
Moussa, M. S. and Gujar, G. T. (2005) Inheritance of Cry1AC resistance in the transgenic Bt cotton selected strain of the cotton bollworm, Helicoverpa armigera (Hubner), Egypt. J. Agric. Res., 83 (3), 1061-1078.
Müller-Cohn, J., Chaufaux, J., Buisson, C. and Gilois, N. (1996) Spodoptera littoralis (Lepidoptera: Noctuidae) resistance to CryIC and cross-resistance to other Bacillus thuringiensis crystal toxins. J Econ Entomol, 89(4):791-797.
Strydom E, Erasmus A, du Plessis H, Van den Berg J (2019) Resistance status of Busseola fusca (Lepidoptera: Noctuidae) populations to single- and stacked-gene Bt maize in South Africa. J Econ Entomol 112(1):305–315. https://doi.org/10.1093/jee/toy306
Tabashnik BE, Gassmann AJ, Crowder DW, Carrière Y (2008) Insect resistance to Bt crops: evidence versus theory. Natl. Biotechnol. 26:199–202
Tabashnik BE, Liu YB, Finson N, Masson L, Heckel DG (1997) One gene in diamondback moth confers resistance to four Bacillus thuringiensis toxins. Proc. Natl. Acad. Sci. USA. 94:1640–1644
Talaei-Hassanloui, R., Bakhshaei, R., Hosseininaveh, V., and Khorramnezhad, A. (2014) Effect of midgut proteolytic activity on susceptibility of lepidopteran larvae to Bacillus thuringiensis subsp. kurstaki. Front Physiol, 4, 406.
Acknowledgements
The authors gratefully thanked the Science and Technology Development for Fund (STDF) for funding this work through the project number 375.
Funding
The research work is funded by the Science and Technology Development for Fund (STDF) through the project number 375.
Author information
Authors and Affiliations
Contributions
SM planned the outline of the research work. AOA prepared the manuscript, while all authors equally did the bioassay experiments. The authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Moussa, S., Biaomy, F., Aiad, K. et al. Bacillus thuringiensis Cry1C resistance development and its processing pattern in Egyptian cotton leaf worm: Spodoptera littoralis (Boisd.) (Lepidoptera:Noctuidae). Egypt J Biol Pest Control 30, 36 (2020). https://doi.org/10.1186/s41938-020-00237-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41938-020-00237-w