Abstract
Background
Monosodium glutamate (MSG E621) is one of the most popular flavouring agents of modern times and is widely used in many commercially packed food and even in house hold cooking. Previous studies revealed that excessive intake of MSG in diet causes obesity, metabolic defects, nephrotoxicity, neurotoxicity and hepatotoxicity in rats, but no reports are available in the literature about the ecotoxicological assessment of MSG by using fishes as a bioindicators. Since fishes are important consumer in aquatic food chain and directly linked with human health status, the present study was aimed to investigate the impact of MSG in freshwater fish Labeo rohita by using histological biomarkers.
Results
Ninety-six h-LC50 of MSG to Labeo rohita was determined (1.5 g/L), and fish exposed to sub-lethal concentration of MSG (1/10th of 96 h-LC50 concentration of MSG (150 mg/L)) showed distinguished behavioural changes like erratic movement, loss of appetite and excessive mucous secretion all over the body as an adaptive syndrome to avoid the direct exposure to MSG in the medium. Histopathological analysis clearly depicts severe damages in the vital organs of fish. In gills, epithelial necrosis, hypertrophy, hyperplasia, primary and secondary gill lamellae degeneration, oedema, fusion of adjacent secondary lamellae and rupture of gill epithelium were observed. The intensity of tissue damage was increased as the exposure period was extended. The liver displayed vein congestion, vacuole formation, degeneration in parenchymal cells and bile stagnation, whereas MSG-treated kidney tissue showed high interstitial inflammation. Among the vital organs gill and liver displayed the highest histopathological alterations.
Conclusions
The present study clearly demonstrated that MSG is toxic to fish and able to cause significant damages in the vital organs as the exposure period was extended. Since the studies on the toxicity of MSG to fish are rare, the present investigation may contribute to the scarce literature on sub-lethal toxicity of MSG to freshwater fishes.
Similar content being viewed by others
Background
Monosodium glutamate (MSG) is the sodium salt of amino acid glutamic acid, and it has 78% of glutamic acid and 22% of sodium salt and water. In many countries, MSG goes by the name “China Salt” (Niaz et al., 2018). It is added as a flavour enhancer to soups, sauces and meal preparation products (Jinap & Hajeb, 2010). In the past, MSG was extracted from foods rich in proteins such as fish, algae and vegetables (Stanska & Krzeski, 2016; Yamaguchi & Ninomiya, 2000). Two isomers of monosodium glutamate are l-glutamate enantiomer and d-glutamate enantiomer. Only the l-glutamate enantiomer has flavour enhancing properties (Kawai et al., 2002). Glutamate is an important metabolic link between the tricarboxylic acid (TCA) cycle and urea cycle involved in cellular energy degeneration and nitrogen disposal (Burrin & Stoll, 2009).
l-glutamic acid was discovered in 1866 by Karl Ritthausen, German scientist who isolated the acid hydrolysate of wheat gluten. Salt of glutamic acid were first revealed in 1908 (Hendry-Unaeze, 2017). Due to the modern lifestyle and addiction to the taste instead of the nutritional quality of food worldwide consumption of readymade and processed food has been increased. These foods are loaded with a variety of preservatives along with monosodium glutamate as a taste enhancer. MSG was produced by the fermentation process with the help of bacterial genera corynebacterium and brevibacterium (Nakayama et al., 2018). For industrial production of MSG, molasses and starch hydrolysate were generally used as raw materials (carbon source) (Sano, 2009). Daily average consumption of MSG as a food additive includes 0.3–1.0 g/day for USA and Europe, 0.6–2.0 g/day in UK (Rhodes et al., 1991), 1.5–3.0 g/day in Taiwan, 1.1–1.6 g/day in Japan, 1.6–2.3 g/day in South Korea (Rhodes et al., 1991) and 0.56–1.0 g/day in Nigeria (Hendry-Unaeze, 2010).
Several studies on rats show that MSG increases obesity and it can produce neurological diseases such as Alzheimer's, arthritis or fibromyalgia (Albrahim & Binobead, 2018). Glutamate has different physiological effects on the gastrointestinal tract and caused obesity in human (Chakraborty, 2019). Use of MSG has also been linked to nephrotoxicity, hepatotoxicity, asthma, urticaria and neoplastic cell growth and differentiation in Albino Wistar rats (Nnadozie et al., 2019).
Several authors have reported the impact of chronic dosing of MSG in albino mice such as fertility impairment in adults like changes in testicular lesions (Alalwani, 2014); significant abnormality in liver and renal functions (Tawfik and Al-Badr, 2012); changes in serum lipids (Nardelli et al., 2011), along with corresponding inflammatory and fibrotic changes in the liver, kidney and neonatal mortality (Nnadozie et al., 2019). Acute toxicity study of MSG (1250 mg/Kg) for eight days to male Wistar rats showed histopathological alterations in the liver like mild disturbance in liver architecture, massive necrosis with little area of regeneration and large congested central vein with ruptured endothelial lining which was invaded by lymphocytic infiltration and few inflammatory cells (Egbuonu et al., 2010).
Aquatic ecosystems are more vulnerable to many kinds of pollutants either from natural calamities or through anthropogenic activities. Such polluted water causes serious physiological and histological alterations in the aquatic organisms especially to fishes. Intractable fermentative wastewater from monosodium glutamate manufacturing plants have high strength of COD (10,000–30000 mg/ L), ammonium (15,000–25,000 mg/L), sulphate (15,000–30000 mg/L) and very low pH (2.0) (Yang et al., 2005). Cheng et al., (1996) reported that untreated MSG effluent is highly toxic to fish Ctenopharyngodon idellus.
Aquatic organisms like fishes tend to accumulate a great variety of pollutants directly from contaminated water and indirectly through the food chain (Ashraf, 2005). Once the toxic substance enters the body of the fish it may affect the organs leading to physiological and pathological disorders and its severity may be dose and time dependent. An array of histopathological alterations is inevitable in fishes exposed to toxicants both in the field and in laboratory conditions (Abdallah & Abdallah, 2008).
The histological biomarkers are useful indicators of the general health of the fish which reflects past exposure to a variety of anthropogenic pollutants (Maharajan et al., 2016). Gills, kidney and liver are responsible for vital functions like respiration, excretion, accumulation and bio transportation of xenobiotics in fish (Gernhofer et al., 2001). Histopathological changes in the gills and liver have been proposed as useful tools for monitoring fish health in polluted water bodies (Ossana et al., 2019). The teleost fish kidney is one of the first organs to be affected by the contaminants in the water (Zeinab et al., 2015).
Fishes are one of the important aquatic organisms which occupy all levels of the aquatic food chain. It also serves as the major protein source to human beings. Rohu (Labeo rohita) is the most important among the three Indian major carp species used in carp polyculture systems. This Indo-Gangetic riverine species is the natural inhabitant of the riverine system of Northern and Central India. Histopathological changes in vital organs of Labeo rohita exposed to triclosan (Hemalatha et al., 2019), toxic effects of malathion to Labeo rohita during acute exposure (Ullah et al., 2018) and amine coated silver nanoparticle induced histopathological alterations in gill and liver of fresh water fish Labeo rohita (Khan et al., 2018) provided the evidence that histological study of fish is one of the important biomarker for assessing the toxicity of pollutants in aquatic environment.
In the last few decades, the discharge of a huge amount of MSG as such from readymade food processing industries, household cooking and effluents from MSG plants to the nearby aquatic environment was increased tremendously. Hence, the ecotoxicological risk assessment of MSG in an aquatic environment is necessary. Based on our literature collection, reports on toxicity of MSG to aquatic organisms, particularly to fishes are rare. Hence, the main objectives of the present investigation are oriented towards the determination of median lethal concentration (LC50) of MSG for 96 h and to evaluate the sub-lethal toxicity of MSG on histopathological changes in Labeo rohita under laboratory conditions.
Methods
Experimental animal and maintenance
Fingerlings of Labeo rohita with the weight of 4–5 g and mean body length of 6–8 cm were used for the present study. Fish fingerlings were procured from Tamil Nadu Fisheries Development Corporation Limited Fish Farm, Aliyar, Tamil Nadu, India. The fingerlings were acclimatized for a week at 25.5 ± 0.2 °C in cement tank disinfected with potassium permanganate prior to the introduction of fish. The fish were maintained under natural light/dark cycle in large cement tank with well aerated systems. The fish were fed ad libitum with 2:1 ratio of rice bran and groundnut oil cake in the form of dough. All physico-chemical parameters of chlorine free fresh water used for the present study (Dissolved oxygen content 5.5 ± 0.1 mg/L; pH 7.34 ± 0.55; Total alkalinity 17.6 ± 8.0 mg/ L; Salinity 0.5 ± 0.35 ppt; Total hardness 20.23 ± 0.35 mg/L; Calcium 11.65 ± 0.99 mg/ L) were determined by following the techniques of APHA (1998) and maintained throughout the study period. Fish were starved for 24 h before and during the experiment.
Test chemical
l-Glutamic Acid Monosodium Salt (MSG) extra pure (99%) was purchased from SRL chemicals Pvt Limited, Mumbai, India. It was in the form of white crystals. Ten grams of MSG was dissolved in 1 L of double distilled water and used as stock solution. It was stored in clean standard flask at room temperature in the laboratory.
Experimental procedure
Determination of median lethal concentration
Preliminary toxicity studies were conducted to determine the LC50 value of MSG. Briefly 6 batch of 10 fish were randomly selected from the stock and exposed to each of the 6 MSG concentrations (1.0 g/L, 1.3 g/L, 1.5 g/L, 1.8 g/L, 2.0 g/L and 2.1 g/L). After 24 h, mortality rate was recorded and cumulative mortality (50%) was observed at 96 h in 1.5 g/L concentration of MSG (Fig. 1). Narrow range concentration was repeated in triplicate to confirm the static renewal acute toxic definitive test (Sprague, 1971) and by following the probit analysis method of Finney (1971) the correlation between MSG concentration and fish mortality (%) followed by regression were calculated. Changes in the behaviour of fish were recorded throughout the study period.
Experimental design
Sub-lethal toxicity study
Based on acute toxicity study, 1/10th of 96 h—LC50 value (150 mg/L) was taken as sub-lethal concentrations. The glass tanks (100L capacity) were filled with 70 L of chlorine free tank water. Seventy fish were randomly selected from the stock (Chi-square test proved no heterogeneity in fish population, data not shown) and housed in two glass tanks and designated as Control (without toxicant) and Experiment (sub-lethal treatment) with 150 mg/L MSG and maintained for 15 days. Behaviour of the fish was noted regularly. The water was changed at every 24 h with newly prepared stock solution (MSG). Uneaten food was quickly removed from the aquaria. This ensured sufficient oxygen supply and devoid of any accumulated metabolic wastes in the aquaria. Fish were starved for 24 h before sampling. Mortality and behaviour of fish were noticed every day. At 5-day intervals for 15 days, 20 fish were randomly selected and killed by mild blow on the head. Then, the organs (Gill, Liver and Kidney) were carefully dissected and stored in neutral-buffered formalin solution (NBF) for histological analysis.
Histological analysis
Gills, liver and kidney were put in 10%neutral-buffered formalin solution (NBF) for 24 h, dehydrated through a graded ethanol series and embedded in paraffin. Tissue Section (7 μm thickness) was stained with haematoxylin and eosin. Histopathological evaluation was done and photographed using the Cilika photomicroscope.
Results
Behavioural study
Fish exposed to acute concentration of MSG showed some behavioural changes such as low intake of feed, sudden movement around the trough, spreading of excess mucus all over the surface of body, anxiety and changes in skin colour. Mortality was noted at 24 h, 48 h, 72 h and 96 h; cumulative mortality (50%) was recorded at 96 h. Pale colour and slipperiness in the skin were observed in dead fish.
During sub-lethal toxicity study, MSG-treated fish showed the similar behavioural changes like acute study, but the changes occurred in slow phase. At the end of 5th day treatment, experimental fish behaved like control fish except slight colour change in their skin. Later on the skin colour of fish became pale as the exposure period was extended. After 10th day fish had more mucus secretion and lost their appetite. Finally, fish exhibited excessive secretion of thick mucus all over the body and floated on the surface of water to engulf the oxygen from air.
Histology of gills
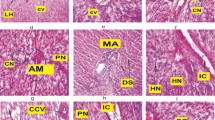
Teleost fish gills are supported by four gill arches. Each gill arch consists of two rows gill filaments. Each gill filament is containing the functional unit of respiratory system called lamella (primary and secondary lamellae).Primary gill lamellae (PGL) has central axis with plenty of chloride cells. Secondary gill lamellae (SGL) are arranged at both the ends of primary lamellae to provide more surface area for efficient gas exchange. Network of blood capillaries run parallel between the supporting pillar cells. Blood capillaries are too narrow, so the blood cells move through them in a single line, so that each red blood cell comes in close contact with dissolved oxygen content in surrounding aquatic medium. Outer epithelial layer of lamella is formed of squamous epithelial cells, whereas inner layer is formed by undifferentiated cells. Primary lamella is covered by thin epithelial layer consist of numerous goblet cells (mucus cells) and pavement cells. Histological study of the gills reveals that fish from control group have intact respiratory lamellae and structural damages were not evident (Fig. 2a).
Changes in the gill architecture of fish Labeo rohita exposed to sub-lethal concentration of MSG. (a–d) a—without toxicant, b–d—with toxicant (MSG). a—Control, b—5th day, c—10th day, d—15th day. PGL Primary Gill Lamellae, PGLD Primary Gill Lamellae Damage, SGL Secondary Gill Lamellae, SGLD Secondary Gill Lamellae Degeneration, ILR Interlamellar Region, WC Water Channels, CA Central Axis, PGLI Primary Gill Lamellae Inflamed, GAD Gill Arches Degeneration, EREC Oedema and rupturing of Epithelial cells, SGLD Secondary Gill Lamellae Degeneration, WC & ILE Water Channels and Interlamellae Effaced, HP Hyperplasia, EN Epithelial Necrosis, HT Hypertrophy
Histopathology of gills
MSG exposure has induced pathological changes in fish gill architecture (Fig. 2b). The changes including primary gill lamellae damage (PGLD), secondary gill lamellae degeneration (SGLD), epithelial necrosis (EN), hypertrophy (HT), hyperplasia (HP), fusion of adjacent secondary lamellae and oedema and rupture of gill epithelial cells (EREC), whereas in 10th day, more inflammation in water channels were noted. As the exposure period was extended more degeneration in gill lamellae (both primary and secondary) with mild oedema in interlamellar region were observed (Fig. 2c, d).
Histology of liver
The fish liver is composed of hepatocytes (parenchymal cells) scattered with some connective tissue extends inwards into parenchyma. Hepatocytes are polygonal, containing clear spherical nucleus. They are present amongst irregularly distributed sinusoids forming cord like structure known as hepatic cords. Fish liver from control group showed no damage in hepatocytes and hepatic cords appeared normal (Fig. 3a).
Changes in the liver architecture of fish Labeo rohita exposed to sub-lethal concentration of MSG. (a–d): a—without toxicant, b, c, d—with toxicant (MSG). a—Control, b—5th day, c—10th day, d—15th day. HC Hepatic Cell, MDP Mild Degeneration in Parenchymal cells, GC Granular Cytoplasm, VC Vein Congestion, BS Bile Stagnation, VF Vacuole Formation, VC Vein Congestion, VF Vacuole formation, PD Parenchymal Degeneration
Histopathology of liver
The changes observed in the liver tissue include degeneration of parenchymal cells (PD), vein congestion (VC) and vacuole formation (VF) (Fig. 3b). On the 10th and 15th day of MSG-treated fish liver showed severe degeneration of parenchymal cells (PD), bile stagnation (BS) and vacuole formation (VF). When compared to 5th and 10th day of treatment, more vacuole formation (VF) was observed at the end of 15th day of treatment. The results depict that severity of damage in liver was directly proportional to the exposure period (Fig. 3c, d).
Histology of kidney
In teleost fishes, the kidney is located in a retroperitoneal position and dark brown in colour. They are structurally differentiated into head kidney and trunk kidney. Histologically the head kidney is composed of hematopoietic components and trunk kidney is rich in nephrons which performs excretory function. The tubules are lined with tall columnar cells with distinct basally located nuclei. The inter-tubular space is filled with lymphoidal tissue. In control fish, the hematopoietic tissue in inter-tubular spaces showed normal distribution of parenchymal cells with distinct nuclei and no renal tissue damage was witnessed (Fig. 4a).
Histopathology of kidney
The kidney of MSG exposed fish showed oedema in interstitial tissue. Mild interstitial inflammation (II) (Fig. 4b) was observed at the end of 5th day MSG-treated fish, and intensity of inflammation was increased as the exposure period was extended (Fig. 4c, d).
Discussion
As a food additive, MSG is regarded as flavouring agent. It can motivate oral-sensory receptors, improves the taste of the meal and increases the appetite. Therefore, MSG is regarded as the leading cause of weight gain (Zanfirescu et al., 2019). Several authors have reported about the toxic effects of MSG administration in humans (Chakraborty, 2019) and animals (Zanfirescu et al., 2019). The effects vary from allergic reaction, flushing, sweating, numbness weakness dizziness and headache in human (Geha et al., 2000), organ damages including female and male genital organs in Wistar rats (Eweka et al., 2010; Nossier et al., 2012) and liver and kidney in adult rats (Tawfik and Al-Badr, 2012). Similarly sub-lethal MSG-treated fish in the present study showed damage in organs like gill, liver and kidney and degeneration of tissues.
In the present study, increased mucus production was observed in MSG-treated fish. Gulping of air from the surface (due to asphyxiation) and excessive secretion of mucus all over the body were the important adaptive responses of fish Labeo rohita to avoid direct contact of MSG in the treatment medium and to ease respiratory stress. In fishes, mucosal surfaces (i.e. skin, gill, gut and olfactory organ) provide the crucial first line defence against the threats present in the immediate environment (Salinas, 2015). The major function of mucus is to entrap and sloughing of the microbes (Wang et al., 2011). Epidermal mucus productions in fishes are nonspecific and are independent of the temperatures (Wendelaar bonga, 1997).
The presence of toxic substances in the environment disrupts the vital functions of gills by altering its structural morphology (Khan et al., 2018) or causes organ damage which lead to asphyxiation. Changes in the gills of fish due to an irritant include inflammation, hyperplasia, lamellar fusion, excessive production of mucus, epithelial lifting, flattening of the secondary lamella and formation of aneurysms (Flores-Lopes and Thomaz, 2011). Extensive architectural loss was observed in the gills of MSG-treated fish (Fig. 2b). After 5 days of MSG exposure (150 mg/L) fish gills showed mild degeneration in both primary and secondary gill lamellae, epithelial necrosis (EN), hypertrophy (HT), hyperplasia (HP), oedema, fusion of adjacent secondary lamellae and rupture of gill epithelium. The observed epithelial necrosis and severe oedema in gills of MSG exposed fish in the present study may be the direct toxic action of MSG and severe oedema induces the lifting of gill epithelium (Schwaiger et al., 2004) which prevents the entry of pollutant into the blood stream of fish.
Alterations such as hyperplasia of the mucosal cells and proliferation of epithelial cells occur as a consequence of infectious conditions or due to the presence of pollutants (Malhotra et al., 2020). Furthermore, the intensity of tissue damages was increased as the exposure time of MSG was extended. 10th and 15th day gill showed more degeneration in primary and secondary gill lamellae than the 5th day treatment. Central axis was partially degenerated (Fig. 2c). Water channels were affected and showed mild oedema. Rupturing of interlamellar region was also noted (Fig. 2d). Similar reports have been well documented in fish (Labeo rohita) exposed to various toxicants such as Triclosan (Hemalatha et al., 2019), Phenol (Butchiram et al., 2013), and Malathion (Ullah et al., 2018).
The liver is the primary detoxifying agent of any xenobiotics (David & Kartheek, 2016). Wide varieties of insecticides and other toxic chemical tend to accumulate in high concentration within the system (Mossa et al., 2018) and cause more damage in liver (Pacheco & Santos, 2002). Histopathological lesions observed in MSG-treated fish were mild degeneration in parenchymal cells (MDP), vacuole formation (VF), vein congestion (VC) and bile stagnation (BS) (Fig. 3c, d). The toxicants cause fish hepatocyte necrosis (Ahmed et al., 2017) and accumulation of bile indicates possible damage to the hepatic metabolism (Fanta et al., 2003). Similar results were observed in Labeo rohita exposed to Malathion (Ullah et al., 2018); Brilliant Green dye (Vigneshpriya et al., 2020). Capkin et al. (2017) reported that vacuolation in hepatocytes facilitates the excessive accumulation of fat in the cytoplasm and also observed hepatic necrosis in Oncorhynchus mykiss exposed to Triclosan. Degeneration of liver tissue and central vein necrosis in the present study might be due to the accumulation of neutrophils and lymphocytes as suggested by David and Kartheek (2016). From the above authors report, we can understand that MSG is as toxic as any other pollutants in the aquatic environment.
Kidney is one of the primary organs to be affected by contaminants in the water. The common alterations in teleostean kidney were necrosis of nephron and extravasations (Thophon et al., 2003). The damage possibly caused malfunctions of this organ; therefore, the removal of the poison could not take place actively (Butchiram et al., 2013). MSG-treated fish kidney displayed mild interstitial inflammation (II) at the end of 5th day (Fig. 4b) and the intensity of inflammation also increased in the kidney of fish (Fig. 4c, d) as the exposure period was extended. Schwaiger et al. (2004), also stated that fish (Rainbow trout) exposed to Diclofenac showed severe interstitial inflammation in the kidney. Similarly, Contini et al. (2017) reported severe inflammation and oxidative stress in kidney interstitial cells of Wistar rat exposed to MSG.
Conclusions
The present study clearly demonstrated that MSG is toxic to fish and able to provoke significant histological damages in the vital organs of fish, especially in gills and liver. Even though mortality was not recorded during sub-lethal study, MSG-induced organ damages can affect the health status of the fish and making fish prone to several infectious diseases. Furthermore, studies on MSG toxicity to fish are rare, and the present findings may contribute to the scarce literature regarding fish exposed to sub-lethal concentration of MSG. In addition to that, our results through a light on the impact of prolonged consumption of such MSG intoxicated fish may cause similar damages and pose a real threat to human health.
Availability of data materials
All data generated or analysed during this study are included in this published article [and its supplementary information files are available with corresponding author on reasonable request].
Abbreviations
- MSG:
-
Monosodium glutamate
- PGL:
-
Primary gill lamellae
- SGL:
-
Secondary gill lamellae
- PGLD:
-
Primary gill lamellae damage
- SGLD:
-
Secondary gill lamellae damage
- WC:
-
Water channels
- CA:
-
Central axis
- PGLI:
-
Primary gill lamellae inflamed
- GAD:
-
Gill arches degeneration
- EN:
-
Epithelial necrosis
- HT:
-
Hypertrophy
- HP:
-
Hyperplasia
- EREC:
-
Oedema and rupture of gill epithelial cells
- ILE:
-
Interlamellae effaced
- HC:
-
Hepatic cells
- MDP:
-
Mild degeneration in parenchymal cells
- PD:
-
Parenchymal degeneration
- BS:
-
Bile stagnation
- GC:
-
Granular cytoplasm
- VC:
-
Vein congestion
- VF:
-
Vacuole formation
- RC:
-
Renal corpuscles
- II:
-
Interstitial inflammation
References
Abdallah, M. A. M., & Abdallah, A. M. A. (2008). Biomonitoring study of heavy metals in biota and sediments in the South Eastern coast of Mediterranean Sea Egypt. Environmental Monitoring and Assessment., 146, 139–145.
Ahmed, S., Giese, B., Schulz, V., & Ahmed, S. (2017). Effects of toxic Microcystis aeruginosa bloom on liver of Nile Tilapia (Oreochromis niloticus). Bangladesh Journal of Zoology., 45(1), 1–10.
Alalwani, A. D. (2014). Monosodium glutamate induced testicular lesions in rats (histological study). Middle East Fertility Society Journal, 19, 274–280.
Albrahim, T., & Binobead, M. A. (2018). Roles of Moringa Oleifera leaf extract in improving the impact of high dietary intake of monosodium glutamate- induced liver toxicity, oxidative stress, genotoxicity, DNA damage and PCNA alterations in male rats. Oxidative Medicine and Cellular Longevity. https://doi.org/10.1155/2018/4501097
APHA. (1998). Standard methods for the examination of water and waste water. American Public Health Association.
Ashraf, W. (2005). Accumulation of heavy metals in kidney and heart tissues of Epinephelus microdon fish from the Arabian Gulf. Environmental Monitoring and Assessment., 101, 311–316.
Burrin, D. G., & Stoll, B. (2009). Metabolic fate and function of dietary glutamate in the gut. American Journal of Clinical Nutrition, 90(3), 850S-856S.
Butchiram, M. S., Vijaya Kumar, M., & Tilak, K. S. (2013). Studies on the histopathological changes in selected tissues of fish Labeo rohita exposed to phenol. Journal of Environmental Biology., 34, 247–251.
Capkin, E., Ozcelep, T., Kayis, S., & Altinok, I. (2017). Antimicrobial agents, triclosan, chloroxylenol, methylisothiazolinone and borax, used in cleaning had genotoxic and histopathologic effects on rainbow trout. Chemosphere, 182, 720–729.
Chakraborty, S. P. (2019). Patho-physiological and toxicological aspects of Monosodium Glutamate. Toxicology Mechanisms and Methods., 29(6), 389–396.
Cheng, S. P., Liu, Y. B., Cui, Y. B., Ding, S. R., & Shi, Y. Z. (1996). Effects of Monosodium Glutamate waste water on the fish Ctenopharynzodon idellus and the Cabbage Brassica capestris. Bulletin of Environmental Contamination and Toxicology., 57, 972–978.
David, M., & Kartheek, R. M. (2016). In vivo studies on heapto-renal imparments in fresh water fish Cyprinus carpio following exposure to sub lethal concentrations of Sodium cyanide. Environmental Science and Pollution Research., 23, 722–733.
del Carmen, C. M., Fabro, A., Millen, N., Benmelej, A., & Mahieu, S. (2017). Adverse effects in kidney function, antioxidant systems and histopathology in rats receiving Monosodium glutamate diet. Experimental and Toxicologic Pathology, 69(7), 547–556.
Egbuonu, A. C. C., Ezeanyika, L. U. S., Ejikeme, P. M., & Obidoa, O. (2010). Histomorphologic alterations in the liver of male wistar rats treated with L-arginine glutamate and monosodium glutamate. Research Journal of Environmental Toxicology., 4(4), 205–213.
El-Greisy, Z. A., Abdel Hakim, A., & El-Gamal. (2015). Experimental studies on the effect of cadmium chloride, Zinc acetate, their mixture and the mitigation with vitamin C supplementation on hatchability, size and quality of newly hatched larvae of common carp, Cyprinus carpio. The Egyptian Journal of Aquatic Research, 41(2), 219–226.
Eweka, A. O., Eweka, A., & Ominiabohs, F. A. E. (2010). Histological studies of the effects of Monosodium Glutamate of the Fallopian tubes of adult female Wistar rats. North American Journal of Medical Sciences., 2(3), 146–149.
Fanta, E., Rios, F. S., Romao, S., Vianna, A. C. C., & Freiberger, S. (2003). Histopathology of the fish Corydoras paleatus contaminated with sublethal levels of organophosphorus in water and food. Ecotoxicology and Environmental Safety, 54, 119–130.
Finney, D. J. (1971). Probit analysis: 3rd (Eds), (pp. 333) Cambridge University Press, London.
Flores-Lopes, F., & Thomaz, A. T. (2011). Histopathologic alterations observed in fish gills as a tool in environmental monitoring. Brazilian Journal of Biology, 71(1), 179–188.
Geha, R. S., Beiser, A., Ren, C., Patterson, R., Greenberger, P. A., Grammer, L. C., Ditto, A. M., Harris, K. E., Shaughnessy, M. A., Yarnold, P. R., Corren, J., & Saxon, A. (2000). Review of alleged reaction to monosodium glutamate and outcome of a multicenter double-blind Placebo controlled study. Journal of Nutrition, 130(4S), 1058S-1062S.
Gernhofer, M., Pawet, M., Schramm, M., Muller, E., & Triebskorn, R. (2001). Ultrastructural biomarkers as tools to characterize the health status of fish in contaminated streams. Journal of Aquatic Ecosystem Stress and Recovery, 8, 241–260.
Hemalatha, D., Nataraj, B., Rangasamy, B., Shobana, C., & Ramesh, M. (2019). DNA damage and physiological responses in an indian major carp Labeo rohita exposed to an antimicrobial agent triclosan. Fish Physiology and Biochemistry, 45(4), 1463–1484.
Hendry-Unaeze, H. N. (2010). Consumer knowledge, attitude and practice towards the use of monosodium glutamate and food grade bullion cubes as dietary constituents. Pakistan Journal of Nutrition., 9(1), 76–80.
Hendry-Unaeze, H. N. (2017). Update on food safety of monosodium l-glutamate (MSG). Pathophysiology, 24(4), 243–249.
Jinap, S., & Hajeb, P. (2010). Glutamate. Its applications in food and contributions to health. Appetite, 55(1), 1–10.
Kawai, M., Okiyama, A., & Ueda, Y. (2002). Taste enhancements between various amino acids and IMP. Chemical Senses, 27(8), 739–745.
Khan, M. S., Qureshi, N. A., Jabeen, F., Shakeel, M., & Asghar, M. S. (2018). Assessment of water borne Amine-coated silver nano particle (Ag-NP)—induced toxicity in Labeo rohita by histological and hematological profiles. Biological Trace Element Research., 182, 130–139.
Maharajan, A., Kitto, M. R., Paruruckumani, P. S., & Ganapriya, V. (2016). Histopathology Biomarker responses in Asian sea bass Lates calcifer (Bloch) exposed to copper. The Journal of Basic and Applied Zoology, 77, 21–30.
Malhotra, N., Ger, T. R., Uapipatanakul, B., Huang, J. C., Chen, K. H. C., & Hsiao, C. D. (2020). Review of copper and copper nano particle toxicity in fish. Nanomaterials, 10(6), 1126.
Mossa, A. T. H., Mohafrash, S. M. M. & Chandrasekaran, N. (2018). Safety of natural insecticides: Toxic effects on experimental animal. BioMed Research International., 1–17.
Nakayama, Y., Hashimoto, K., Sawada, Y., Sokabe, M., Kawasaki, H., & Martinac, B. (2018). Corynebacterium glutamicum mechanosensitive channels: Towards unpuzzling “glutamate efflux” for amino acid production. Biophysical Reviews, 10(5), 1359–1369.
Nardelli, T. R., Ribeiro, R. A., Balbo, S. L., Vanzela, E. C., Carneiro, E. M., Boschero, A. C., & Bonfleur, M. L. (2011). Taurine prevents fat deposition and ameliorates Plasma lipid profile in Monosodium glutamate-obese rats. Amino Acids, 41(4), 901–908.
Niaz, K., Zaplatic, E., & Spoor, J. (2018). Extensive use of monosodium glutamate: An Threat to public health? EXCLI Journal, 17, 273–278.
Nnadozie, J. O., Chijioke, U. O., Okafor, O. C., Olusina, D. B., Oli, A. N., Nwonu, P. C., Mbagwu, H. O., & Chijioke, C. P. (2019). Chronic toxicity of low dose monosodium glutamate in albino Wistar rats. BioMed Central Research Notes, 12(593), 1–7.
Nossier, N. S., Ali, M. H. M., & Ebaid, H. M. (2012). A histological and morphometric study of monosodium glutamate toxic effect on testicular structure and potentiality of recovery in adult albino rats. Research Journal of Biological Sciences., 2(2), 66–78.
Ossana, N. A., Baudou, F. G., Castane, P. M., Tripoli, L., Soloneski, S. & Ferrari, L. (2019). Histological, genotoxic and biochemical effects on Cnesterodon decemmaculatus (Jenyns 1842) (Cyprinodontiformes, Poeciliidae): Early response bioassays to assess the impact of receiving waters, Journal of Toxicology., 1–13.
Pacheco, M., & Santos, M. A. (2002). Biotransformation, genotoxic and histopathological effects of environmental contaminants in European eel (Anguilla anguilla L.). Ecotoxicology Environmental Safety, 53(3), 331–347.
Rhodes, J., Titherley, A. C., Norman, J. A., Wood, R., & Lord, D. W. (1991). A survey of the monosodium glutamate content of foods and an estimation of the dietary intake of monosodium glutgamate. Food Additives and Contaminants, 8(3), 265–274.
Salinas, I. (2015). The mucosal immune system of teleost fish. Biology, 4, 525–539.
Sano, C. (2009). History of glutamate production. The American Journal of Clinical Nutrition, 90(3), 728S-732S.
Schwaiger, J., Ferling, H., Mallow, U., Wintermayr, H., & Negele, R. D. (2004). Toxic effects of the non-steroidal anti-inflammatory drug diclofenac: Part I: Histopathological alterations and bioaccumulation in rainbow trout. Aquatic Toxicology, 68, 141–150.
Sprague, J. B. (1971). Measurement of pollutant toxicity to fish-III sublethal effects and safe concentrations. Water Research, 5(6), 245–266.
Stanska, K., & Krzeski, A. (2016). The umami taste: From discovery to clinical use. Otolaryngologia Polska, 70(4), 10–15.
Tawfik, M. S., & Al-Badr, N. (2012). Adverse effects of monosodium glutamate on liver and kidney functions in adult rats and potential protective effect of vitamin C and B. Food and Nutrition Science, 3, 651–659.
Thophon, S., Kruatrache, M., Upatham, E. S., Pokethitiyook, P., Sahaphong, S., & Jaritkhuan, S. (2003). Histopathological alterations of white sea bass, Lates calcacifer, in acute and subacute cadmium exposure. Environmental Pollution, 121, 307–320.
Ullah, S., Li, Z., Hasan, Z., Khan, S. U., & Fahad, S. (2018). Malation induced oxidative stress leads to histopathological and biochemical toxicity in the liver of Rohu (Labeo rohita, Hamilton) at acute concentration. Ecotoxicology and Environmental Safety, 161, 270–280.
Vigneshpriya, D., Krishnaveni, N., Renganathan, S., & Priyadarshini, R. S. S. (2020). Impact of untreated and Sargassumwightii-treated brilliant green dye exposure on Indian major carp, Labeo rohita Ham.: Hematology, biochemistry, enzymology and histopathology. International Journal of Phytoremediation, 22(8), 819–826.
Wang, S., Wang, Y., Ma, J., Ding, Y., & Zhang, S. (2011). Phosvitin plays a critical role in the immunity of zebra fish embryos via acting as a pattern recognition receptor and an antimicrobial effector. The Journal of Biology and Chemistry, 286(25), 22653–22664.
WendelaarBonga, S. E. (1997). The stress response in fish. Physiological Review, 77(3), 591–625.
Yamaguchi, S., & Ninomiya, K. (2000). Umami and food palatability. The Journal of Nutrition, 130(4S), 921S-926S.
Yang, Q., Yang, M., Zhang, S., & Lv, W. (2005). Treatment of wastewater from a monosodium glutamate manufacturing plant using successive yeast and activated sludge systems. Process Biochemistry, 40, 2483–2488.
Zanfirescu, A., Ungurianu, A., Tsatsaki, A. M., Qnituleseu, G. M., Kouretas, D., Veskoukis, A., Tsoukalas, D., Engin, A. B., Aschner, M., & Margina, D. (2019). A review of the alleged health hazards of Monosodium Glutamate. Comprehensive Reviews in Food Science and Food Safety, 18, 1111–1134.
Acknowledgements
There is none.
Funding
Not funded.
Author information
Authors and Affiliations
Contributions
NP performed the histological studies and wrote the first draft. GN conceived the idea, designed the work and drafted the final manuscript. RM critically analysed the toxicity studies and improved the manuscript. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The experiments and the handling of the fish were carried out as per the guidelines of the CPCSEA and approved by ethics Committee of Bharathiar University for the Purpose of Control and Supervision of Experiment on Animals (CPCSEA-722/02/a/CPCSEA), Government of India.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Perumalsamy, N., Nandagopalan, G. & Mathan, R. Histopathological alterations in the vital organs of Indian major carp Labeo rohita exposed to monosodium glutamate (MSG). JoBAZ 85, 10 (2024). https://doi.org/10.1186/s41936-024-00363-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41936-024-00363-z