Abstract
Background
Since many research works have been carried out to identify the pharmacological properties of conotoxins from many Conus sp. The pharmacological properties of higher molecular weight toxin from the venom of Conus sp. have been poorly elucidated. Therefore, the present investigation was made to isolate the higher molecular weight toxin from the venom of Conus virgo and to test its antitumor effect on rat glioma cell line.
Results
The partially purified fraction 1 showed better activity (IC50 50 μg/ml) than fraction 2 (IC50 150 µg/ml) after 24 h incubation. The decreased elevation of creatine kinase was documented on fraction 1treated C6 cells. Vero cells showed minimal cell damage at 150 μg/ml 24 h as well. The nuclear morphology and nuclear fragmentation showed the presence of nuclear damage in C6 cells. Western blot analysis revealing the upregulation of the p53-mediated intrinsic apoptotic pathway was documented.
Conclusions
The present investigation emphasizes that the partially purified fraction 1 has triggered p53 mediated apoptosis by synergism of two higher molecular weight toxins in Rat glioma cell.
Similar content being viewed by others
Background
Malignant gliomas are the most common primary central nervous system (CNS) tumors in adults, accounting for 78% of all primary malignant CNS tumors (Sontheimer, 2003). Gliomas are a disease that can occur in all age groups, especially in elderly population, though a small percentage of populations are genetically predisposed to develop glioblastomas; the disease occurs sporadically with no known underlying cause (Demuth & Berens, 2004). Gliomas are relatively uncommon but lethal cancers, which are responsible for approximately 2% of all types of cancer deaths, and among the brain tumors, malignant glial neoplasms comprise 50% of brain tumor cases (Kaye & Hill, 1993). Understanding the molecular pathogenesis of glioma may lead to a rational development of new therapies, and new treatments have emerged to target molecules controlling the signaling pathways with the goal of increasing specific efficacy and minimizing toxicity (Sathornsumetee et al., 2007).
The venom (conotoxin) of cone snails is a complex containing between 50 and 200 different peptides, each being only 10 to 35 amino acids in length that is distinct for each species (Olivera et al., 1999). These toxins act as a combination drug strategy or a ‘cabal,’ which first immobilizes the prey and then disrupts its neuromuscular transmission (Olivera, 1997). Conotoxin diversity of superfamilies targets a wide range of voltage-gated ion channels and ligand-gated ion channels (Milne et al., 2003). Biomedical research of ‘conotoxins’ has gained traction during the past 25 years, but today less than two per cent of toxins have so far been characterized (Kaas et al., 2010). The first approved drug derived from C. magus, namely Ziconotide, is an N-type calcium channel blocker developed for the treatment of severe chronic pain and the prevention of stroke (Staats et al., 2004). This drug is able to provide effective relief from intractable pain without any side effects (Garber, 2005). Current research and potential development for other toxins lie in the diagnosis and treatment of conditions as diverse as cancer, hypertension, epilepsy, arrhythmia, asthma, multiple sclerosis and diabetic neuropathy (Livett et al., 2004).
The present study was aimed to evaluate the antitumor potential of partially purified higher molecular weight toxin from C. virgo on rat glioma cell line. This finding could create new path in the pharmacological properties of higher molecular weight toxins from Cone snail, and it could be an alternative source for glioma chemotherapy.
Methods
Reagents
DMEM, Trypsin–EDTA, FBS, MTT, sodium bicarbonate, DMSO and antibiotic–antimycotic solution were purchased from HiMedia Laboratories, Mumbai, India. 96-well plates, 6-well plates, tissue culture flasks (25 and 75 mm2) and centrifuge tubes (15 and 50 ml) were purchased from Tarsons Products Pvt. Ltd., Kolkata, India. DEAE-cellulose column was purchased from Sigma-Aldrich, USA.
Glioma (C6) cell line and maintenance
Rat glioma cell line (C6) was obtained from NCCS, Pune, India. The cell line was maintained in DMEM medium supplemented with 10% fetal bovine serum and 1% antibiotic–antimitotic solution. C6 cells were stored in a tissue culture flask at 37 °C in a 5% CO2 fully humidified CO2 incubator. The flask with 70–80% confluence was used for the experiments.
Collection and identification of cone snail
The cone snails were collected from the Southeast coast of Tamil Nadu near Kasimedu, India. The snail were carefully transported to the laboratory and identified based on the morphological key characteristics explained by Franklin et al. (2009).
Isolation of crude venom
The isolation of crude venom from Conus virgo was performed by the method of McIntosh et al. (1995), with slight modification briefly. First, the venom apparatus was dissected out from the visceral mass of the species and from that the venom duct was dissected out; then, by gently squeezing the venom duct, the milky venom was collected into a clean Eppendorf tube containing TBS (10 mM). It was centrifuged at 5000 rpm for 10 min. The supernatant was collected and stored at − 20 °C for further analysis.
Determination of protein concentration and profile
The total protein concentrations of crude and partially purified venom were determined based on the procedure reported by Lowry et al. (1951). The protein profile of crude and partially purified venom was analyzed by 10% native polyacrylamide gel electrophoresis (native PAGE) according to the method of Maurer (1978).
Partial Purification of venom
The crude venom of C. virgo was partially purified using DEAE cellulose anion exchange chromatography. Briefly, the matrix was packed in an 8 cm diameter × 24 cm height column (Bio-Rad, USA). The column was washed using 4 M NaCl. Then, the matrix was again re-equilibrated with 10 mM Tris–HCl (pH 7.8) buffer, and the crude venom was passed through the matrix and incubated for 30 min to bind the matrix. After binding the unbounded molecules eluted with the equilibration buffer, the bounded molecules were eluted with the 0.0–0.5 M NaCl gradient system and the eluents of every fraction were continuously monitored at 280 nm spectrophotometrically. Totally, 50 fractions were collected and each fraction contained 1 ml of eluents.
Cell viability assay
Cell viability of the C6 cells was assessed by MTT assay using the method of Mosmann (1983). Briefly, the glioma cells were seeded in 96-well plates (1 × 105cells/ml) with DMEM medium. The cells were treated with crude venom at different concentrations of 1, 5, 10, 15 and 20 μg/ml along with the medium; likewise, the partially purified venom protein fractions 1 and 2 at the concentrations of 50, 75, 100, 125 and 150 μg/ml were incubated for 24 h. After the incubation, 10 μl of 0.5 mg/ml MTT solution was added to each well and the cells were further incubated for 4 h. Then, the MTT solution was aspirated and 100 µl of DMSO was added. A microplate reader was employed to measure the absorbance at 570 nm. Growth inhibition rate was calculated as percentage. The percentage of cell viability was calculated using the following formula:
Estimation of creatine kinase (CK)
The creatine kinase activity of the control and treated C6 cells was estimated by the method of Okinaka et al. (1961).
Cell morphological study
The general morphological structure of the cells was examined to determine the effect of fraction 1 on C6 cell line. The C6 cells were seeded as mentioned above and plated at 5 × 104 cells/well into a 6-well chamber plate. The experimental plates received fraction 1 at the concentrations of 50 and 150 µg/ml, and the control cells received the medium alone. After 24 h incubation, the cells were observed under an inverted light microscope (Radical, India) at 20 × magnification.
Propidium iodide staining
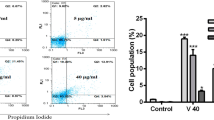
Fluorescent staining of C6 nuclei was performed following the method of Ip et al. (2012). The nuclear morphological structure of the cells was examined to determine the effect of fraction 1 venom protein. The cells were seeded at a density of 5 × 105 cells/well in a 6-well plate. The cells were treated with 50 and 150 µg/ml of fraction 1 venom protein for 24 h and the control cells received the medium alone. After incubation, the cells were washed with PBS, fixed in methanol/acetic acid (3:1 v/v) for 10 min and stained with 50 μg/ml of propidium iodide for 20 min. The cells were visualized under a fluorescence microscope at 20 × magnification.
DNA fragmentation analysis by agarose gel electrophoresis
DNA extraction and agarose gel electrophoresis were performed following the method of Ip et al. (2012). Rat glioma cells (3 × 106 cells/ml) were plated per well in 6-well plates. The control cells received the medium alone and the treatment plates received appropriate concentrations (50 and 150 µg/ml) of fraction 1 venom protein. After completion of 24 h incubation, the DNA was extracted from the cell lysate by phenol–chloroform extraction method. The DNA was quantified by UV–visible spectroscopy and 10 μg of DNA was electrophoresed in 1% agarose gel containing ethidium bromide in a mini gel tank containing TBE buffer for 1 h under 90 V. Then, the gel was examined under a UV transilluminator (Bio-Rad) and photographed.
Western blot analysis
Western blot analysis was performed following the method of Towbin et al. (1979). The C6 cells were treated with fraction 1 at minimal and maximal concentrations and then harvested and homogenized in RIPA buffer. An equal concentration of proteins (60 μg) from each sample was subjected to 10% SDS-PAGE, following the method of Laemmli (1970), and transferred onto PVDF membranes (Millipore Corp., USA). To assess the expression of specific proteins, the membranes were incubated with blocking buffer for 1–2 h at 37 °C and then incubated again overnight at 4 °C in 10 ml TBS/Tween (0.1% v/v) containing 1% BSA with one of the respective primary antibodies p53 (goat polyclonal antibody 1:100), Bax and Bcl2 (mouse monoclonal antibody 1:200). Then, the membrane washed with washing buffer for 5–10 min and the corresponding HRP-conjugated secondary antibody (1:1000) was incubated for 1 h at 37 °C; finally, the expression of immunoreactive bands was detected using DAB substrate.
Statistical analysis
The data were expressed as mean ± S.D. Statistical significance was evaluated using GraphPad Prism 5. The hypothesis testing methods used include one-way analysis of variance (ANOVA) followed by Tukey’s multiple comparison test. p values of less than 0.05 and 0.001 were considered statistically significant.
Results
Quantitative and Qualitative analyses of crude venom protein of C. virgo
Protein concentration in the collected crud venom was calculated as 3.081 mg/ml. The protein profile of the crude venom was determined through native PAGE upon staining with CBB (Fig. 1B. a). The presence of fifteen protein bands was clearly observed in the resolving gel upon electrophoresis, which includes five slow-moving bands, five relatively fast-moving bands, two at the middle of the gel and three fast-moving bands toward the anode region.
Purification of venom protein from C. virgo using DEAE- Cellulose chromatography. A Chromatogram of partially purified venom protein. B Native-PAGE analysis of purified venom protein from C. virgo. A Crude venom upon staining with Coomassie brilliant blue; B partially purified venom protein upon staining with silver nitrate; Lane 1—fraction 1; and Lane 2—fraction 2
Partial purification of C. virgo venom
The partial purification of venom from C. virgo was performed using DEAE-cellulose anion exchange column chromatography. The elution profile depicted in Fig. 1A shows the emergence of two shape peaks upon eluting the bounded proteins from the DEAE-cellulose column with 0.190 and 0.470 mM NaCl, respectively. The effluents were collected as a 1 ml fraction. The eluents 19–23 were pooled together giving fraction 1, and effluents 47–50 were pooled together giving fraction 2. The total protein contents were estimated at 0.68 and 1.14 mg/ml concentrations, respectively, fraction 1 shows two bands upon silver staining which is a sensitive method to identify homogenous protein band, and fraction 2 shows a single band in electrophorogram of native PAGE (Fig. 1B).
Cell viability of crude venom
The cell viability of crude venom from C. virgo was assessed using MTT assay. Decreased cell viability was observed when increasing the concentration of the crude venom (Fig. 2). The IC50 value was found to be 1.5 μg/ml for 24 h incubation. There was not much reduction in cell viability beyond the concentration of 3 µg/ml.
Cytotoxicity assessment of partially purified fractions
A significant decrease in cell viability was observed in both the fractions 1 and 2, but the cell viability of partially purified venom fraction 1 showed better activity than fraction 2 (Fig. 3A). The IC50 values for C6 cell line were found to be 50 μg/ml for fraction 1 and 150 μg/ml for fraction at 24 h incubation. The R2 values for the both fractions were found to be 0.9446 and 0.9058, respectively. The CK activity reveals that upon treatment of both fractions the activity was decreased, but in the case of fraction 1 at 150 μg/ml concentration the level of creatine phosphokinase was significantly less when compared to fraction 1 (Fig. 3B). Moreover, the cytotoxicity of fraction 1 was evaluated in Vero cells; the maximum concentration (125 μg/ml) showed 80% cell viability. Therefore, fraction 1 was taken for further assays (Fig. 3C).
Cell morphology of C6 cells
The morphological observations of C6 cells upon the treatment of fraction 1 and control cells were observed under a light microscope (Radical, India). The control cells were appeared with epithelial irregular, axon, dendrites and axon terminal confluent aggregates as well as clear nucleus at the center of the cell. Upon the treatment of crude venom, the polygonal cells tended to shrink and appeared spherical in shape. The cell shrinkage increased progressively with an increase in the dose and an enlargement of the nucleus was observed. These changes may be due to the growth inhibitory effect of fraction 1 venom from C. virgo on rat glioma cells (Fig. 4A).
Effect of venom protein (fraction 1) on Morphological changes in C6 cells. A Cytomorphological changes of C6 cells at 24 h incubation. Scale bar 20x. (A) Control cells. (B) Low concentration (50 µg/ml). (C) High concentration (150 µg/ml). B Effect of venom protein (fraction 1) on nuclear changes of C6 cells at 24 h incubation. Scale bar 20x. (A) Control cells. (B) Low concentration (50 µg/ml). (C) High concentration (150 µg/ml). The arrow mark represents nuclear changes/damages
Propidium iodide staining
The partially purified venom induces a morphological variation in the nucleus of C6 cells which was analyzed using propidium iodide staining, to emphasize cytonuclear localization of DNA damage in a C6 cells. In the control cells, the nucleus was very clear and intact but upon the treatment of fraction 1 for 24 h the nucleus began to enlarge and also nuclear condensation was observed. The number of positively stained nuclear destructive cells was observed (Fig. 4B).
DNA fragmentation assay
DNA fragment analysis is a typical assay to find out the venom-induced apoptosis. In the present study, the C6 cells were treated with venom protein (fraction 1) at 50 and 150 µg/ml concentrations 24 h incubation. The control cells were showed intact DNA, whereas in venom the cells treated at a lower concentration (50 µg/ml) showed the fragmentation streaking up to the middle of agarose gel electrophoresis and those treated at the higher concentration (150 µg/ml) showed increasing streaking up to three-fourth of gel electrophoresis (Fig. 5A).
Fraction 1-induced apoptosis on C6 cells for 24 h incubation. A DNA fragmentation, Lane A—Control C6 cells, Lane B—50 μg/ml concentration, Lane C—150 μg/ml concentration. B Western blot analysis, Lane 1: control, Lane 2: 50 μg/ml, Lane 3: 150 μg/ml. C Densitometric analysis. The densitometric analysis was performed using ImageJ software and expressed as relative intensity units. Results are given as statistically significant at p ˂ 0.05. *donates between control and cells treated with fraction 1 for 24 h incubation
Western blot analysis
The fraction 1 venom protein-induced apoptosis was further confirmed with the analysis of the expression of apoptotic regulatory proteins Bax and Bcl-2. The increasing expression of Bax and the decreasing expression of Bcl-2 clearly reveal the fraction 1 venom-induced apoptosis through the intrinsic apoptosis pathway. The tumor suppressor protein p53 was also analyzed, and an increased expression of p53 was observed upon the treatment of 50 and 150 µg/ml concentrations (Fig. 5B, C).
Discussion
Over millions of years, evolutionary process in the venom makes the conopeptides as a rich source for targeting the specific ion channels for their prey using hunting techniques. These peptides are high affinities and selectivity binds with cell surface receptors especially in the neuronal cells and other neurosecretory cells. This high potency is used as a research tool in the field of neuroscience and pharmacology. Among them, some are in various stages of clinical trials for the treatment of human diseases (Nelson, 2004). In many cases, cytotoxic activity was carried out using different cell lines in different Conus sp. crude venom so far. All the research work well documented for conopeptide alone and was yet isolated or being isolated smaller molecular weight conopeptide from genus Conus. The higher molecular weight venom has been studied poorly, and also the pharmacological properties are not yet elucidated. So in the present study, we partially purified the higher molecular weight protein from C. virgo and emphasized its antitumor potential in rat glioma cell line.
The crude venom of C. virgo showed the IC50 value of 0.75 µg/ml concentration on rat glioma cells at 24 h, which is similar to the values observed in other studies; crude venom isolated from C. amadis showed an IC50 value of 25 µg/ml against HEp-2 cells at 48 h (Ramesh et al., 2014). Conus betulinus showed an IC50 value of 12.5 µg/ml against HeLa cells at 72 h and showed minimal toxicity against Vero cells, and 60% cell viability was observed at the concentration of 50 µg/ml (Sadhasivam Et Al., 2014). The present study strongly reveals that it has potent activity against C6 cells in the crude venom of Conus virgo and the partially purified venom protein also exhibited the cytotoxic activity. Fraction 1 possessing two proteins was eluted in 115 mM of NaCl, and fraction 2 was eluted in 490 mM of NaCl, which possessed one protein band, which was homogenously determined by native PAGE upon silver staining. The cell viability was assessed for both fractions, of which fraction 1 showed better activity than fraction 2. The IC50 value of fraction 1 was 50 µg/ml and that of fraction 2 was 150 µg/ml which were obtained for 24 h incubation. An earlier report demonstrated that the dissected venom of C. virgo contained gelatinolytic protease with the molecular weight of ~ 97–100 kDa (Möller et al., 2013). The cytotoxicity of fraction 1 showed minimal cell death/damage (80%) on Vero cells even at maximum concentration (150 µg/ml).
Being one of the hallmarks of cytotoxicity, loss of plasma membrane asymmetry represents an early event of apoptosis resulting in the translocation of phosphatidylserine from the inner to the outer surface, while membrane integrity remains unchanged (Van Engeland et al., 1998). The morphological changes including cell shrinkage, detachment of cells and loss of colony formation ability were observed. In our study, the C6 cells treated with fraction 1 showed cytomorphological changes like cell shrinkage, loss of colony formation, cytoplasmic condensation and partial detachment at the concentrations of 50 (minimum) and 150 (maximum) µg/µl. Similarly, bee venom (10 µg/ml)-induced morphological changes in H1299 cells were reported (Jang et al., 2003), which also showed cytotoxicity (IC50 10 µg/ml) against human cervical epidermoid carcinoma Ca Ski cells (Ip et al., 2008), human bladder cancer cells at an IC50 of 8 µg/ml (Ip et al., 2012). The snake’s venom toxin from Vipera lebetina turanica showed cytotoxicity against SK-N-MC and SK-N-SH human neuroblastoma cells, with the IC50 value being 5 µg/ml for both cells (Park et al., 2009). The PI staining performed in the present study revealed that the C6 cell line treated with fraction 1 showed significant nuclear changes at maximum concentration; these changes may be due to the induction of apoptosis upon fraction 1 treatment. Similar results were reported in studies on various animals. Melittin, a major component of bee venom, induces apoptosis in HCC cells at the concentration of 5 or 10 µg/ml, which was examined by Annexin V/PI double staining (Wang et al., 2009). The venom of Bothrops alternatus (snake’s) at 10 µg/ml induced detectable changes in C2C12 mouse myoblast cells, including the reduction of nuclear size, which often displayed diverse degrees of chromatin condensation, cell rounding and some areas devoid of cells. Furthermore, cell shrinkage and the formation of blabbing of cell surface finally resulted in the generation of apoptotic bodies (Bustillo et al., 2009). DNA fragmentation assay is a classical and indirect method to identify the apoptosis induced/triggered by any cytotoxic drugs or toxins. From DNA fragmentation analysis conducted in our study, intact DNA was observed in control cells, and upon treatment with fraction 1 at 50 µg/ml, the DNA fragmentation appeared as a striking pattern in agarose gel electrophoresis, which was increased with increasing (150 µg/ml) the concentration. Similar results were observed in the earlier reports which revealed that the synovial fibroblasts upon treatment with bee venom at 24 h showed 3 DNA fragment bands. In human cervical epidermoid carcinoma Ca Ski cells, the DNA fragments were observed after 48 h when treated with bee venom (Ip et al., 2008).
Creatine kinase (CK) is a group of isoenzymes which play a key role in energy metabolism particularly for high energy-consuming tissues such as the brain, skeletal muscle and heart. The increased level of creatine kinase indicated the tissue damage, during excitation of cells the increased level of cellular ATP turnover occurs, and these rapid changes of the creatine/phosphocreatine/creatine kinase (Cr/PCr/CK) are necessary for energy buffering and transport system between the sites of ATP production also consumption by ATPases to avoid large fluctuations of cellular ATP/ADP levels in these excitable tissues (Wallimann et al., 1992). In the present investigation fractions 1 and 2, both showed a significant decrease in the CK level upon treatment at 50 and 150 μg/ml concentrations, but fraction 1 showed good activity than fraction 2 which clearly indicated that fraction 1venom reduced the pathological marker enzyme activity; similar results were documented in many in vivo studies (Funchal et al., 2006; Narasimhan et al., 2011; Subramanian et al., 2010).
p53 is a tumor suppressor protein which is described as the guardian of the genome preventing them from the mutation (Lane, 1992). It has been reported that most cancers have mutation in p53 including glioma (Muller & Vousden, 2014; Nagpal et al., 2006), which leads in the mutant forms of p53 trans activating various target genes such as multiple drug resistance gene 1 (MDR1), c-myc, proliferating cell nuclear antigen (PCNA), interleukin 6 (IL-6), insulin-like growth factor 1 (IGF-1), fibroblast growth factor (FGF) and epidermal growth factor receptor (EGFR) (Chin et al., 1992; Cadwell & Zambetti, 2001; Ludes-Meyers et al., 1996; Frazier et al., 1998). Thus, the p53 mutant leads to the progression of aggressive cancers. Moreover, it also activates the intrinsic mitochondrial pathway which promotes the downstream process of apoptosis (Benchimol, 2001). In the present study, we reported that the fraction 1 protein activates the p53-dependent apoptosis through the activation of Bax and Bcl-2 expression ratio. Similar results were reported in C. californicus, the peptide of which induced apoptosis in lung cancer cells (H1299 and H1437) through Bcl-2 and Bax cascades (Oroz-Parra et al., 2016). Bee venom induced apoptosis in Ca Ski cells (Ip et al., 2008) and snake’s venom in neuroblastoma cells (Park et al., 2009). The entire result, DNA fragmentation, cell morphological observation and Western blot analysis, taken together, clearly reveal fraction 1-induced intrinsic apoptosis through the activation of p53-dependent pathway. Further characterization and experimental studies are needed to explore the molecular intervention of antitumor potential on glioma.
Conclusions
Fraction 1 has potential antitumor properties which are clearly documented in the Rat glioma cell line. The overexpressed CK level was downregulated upon the treatment of fraction 1 conotoxin, and it triggers the p53-mediated intrinsic apoptosis by Bax/Bcl2 ratio and also showed less toxicity on non-target Vero cells.
Availability of data and materials
Data will not be shared, only by request from corresponding author.
Abbreviations
- DMEM:
-
Dulbecco’s modified eagle medium
- Trypsin–EDTA:
-
Ethylenediaminetetraacetic acid
- FBS:
-
Fetal bovine serum
- MTT:
-
3-(4,5-Dimethylthiazol-2yl)-2,5-diphenyltetrazolium bromide
- DMSO:
-
Dimethyl sulfoxide
- DEAE-cellulose:
-
Diethylaminoethyl cellulose
- NCCS:
-
National Centre for Cell Science
- RIPA:
-
Radioimmunoprecipitation assay buffer
- BSA:
-
Bovine serum albumin
- DAB:
-
3,3'-Diaminobenzidine
- Bcl-2:
-
B-cell lymphoma 2
- BAX:
-
Bcl-2-associated X protein
References
Benchimol, S. (2001). p53-dependent pathways of apoptosis. Cell Death and Differentiation, 8(11), 1049–1051.
Bustillo, S., Lucero, H., Leiva, L. C., Acosta, O., Kier Joffé, E. B., & Gorodner, J. O. (2009). Cytotoxicity and morphological analysis of cell death induced by Bothrops venoms from the northeast of Argentina. Journal of Venomous Animals and Toxins including Tropical Diseases, 15(1), 28–42.
Cadwell, C., & Zambetti, G. P. (2001). The effects of wild-type p53 tumor suppressor activity and mutant p53 gain-of-function on cell growth. Gene, 277(1), 15–30.
Chin, K. V., Ueda, K., Pastan, I., & Gottesman, M. M. (1992). Modulation of activity of the promoter of the human MDR1 gene by Ras and p53. Science, 255(5043), 459–462.
Demuth, T., & Berens, M. E. (2004). Molecular mechanisms of glioma cell migration and invasion. Journal of Neurooncology, 70(2), 217–228.
Franklin, J. B., Subramanian, K. A., Fernando, S. A., & Krishnan, K. S. (2009). Diversity and distribution of conidae from the TamilNadu coast of India (Mollusca: Caenogastropoda: Conidae). Zootaxa, 2250, 3–63.
Frazier, M. W., He, X., Wang, J., Gu, Z., Cleveland, J. L., & Zambetti, G. P. (1998). Activation of c-myc gene expression by tumor-derived p53 mutants requires a discrete C-terminal domain. Molecular and Cellular Biology, 18, 3735–3743.
Funchal, C., Schuck, P. F., dos Santos, A. Q., Jacques-Silva, M. C., Gottfried, C., Pessoa-Pureur, R., & Wajner, M. (2006). Creatine and antioxidant treatment prevent the inhibition of creatine kinase activity and the morphological alterations of C6 glioma cells induced by the branched-chain α-keto acids accumulating in maple syrup urine disease. Cellular and Molecular Neurobiology, 26(1), 67–79.
Garber, K. (2005). Peptide leads new class of chronic pain drugs. Nature Biotechnology, 23(4), 399.
Ip, S. W., Wei, H. C., Lin, J. P., Kuo, H. M., Liu, K. C., Hsu, S. C., Yang, J. S., Yang, M. D., Chiu, T. H., Han, S. M., & Chung, J. G. (2008). Bee venom induced cell cycle arrest and apoptosis in human cervical epidermoid carcinoma Ca Ski cells. Anticancer Research, 28(2A), 833–842.
Ip, S. W., Chu, Y. L., Yu, C. S., Chen, P. Y., Ho, H. C., Yang, J. S., Huang, H. Y., Chueh, F. S., Lai, T. Y., & Chung, J. G. (2012). Bee venom induces apoptosis through intracellular Ca2+-modulated intrinsic death pathway in human bladder cancer cells. International Journal of Urology, 19(1), 61–70.
Jang, M. H., Shin, M. C., Lim, S., Han, S. M., Park, H. J., Shin, I., Lee, J. S., Kim, K. A., Kim, E. H., & Kim, C. J. (2003). Bee venom induces apoptosis and inhibits expression of cyclooxygenase-2 mRNA in human lung cancer cell line NCI-H1299. Journal of Pharmacological Sciences, 91(2), 95–104.
Kaas, Q., Westermann, J. C., & Craik, D. J. (2010). Conopeptide characterization and classifications: An analysis using ConoServer. Toxicon, 55(8), 1491–1509.
Kaye, A. H., & Hill, J. S. (1993). Photodynamic therapy of brain tumours. Annals of the Academy of Medicine, Singapore, 22(3 Suppl), 470–481.
Laemmli, U. K. (1970). Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature, 227(5259), 680–685.
Lane, D. P. (1992). Cancer. p53, guardian of the genome. Nature, 358, 15–16.
Livett, B. G., Gayler, K. R., & Khalil, Z. (2004). Drugs from the sea: Conopeptides as potential therapeutics. Current Medicinal Chemistry, 11(13), 1715–1723.
Lowry, O. H., Rosebrough, N. J., Farr, A. L., & Randall, R. J. (1951). Protein measurement with the Folin phenol reagent. Journal of Biological Chemistry, 193, 265–275.
Ludes-Meyers, J. H., Subler, M. A., Shivakumar, C. V., Munoz, R. M., Jiang, P., Bigger, J. E., Brown, D. R., Deb, S. P., & Deb, S. (1996). Transcriptional activation of the human epidermal growth factor receptor promoter by human p53. Molecular and Cellular Biology, 16, 6009–6019.
Maurer, H. R. (1978). Disc electrophoresis and related techniques of polyacrylamide gel electrophoresis. Walter de Gruyter.
McIntosh, J. M., Ghomashchi, F., Gelb, M. H., Dooley, D. J., Stoehr, S. J., Giordani, A. B., Naisbitt, S. R., & Olivera, B. M. (1995). Conodipine - M, a novel phospholipase A2 isolated from the venom of the marine snail Conus magus. Journal of Biological Chemistry, 270(8), 3518–3526.
Milne, T. J., Abbenante, G., Tyndall, J. D., Halliday, J., & Lewis, R. J. (2003). Isolation and characterization of a cone snail protease with homology to CRISP proteins of the pathogenesis-related protein superfamily. Journal of Biological Chemistry, 278(33), 31105–31110.
Möller, C., Vanderweit, N., Bubis, J., & Marí, F. (2013). Comparative analysis of proteases in the injected and dissected venom of cone snail species. Toxicon, 65, 59–67.
Mosmann, T. (1983). Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. Journal of Immunological Methods, 65(1–2), 55–63.
Muller, P. A., & Vousden, K. H. (2014). Mutant p53 in cancer: New functions and therapeutic opportunities. Cancer Cell, 25(3), 304–317.
Nagpal, J., Jamoona, A., Gulati, N. D., Mohan, A., Braun, A., Murali, R., & Jhanwar-Uniyal, M. (2006). Revisiting the role of p53 in primary and secondary glioblastomas. Anticancer Research, 26(6C), 4633–4639.
Narasimhan, R., Vaithiyanathan, M., & Janardanam, V. (2011). Neuroprotective effect of sesamol in glioma induced in rats. Biomedicine International, 2(1), 14.
Nelson, L. (2004). Venomous snails: One slip, and you’re dead. Nature, 429(6994), 798–799.
Okinaka, S., Kumagai, H., Ebashi, S., Sugita, H., Momoi, H., Toyokura, Y., & Fujie, Y. (1961). Serum creatine phosphokinase: Activity in progressive muscular dystrophy and neuromuscular diseases. Archives of Neurology, 4(5), 520–525.
Olivera, B. M. (1997). EE Just lecture, 1996 Conus venom peptides, receptor and ion channel targets, and drug design: 50 million years of neuropharmacology. Molecular Biology of the Cell, 8(11), 2101–2109.
Olivera, B. M., Walker, C., Cartier, G. E., Hooper, D., Santos, A. D., Schoenfeld, R., Shetty, R., Watkins, M., Bandyopadhyay, P., & Hillyard, D. R. (1999). Speciation of cone snails and interspecific hyperdivergence of their venom peptides: Potential evolutionary significance of introns. Annals of the New York Academy of Sciences, 870(1), 223–237.
Oroz-Parra, I., Navarro, M., Cervantes-Luevano, K. E., Álvarez-Delgado, C., Salvesen, G., Sanchez-Campos, L. N., & Licea-Navarro, A. F. (2016). Apoptosis activation in human lung cancer cell lines by a novel synthetic peptide derived from Conus californicus Venom. Toxins, 8(2), 38.
Park, M. H., Son, D. J., Kwak, D. H., Song, H. S., Oh, K. W., Yoo, H. S., Lee, Y. M., Song, M. J., & Hong, J. T. (2009). Snake venom toxin inhibits cell growth through induction of apoptosis in neuroblastoma cells. Archives of Pharmacal Research, 11, 1545–1554.
Ramesh, S., Dilipan, E., & Mayavu, P. (2014). Effects of drugs against Antioxidant and Cytotoxic (HEp2 Cell Line) activity compounds from marine animals Conus amadis venom (GMELIN, J F, 1791). International Journal of Pharmacy and Pharmaceutical Sciences, 6(7), 638–643.
Sadhasivam, G., Muthuvel, A., Rajasekaran, R., Pachaiyappan, A., & Thangavel, B. (2014). Studies on biochemical and biomedical properties of Conus betulinus venom. Asian Pacific Journal of Tropical Disease, 4, 102–110.
Sathornsumetee, S., Reardon, D. A., Desjardins, A., Quinn, J. A., Vredenburgh, J. J., & Rich, J. N. (2007). Molecularly targeted therapy for malignant glioma. Cancer, 110(1), 13–24.
Sontheimer, H. (2003). Malignant gliomas: Perverting glutamate and ion homeostasis for selective advantage. Trends in Neurosciences, 26(10), 543–549.
Staats, P. S., Yearwood, T., Charapata, S. G., Presley, R. W., Wallace, M. S., Byas-Smith, M., Fisher, R., Bryce, D. A., Mangieri, E. A., Luther, R. R., & Mayo, M. (2004). Intrathecal ziconotide in the treatment of refractory pain in patients with cancer or AIDS: A randomized controlled trial. JAMA, 291(1), 63–70.
Subramanian, U., Poongavanam, S., & Vanisree, A. J. (2010). Studies on the neuroprotective role of Piper longum in C6 glioma induced rats. Investigational New Drugs, 28(5), 615–623.
Towbin, H., Staehelin, T., & Gordon, J. (1979). Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: Procedure and some applications. Proceedings of the National Academy of Sciences, 76(9), 4350–4354.
van Engeland, M., Nieland, L. J., Ramaekers, F. C., Schutte, B., & Reutelingsperger, C. P. (1998). Annexin V-affinity assay: A review on an apoptosis detection system based on phosphatidylserine exposure. Cytometry, 31(1), 1–9.
Wallimann, T., Wyss, M., Brdiczka, D., Nicolay, K., & Eppenberger, H. M. (1992). Intracellular compartmentation, structure and function of creatine kinase isoenzymes in tissues with high and fluctuating energy demands: The “phosphocreatine circuit” for cellular energy homeostasis. Biochemical Journal, 281, 21.
Wang, C., Chen, T., Zhang, N., Yang, M., Li, B., Lü, X., Cao, X., & Ling, C. (2009). Melittin, a major component of bee venom, sensitizes human hepatocellular carcinoma cells to tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)-induced apoptosis by activating CaMKII-TAK1-JNK/p38 and inhibiting IκBα kinase-NFκB. Journal of Biological Chemistry, 284(6), 3804–3813.
Acknowledgements
All the authors are thankful to Prof. N. Munuswamy, Department of Zoology, University of Madras, for his valuable suggestions and moral support.
Funding
This study was funded by the UGC non-NET grant from University Grants Commission, New Delhi, India.
Author information
Authors and Affiliations
Contributions
CA and GD contributed to the design of the work and interpretation of the data. DA and LA drafted the work. KN contributed to the acquisition and analysis of the data. CA contributed to the revision of the work. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ganesan, D.S., Annadurai, D., Vadakkuvaselvi, L. et al. Antitumor effect of partially purified venom protein from Conus virgo on rat glioma cell line. JoBAZ 83, 25 (2022). https://doi.org/10.1186/s41936-022-00288-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41936-022-00288-5