Abstract
Background
Chrysomya albiceps (Wiedemann, 1819) (blowflies), family Calliphoridae, is important in forensic entomology, where the minimum and maximum postmortem intervals (PMI) are estimated on the basis of the developmental stages of Diptera larvae that consume dead tissue. The present study was designed to estimate the effects of different ambient temperatures (20, 25, and 30 °C) under controlled laboratory conditions on the developmental stages of C. albiceps from the Jazan region, Saudi Arabia.
Results
The present study showed that the larval body weight and length were significantly increased when larvae were reared at 30 °C compared with corresponding values at 24 h, 48 h, and 72 h at rearing temperatures of 20 °C and 25 °C; however, the weight and length were significantly decreased compared with corresponding values at 96 h at 20 °C and 25 °C. The pupation time was inversely related to the rearing temperature, occurring at 144, 124, and 120 h at rearing temperatures of 20 °C, 25 °C, and 30 °C, respectively. The pupal weight and length were significantly increased in larvae reared at 30 °C compared with those reared at 20 °C and 25 °C. At 20 °C, 25 °C, and 30 °C, larval durations of 5.00, 5.00, and 4.00 days were recorded, respectively. Pupae and adults showed gradual decreases in life stage durations, at 6.00, 5.30, and 4.80 days in pupae and 20.00, 18.70, and 16.90 days in adults, with increasing rearing temperatures. Average adult longevity at 30 °C (194.40 h) was significantly less than adult longevity at 20 °C (216.00 h) and 25 °C (204.60 h). The results showed an inverse relation between durations of developmental stages and rearing temperatures.
Conclusions
Insect laboratory colonization for the determination of biological characteristics of insects is economically viable for forensic entomology and as a technique for evaluating insect evidence.
Similar content being viewed by others
Background
Forensic science is one of the most important aspects in the investigation of any crime. According to the National Institute of Justice, forensic science is the application of sciences to matters of the law (Bruinsma and Weisburd 2014). The forensic science manipulate with the usage of a broad spectrum of scientific branches like physics, chemistry, and biology to achieve relevant information to criminal and legal evidence. Forensic science is any scientific and specialized skills and tools that the crime scene investigators and lab technicians are able to collect and analyze as an evidence in order to solve a crime or successfully convict the offender (Morgan 2019).
Forensic entomology evaluates the succession of necrophagous insects and the larval age of relevant insects collected from decomposed remains to estimate the time elapsed since death; entomological analysis is a tool in forensic investigations and can provide evidence of the postmortem interval (PMI) in a court of law.
Forensic entomology examines insect evidence for forensic and legal purposes to estimate the minimum time since death. The collected entomological evidence can provide necessary and important information about the movement or storage of remains following death, submersion interval, time of decapitation and/or dismemberment, identification of specific trauma sites, and postmortem artifacts on the body. The use of entomotoxicology can link a suspect to the scene of a crime, determine sexual molestation, or identify a suspect by examining the insects recovered from infested wounds to determine the period of neglect of living humans and animals (Catts 1992; Campobasso and Introna 2001; Amendt et al. 2007). Determination of the time since death is temperature dependent because temperature affects insect development as well as insect access to corpses (Campobasso et al. 2001; Myskowiak and Doums 2002).
Dipterans are the most important insect order in forensic investigations. These insects utilize temporary microhabitats of the corpse to feed and lay their eggs; they are normally the first arthropods to colonize a corpse (Baia et al. 2016).
The first group of insects to arrive and lay eggs on and in dead bodies in the early stage of decomposition are usually blowflies (Diptera: Calliphoridae), which are attracted by odor (Amendt et al. 2007; Gullan and Cranston 2014). The development rates of blowflies can be used to estimate the time elapsed since death within the first few weeks after death (Anderson 2000). The stage of development of the oldest immature insects on the body and the average environmental temperatures at the crime scene while the body was in situ allow the calculation of an accurate PMI (Amendt et al. 2011).
Chrysomya albiceps (Wiedemann, 1819) (Diptera: Calliphoridae) is one of the most studied blowflies and is recognized as a pioneer species in the colonization of corpses and carcasses. Similar to other muscoids, it is attracted by the odor produced during corpse decomposition (Lane 1975; Goddard and Lago 1985; Vasconcelos et al. 2013). C. albiceps was the dominant species recorded on indoor and outdoor carcasses in stages of bloating, decay, and advanced decay in a recently published study in Riyadh, Saudi Arabia (Al-Khalifa et al. 2020). Mashaly et al. (2017) found that C. albiceps was the most abundant species of carrion fly on sheep carcasses in seven cities.
C. albiceps (Wiedemann, 1819) is a native tropical and subtropical African species with a worldwide distribution that has expanded since the turn of the century (Laurence 1981; Hall and Wall 1995; Grassberger et al. 2003; Gomes et al. 2009; Kotrba et al. 2012; Klekovska et al. 2017; Makovetskaya and Verves 2018). Blowflies identified and represented in the Middle East (Akbarzadeh et al. 2015) have been reported, and C. albiceps has been previously collected and identified in the area of interest of the present study (Jazan, Saudi Arabia) (Bosly 2010; Setyaningrum and Al Dhafer 2014; Dawah et al. 2019). In addition to its forensic medical and veterinary importance, larvae can cause primary and secondary cutaneous myiasis in humans and livestock (Alahmed 2004; Alahmed et al. 2006; Stevens and Wallman 2006; Amendt et al. 2011). The worldwide distribution of C. albiceps has allowed its use in different important studies because of easy collection and laboratory handling (Vélez and Wolff 2008; Pujol-Luz and Barros-Cordeiro 2012). C. albiceps is important in forensic entomology since it can be used to determine the PMI by calculating the age of the oldest larval stage feeding on a corpse (Gomes and Von Zuben 2005; Gomes et al. 2006; Mendonça et al. 2010; Salazar-Souza et al. 2018). Hence, the developmental stage of the insect helps specialists determine the time since death, which is temperature dependent (Li et al. 2016).
To date, entomology has not been used in legal investigations in the Kingdom of Saudi Arabia and most Arab countries in the Middle East. Development data of insects associated with corpse decomposition can help estimate the PMI in forensic investigations that requires judicial requirements. Forensic entomology is recognized as an important tool for legal investigations in many countries, while in our region, it has not yet been used as legal evidence in court. The reason for the lack of application of entomological evidence may be the insect fauna, which changes from one region to another, or the lack of standardized reference protocols regarding the developmental stages of arthropods (Vasconcelos et al. 2019).
The present study aimed to explore the effect of different ambient temperatures on the developmental stages of forensically important C. albiceps under laboratory conditions. That is because the use of laboratory colonization technique may add for evaluating insect evidence, and the present study is the first to be done in the Jazan region, southwest Kingdom of Saudi Arabia.
Methods
Stock colony
The stock colony was established from collected flies from governmental slaughterhouses in Abu Arish (16° 58’ N-42° 47’ E), which is a city located in the eastern Jazan region (southwestern Saudi Arabia), with previous collection experience (Bosly 2010). C. albiceps flies were identified at the Biology Department, Faculty of Science, and were reared in the laboratory for ten generations. Flies were placed in polypropylene breeding cages (45 × 30 × 20 cm), and adults had access to food ad libitum; the diet consisted of skimmed powder milk and 10 g of sugar in 100 ml of water in Petri dishes (Al-Shareef and Al-Qurashi 2016; Salazar-Souza et al. 2019). Larvae were placed in beakers within transparent boxes containing sand and sawdust to prevent the postfeeding larvae from escaping. Larvae were provided fresh rat muscle ad libitum as a rearing medium/substrate in the cages. Rats were supplied by the Animal Research Center . The rearing laboratory conditions were 25 ± 1 °C, with 75% RH and a photoperiod of 12 h/12 days (light/dark).
Experimental procedures
First instars hatched from an egg mass containing approximately 50–60 eggs were transferred directly and individually with the aid of a brush (number 0) to plastic vials (15 × 12 × 11 cm3) containing 20 g of normal untreated rat muscle. The vials were covered with muslin secured with a rubber band, and the muscle tissue was replaced daily with sterilized muscle shavings. The vials were kept in climatic chambers maintained at 20, 25, and 30 °C, with the aforementioned humidity and photoperiod. The experimental procedures were performed in three replicates under the temperature conditions of interest. The replicates were observed every 24 h, and the larvae and pupal weights (mg) and lengths (mm) were calculated and recorded.
The ambient temperatures range used in the present study were chosen to investigate the most affecting temperature within this range on the insect’s developmental changes because the optimal temperature range for most species of blowflies (Diptera: Calliphoridae) is between 20 and 30 °C, with development and survival compromised at temperatures outside this range (Grassberger and Reiter 2001; Richards et al. 2008a). C. albiceps (Wiedemann, 1819) is reported to be the most temperature-tolerant species and has been found to be capable of surviving and developing at temperatures ranging from 11 to 50 °C (Richards et al. 2008b).
Measurements of body weights and lengths were carried out regularly every 24 h. Thirty randomly selected larvae were immersed in hot water (70–80 °C) for 3–5 min to prevent shrinkage before preservation in 75% alcohol according to the method described by Adams and Hall (2003). The weight of each dry larva was recorded by using a sensitive electrical balance with a sensitivity of 0.001 g-1. The larval length was measured to the nearest 0.01 mm under a stereoscopic binocular microscope. In all replicates, larval samples from each temperature group were allowed to complete their development cycle to estimate the duration of all insect stages at all temperatures under investigation. The times of pupation and adult emergence were recorded in each group, and the group mean developmental period was calculated to assess longevity.
Statistical analysis
Data analysis was performed using one-way ANOVA (with a least significant difference (LSD) test), and significant differences were defined as those with P < 0.05. Statistical analysis was performed using the Statistical Package for Social Science (SPSS) for Windows software, Release 22.0 (SPSS, Chicago, IL, USA).
Results
The data in Table 1 show the means and standard errors of C. albiceps larval body weights every 24 h at constant temperatures. There was a significant increase in the larval body weight in the group reared at 25 °C compared with the corresponding weights in larvae reared at 20 °C at 24, 48, 72, 96, and 120 h. However, the larvae exhibited a significant decrease in body weight at 120 h compared with the recorded body weights at 96 h in larvae reared at both 20 °C and 25 °C. The larval body weights of those reared at 30 °C were significantly higher than the body weights of those reared at 20 °C and 25 °C at 24 h, 48 h, 72 h, and 96 h. There was a significant decrease in body weight at 96 h compared with the body weight at 72 h. Larvae reared at 30 °C initiated pupation at 120 h, which was earlier than the time of pupation in those reared at 20 °C (144 h) and 25 °C (124 h).
Regarding the effect of temperature on larval body length (Table 2 and Fig. 1), the results showed a significant increase in the body length of larvae reared at 25 °C compared with the body length of larvae reared at 20 °C after 48 h, 72 h, and 120 h. Moreover, the larval body length of those reared at 30 °C was significantly higher than the corresponding body lengths of larvae reared at 20 °C and 25 °C at 24 h and 48 h. There was a significant decrease in body length compared with the corresponding body lengths of larvae reared at 20 °C and 25 °C at 96 h.
The data presented in Table 3 show the means and standard errors of pupal body weights, lengths, and durations of C. albiceps life stages at different constant temperatures. The mean pupal weight and length in those reared at 25 °C were significantly increased compared with those in pupae reared at 20 °C. The pupal duration was significantly decreased in those reared at 25 °C compared with that in those reared at 20 °C. The mean weight and length of pupae reared at 30 °C were significantly increased compared with those in pupae reared at 20 °C and 25 °C. The pupal duration was significantly decreased in those reared at 30 °C compared with that in those reared at 20 °C and 25 °C.
The results of the effects of temperature on the mean and standard error of C. albiceps adult longevity are presented in Table 4. In those reared at 25 °C, the average adult longevity was significantly decreased compared with that in those reared at 20 °C. In those reared at 30 °C, the average adult longevity was decreased significantly compared with the average adult longevity in those reared at 20 °C and 25 °C.
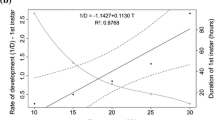
The results regarding the effects of temperature on the C. albiceps mean developmental duration in days at each stage are represented in Fig. 2. The larval period lasted 5.00, 5.00, and 4.00 days at 20 °C, 25 °C, and 30 °C, respectively. C. albiceps pupae and adults showed gradual decreases in developmental durations with increasing rearing temperatures. The pupal durations were 6.00, 5.30, and 4.80 days, and the adult durations were 20.00, 18.70, and 16.90 days at 20 °C, 25 °C, and 30 °C, respectively.
Discussion
The goal of the present study was to evaluate the effects of different ambient temperatures on the developmental stages of C. albiceps under laboratory conditions for the first time in the Jazan region, Saudi Arabia. The developmental data of forensically important C. albiceps under laboratory conditions should be considered reliable, as several studies demonstrated that prediction of the developmental rate at constant laboratory temperatures was quite similar to that under normal temperature conditions (Bohem 2015). Muscles were selected as the larval feeding source to provide optimal nutrition according to different studies that revealed that diet plays important roles in the development and developmental periods of forensically important C. albiceps. These studies also found that a diet of muscular tissue resulted in the fastest developmental rate at a constant temperature due to the availability of required nutrients (Rabêlo et al. 2011; Beuter and Mendes 2013; Thyssen et al. 2014). Regarding larval growth, the study showed that larval body weight gain and length were positively related to temperature increases from 20 to 25 °C to 30 °C. At 24, 48, 72, and 96 h, the larvae reared at 20 °C had slightly lower larval body weights, at 2.30, 9.40, 36.60, and 43.00 mg, than those reared at 25 °C at 3.00, 12.40, 49.00, and 79.60 mg, respectively. Then, the body weights decreased to 39.77 mg and 71.90 mg at 120 h in larvae reared at 20 °C and 25 °C, respectively. Larvae reared at 30 °C had significantly higher body weights than those reared at the aforementioned rearing temperatures. The larvae weighed 5.00 mg at 24 h and 30.20 mg at 48 h, with body weight peaking at 72 h (85.30 mg). The body weight was decreased at 96 h (78.60 mg), which is in line with earlier pupation in this group. Larval body length followed the same patterns as larval body weight; there was a significant increase in larval body length in larvae reared at 30 °C compared with that in those reared at 20 °C and 25 °C from 24 h until 72 h. The body length was decreased at 96 h.
These results may be explained by the nature of C. albiceps, which is a tropical and subtropical species that prefers high temperatures and humidity levels (Saleh et al. 2014; Greenberg and Povolny 2019); most insect species have low activity levels at low temperatures (Scholtz and Caveney 1992). When substrate is available, a high ambient temperature may stimulate faster feeding by individual maggots, and the metabolic rate may be markedly increased, which could result in increased body weight and length. In addition, temperature has been suggested to affect the development through the larval central nervous system, promoting thermotaxis and an increased feeding rate due to the stimulation of temperature-sensitive neurons in the CNS of larvae that accelerate the larval growth (Hückesfeld et al. 2011).
The effects of temperature on larval growth in the present study are consistent with those in earlier studies on C. albiceps (Queiroz 1996; Vélez and Wolff 2008; Al-Shareef and Al-Qurashi 2016; Salimi et al. 2018), supporting that higher temperatures accelerate larval growth, as also observed in other forensically important flies, such as Chrysomya megacephala (Fabricius, 1794) (Bansode et al. 2016). The decrease in body length and weight at the last sampling period at each rearing temperature indicated the postfeeding phase, which is characteristic of the blowfly (Greenberg and Kunich 2002). The present study recorded the onset of pupation at 120 h in the larvae reared at 30 °C, while the larval period extended to 144 h in the larvae reared at 20 °C and at 124 h in those reared at 25 °C. The larval developmental period decreased from 5 days at 20 °C and 25 °C to 4 days at 30 °C. Hence, the present results of the inverse relationship of the developmental period and temperature are in agreement with those previously reported for C. albiceps. Growth acceleration resulted in shortening of the developmental period due to an elevated minimum growth temperature threshold. Queiroz (1996) found that the basal temperature required for rearing C. albiceps under laboratory conditions at 60 + 10% RH and a 14-h photoperiod was 15.04 °C in the larval stage, 17.39 °C in the pupal stage and 15.38 °C in the larva-to-adult phase. Later studies that analyzed C. albiceps under four constant rearing temperatures (20, 25, 30, and 35 °C) reported total larval stage durations of 9, 6, 4.83, and 4.75 days, respectively (Al-Shareef and Al-Qurashi 2016). The larval stages of those reared at 25 °C and 30 °C were 6 and 4 days, respectively (Salimi et al. 2018); and the larval stages of those reared at 18, 22, 27, and 32 °C were 21.30, 10.61, 5.0, and 4.0 days, respectively (Queiroz 1996). These results are similar to those of studies on forensically important species of family Calliphoridae, such as Chrysomya pinguis (times from egg to intrapuparial development at rearing temperatures ranging from 16 to 34 °C ranged from 388.00 to 91.20 h) (Zhang et al. 2019), Lucilia cuprina (time from egg to prepupal development at rearing temperatures of 20 °C, 20 °C, and 30 °C were 194.00, 128, and 83.50 h, respectively) (Bansode et al. 2016), and Calliphora vicina (Bharti 2009).
In the present study, increasing temperatures from 20 and 25 to 30 °C were inversely associated with the pupation period, and the lengths of the pupal period for C. albiceps at 20 °C, 25 °C, and 30 °C were 6.00, 5.30, and 4.80 days, respectively. The latter data are in line with those previously reported for C. albiceps in laboratory studies, where under four constant rearing temperatures at 20, 25, 30, and 35 °C, pupal periods of 7, 5.5, 4.00, and 1.50 days were recorded, respectively (Al-Shareef and Al-Qurashi 2016). Additionally, at 25 °C and 30 °C, the pupal periods were determined to be 5 and 3.5 days, respectively (Salimi et al. 2018). Under laboratory conditions (28 °C and 40% RH), the C. albiceps pupation period ranged from 4 to 6 days (Shiravi et al. 2011). Additionally, the maximum pupal periods recorded for C. albiceps were 5 and 4.5 days at 30 °C and 32 °C (Augul and Jassim 2009), and the developmental pupation periods recorded at rearing temperatures of 20, 25, 30, and 35 °C with 60% RH and 14:10 (light:dark) h were 12.90, 8.10, 5.9, and 4.6 days, respectively (Grassberger et al. 2003). A study on Chrysomya megacephala reported a reduction in the pupariation period from 168.00 h at 22 °C to 105.47 h at a rearing temperature of 31 °C (Yang et al. 2016). The present results showed that adults reared at laboratory temperatures of 20 and 25 °C had significantly increased longevity (216.00 and 204.00 h, respectively) and an increased adult duration, at 9.0 and 8.4 days, compared with those reared at 30 °C, where longevity was 194.40 h and the duration was 8.1 days, in line with previous studies (Al-Shareef and Al-Qurashi 2016; Salimi et al. 2018). Additionally, a study estimated that the biological upper limit for the complete development of Sarcosenia chlorogaster was approximately 31 °C (Lecheta et al. 2015). The present data from adults are in agreement with the study results of Augul and Jassim (2009), in which adult longevity at laboratory temperatures of 24, 30, and 32 °C in both sexes of C. albiceps decreased with increasing temperatures. Moreover, females did not deposit eggs at 24 °C, while females reared at 30 °C deposited eggs. From the recorded data of that study, the authors concluded that rearing at 30 °C was optimal for C. albiceps.
The results of the present study are applicable to real-life scenarios because Jazan is among the tropical cities with average temperature 30.7 ± 2.28 °C within the range of 23.0–35.1 °C (Al-Mekhlafi et al. 2021). Hence, the results of the present study confirmed the optimal rearing for C. albiceps at 30 °C and in line with that discussed in previous studies. In Nigeria, Ekanem and Dike (2010) identified C. albiceps and other flies at temperatures of 28.6 °C (unshaded area) and 26.5 °C (shaded area). Additionally, in South Africa, C. albiceps and others were recorded at a temperature of 30.4 °C (Richards et al. 2009). C. albiceps is a cosmopolitan species that is active in different regions with a similar temperature range, making it possible to compare its behavior in different locations.
Conclusions
The results showed an obvious inverse relation between developmental stage and rearing temperature, as the developmental duration in each insect stage decreased with increasing rearing temperatures. The shortest developmental durations were recorded at 30 °C, and the longest developmental durations were recorded at 20 °C in the larval, pupal, and adult stages. Hence, insect laboratory colonization can serve as a basis for different entomological studies, and determination of the biological aspects of insects is useful in forensic entomology to guide the interpretation of insect evidence. However, it is important to be cautious when applying insect data as evidence in forensic investigations.
Availability of data and materials
All data generated or analyzed during this study are included in this article.
Abbreviations
- JUMRC:
-
Medical Research Center
- RH:
-
Relative humidity (%)
- PMI:
-
Postmortem interval
References
Adams ZJ, Hall MJ (2003) Methods used for the killing and preservation of blowfly larvae, and their effect on post-mortem larval length. Forensic Sci Int 138:50–61
Akbarzadeh K, Wallman JF, Sulakova H, Szpila K (2015) Species identification of Middle Eastern blowflies (Diptera: Calliphoridae) of forensic importance. Parasitol Res 114:1463–1472
Alahmed A (2004) Myiasis in sheep farms in Riyadh Region, Saudi Arabia. J Egypt Soc Parasitol 34:153–160
Alahmed A, Al Dawood A, Kheir S (2006) Seasonal activity of flies causing myiasis in livestock animals using sticky traps baited with swormlure-4 in Riyadh region, Saudi Arabia. Sci J King Faisal Univ (Basic and Applied Sciences) 7:1427H
Al-Khalifa MS, Mashaly AM, Al-Qahtni AH (2020) Insect species colonized indoor and outdoor human corpses in Riyadh, Saudi Arabia. J King Saud Univ-Sci 32:1812–1817
Al-Mekhlafi HM, Madkhali AM, Ghailan KY, Abdulhaq AA, Ghzwani AH, Zain KA, Atroosh WM, Alshabi A, Khadashi HA, Darraj MA, Eisa ZM (2021) Residual malaria in Jazan region, southwestern Saudi Arabia: the situation, challenges and climatic drivers of autochthonous malaria. Malar J 20(1):315. https://doi.org/10.1186/s12936-021-03846-4 PMID: 34256757; PMCID: PMC8276496
Al-Shareef LA, Al-Qurashi SI (2016) Study of some biological aspects of the blowfly Chrysomya albiceps (Wiedemann 1819)(Diptera: Calliphoridae) in Jeddah, Saudi Arabia. Egypt J Forensic Sci 6:11–16. https://doi.org/10.1016/j.ejfs.2015.06.003 (https://www.sciencedirect.com/science/article/pii/S2090536X15000507?via%3Dihub)
Amendt J, Campobasso CP, Gaudry E, Reiter C, LeBlanc HN, Hall MJ (2007) Best practice in forensic entomology—standards and guidelines. Int J Leg Med 121:90–104
Amendt J, Richards CS, Campobasso CP, Zehner R, Hall MJ (2011) Forensic entomology: applications and limitations. Forensic Sci Med Pathol 7:379–392
Anderson GS (2000) Minimum and maximum development rates of some forensically important Calliphoridae (Diptera). J Forensic Sci 45:824–832
Augul R, Jassim S (2009) Study of some biological and ecological aspects of the fly Chrysomya albiceps (Wiedemann)(Diptera; Calliphoridae). J al-anbar Univ Pure Sci 3:1–4
Baia TC, Campos A, Wanderley BMS, Gama RA (2016) The effect of flunitrazepam (Rohypnol®) on the development of Chrysomya megacephala (Fabricius, 1794) (Diptera: Calliphoridae) and its implications for forensic entomology. J Forensic Sci 61:1112–1115
Bansode S, More V, Zambare S (2016) Effect of different constant temperature on the life cycle of a fly of forensic importance Lucilia cuprina. Entomol Ornithol Herpetol 5:2161–0983
Beuter L, Mendes J (2013) Development of Chrysomya albiceps (Wiedemann) (Diptera: Calliphoridae) in different pig tissues. Neotrop Entomol 42:426–430
Bharti M (2009) Studies on life cycles of forensically important flies, Calliphora vicina and Musca domestica nebulo at different temperatures. J Entomol Res 33:273–275 ISSN: 0378-9519
Bohem ASZ (2015) Influence of constant and changing temperatures on the larval development of Calliphora vicina (Diptera: Calliphoridae). Acta Soc Zool Bohem 79:149–154 ISSN 1211-376X
Bosly HA (2010) Prevalence of dipterous flies with veterinary importance in selected sheep’s farms and slaughter houses in Jazan, Saudi Arabia. Egypt Acad J Biol Sci A, Entomol 3:63–73 ISSN: 1687–8809. http://entomology.eajbs.eg.net/pdf/vol3-num2/8.pdf
Bruinsma G, Weisburd D (eds) (2014) Forensic science, in: Encyclopedia of Criminology and Criminal Justice. Springer New York, New York, p 1754. https://doi.org/10.1007/978-1-4614-5690-2_100262
Campobasso CP, Di Vella G, Introna F (2001) Factors affecting decomposition and Diptera colonization. Forensic Sci Int 120:18–27
Campobasso CP, Introna F (2001) The forensic entomologist in the context of the forensic pathologist’s role. Forensic Sci Int 120:132–139
Catts E (1992) Problems in estimating the postmortem interval in death investigations. J Agric Entomol (USA) 9:245–255
Dawah HA, Abdullah MA, Ahmad SK (2019) An overview of the Calliphoridae (Diptera) of Saudi Arabia with new records and updated list of species. J Entomol Res Soc 21:65–93 http://entomol.org/journal/index.php/JERS/article/view/1477
Ekanem MS, Dike MC (2010) Arthropod succession on pig carcasses in southeastern Nigeria. Pap Avulsos Zool 50:561–570. https://doi.org/10.1590/s0031-10492010003500001
Goddard J, Lago PK (1985) Notes on blow fly (Diptera: Calliphoridae) succession on carrion in Northern Mississippi. J Entomol Sci 20:312–317
Gomes L, Godoy WAC, Von Zuben CJ (2006) A review of postfeeding larval dispersal in blowflies: implications for forensic entomology. Naturwissenschaften 93:207
Gomes L, Gomes G, Von Zuben CJ (2009) The influence of temperature on the behavior of burrowing in larvae of the blowflies, Chrysomya albiceps and Lucilia cuprina, under controlled conditions. J Insect Sci 9:14
Gomes L, Von Zuben CJ (2005) Postfeeding radial dispersal in larvae of Chrysomya albiceps (Diptera: Calliphoridae): implications for forensic entomology. Forensic Sci Int 155:61–64
Grassberger M, Friedrich E, Reiter C (2003) The blowfly Chrysomya albiceps (Wiedemann) (Diptera: Calliphoridae) as a new forensic indicator in Central Europe. Int J Leg Med 117:75–81
Grassberger M, Reiter C (2001) Effect of temperature on Lucilia sericata (Diptera: Calliphoridae) development with special reference to the isomegalenand isomorphen-diagram. Forensic Sci Int 120:32–36
Greenberg B, Kunich JC (2002) Entomology and the law: flies as forensic indicators, 1st edition. Cambridge University Press. ISBN: 0521809150.
Greenberg B, Povolny D (2019) Chapter 3. Bionomics of flies. In: In Flies and Disease. Princeton University Press, Princeton. https://doi.org/10.1515/9780691196763-005
Gullan PJ, Cranston PS (2014) The insects: an outline of entomology. Wiley-Blackwell. ISBN: 978-1-118-84615-5.
Hall M, Wall R (1995). Myiasis of Humans and Domestic Animals. Adv Parasitol 35:257–334. https://doi.org/10.1016/S0065-308X(08)60073-1
Hückesfeld S, Niederegger S, Schlegel P, Heinzel H-G, Spieß R (2011) Feel the heat: the effect of temperature on development, behavior and central pattern generation in 3rd instar Calliphora vicina larvae. J Insect Physiol 57:136–146
Klekovska D, Slavevska-Stamenković V, Smiljkov S, Hinić J, Rebok K, Janeska B (2017) Forensic use of Chrysomya albiceps (Wiedemann, 1819): the first cases indicating postmortem interval for human corpses in Republic of Macedonia. J Entomol Zool Stud 5:320–323
Kotrba M, Reckel F, Grunwald J, Balke M, Swoboda S (2012) Chrysomya albiceps–a forensically important blow fly new for Bavaria (Diptera: Calliphoridae). Mitteilungen der münchener entomologischen Gesellschaft 102:99–103
Lane RP (1975) An investigation into blowfly (Diptera: Calliphoridae) succession on corpses. J Nat Hist 9:581–588. https://doi.org/10.1080/00222937500770461
Laurence B (1981) Geographical expansion of the range of Chrysomya blowflies. Expansión del ámbito geográfico de las moscas califóridas Chrysomya Transactions of the Royal Society of Tropical Medicine and Hygiene 75:130–131
Lecheta MC, Thyssen PJ, Moura MO (2015) The effect of temperature on development of Sarconesia chlorogaster, a blowfly of forensic importance. Forensic Sci Med Pathol 11:538–543
Li L, Wang Y, Wang J, Ma M, Lai Y (2016) Temperature-dependent development and the significance for estimating postmortem interval of Chrysomya nigripes Aubertin, a new forensically important species in China. Int J Leg Med 130:1363–1370
Makovetskaya K, Verves Y (2018) First records of Chrysomya albiceps (Wiedemann, 1819) (Diptera: Calliphoridae) in Belarus with analysis of distribution of this species in Europe DIPTERON. Bull Dipterol Sect Polish Entomol Soc 34:60–67. https://doi.org/10.5281/zenodo.2501558
Mashaly A, Alajmi R, Mustafa AE-Z, Rady A, Alkhedir H (2017) Species abundance and identification of forensically important flies of Saudi Arabia by DNA barcoding. J Med Entomol 54:837–843
Mendonça PM, Santos-Mallet JRD, Queiroz MMDC (2010) Ultramorphological characteristics of immature stages of Chrysomya albiceps (Wiedemann 1819) (Diptera: Calliphoridae), a fly specie of forensic importance. Microsc Res Tech 73:779–784
Morgan RM (2019) Forensic science. The importance of identity in theory and practice. Forensic Sci Int: Synergy 1:239–242. https://doi.org/10.1016/j.fsisyn.2019.09.001
Myskowiak J-B, Doums C (2002) Effects of refrigeration on the biometry and development of Protophormia terraenovae (Robineau–Desvoidy) (Diptera: Calliphoridae) and its consequences in estimating post-mortem interval in forensic investigations. Forensic Sci Int 125:254–261
Pujol-Luz JR, Barros-Cordeiro KB (2012) Intra-puparial development of the females of Chrysomya albiceps (Wiedemann) (Diptera, Calliphoridae). Rev Bras Entomol 56:269–272
Queiroz MMC (1996) Temperature requirements of Chrysomya albiceps (Wiedemann, 1819) (Diptera, Calliphoridae) under laboratory conditions. Mem Inst Oswaldo Cruz 91:785–788
Rabêlo KC, Thyssen PJ, Salgado RL, Araújo MS, Vasconcelos SD (2011) Bionomics of two forensically important blowfly species Chrysomya megacephala and Chrysomya putoria (Diptera: Calliphoridae) reared on four types of diet. Forensic Sci Int 210:257–262
Richards CS, Paterson ID, Villet MH (2008a) Estimating the age of immature Chrysomya albiceps (Diptera: Calliphoridae), correcting for temperature and geographical latitude. Int J Leg Med 122:271–279
Richards CS, Price BW, Villet MH (2008b) Thermal ecophysiology of seven carrion-feeding blowflies in Southern Africa. Entomol Exp Appl 131:11–19
Richards CS, Price BW, Villet MH (2009) Thermal ecophysiology of seven carrion-feeding blowflies in Southern Africa. Entomol Exp Appl 131:11–19. https://doi.org/10.1111/j.1570-7458.2009.00824.x
Salazar-Souza M, Couri MS, Aguiar VM (2018) Chronology of the intrapuparial development of the blowfly Chrysomya albiceps (Diptera: Calliphoridae): application in forensic entomology. J Med Entomol 55:825–832
Salazar-Souza M, de Alcantara Azevedo WT, Couri MS, Aguiar VM (2019) Diets of animal origin and their influence on the development of the immatures of Chrysomya albiceps (Diptera: Calliphoridae): implications for forensic entomology. Austral Entomol 58:638–645
Saleh V, Soltani A, Dabaghmanesh T, Alipour H, Azizi K, Moemenbellah-Fard MD (2014) Mass rearing and life table attributes of two cyclorrhaphan flies, Lucilia sericata Meigen (Diptera: Calliphoridae) and Musca domestica L. (Diptera: Muscidae) under laboratory conditions. Aust J Entomol 11:291–298
Salimi M, Rassi Y, Oshaghi M, Chatrabgoun O, Limoee M, Rafizadeh S (2018) Temperature requirements for the growth of immature stages of blowflies species, Chrysomya albiceps and Calliphora vicina (Diptera: Calliphoridae) under laboratory conditions. Egypt J Forensic Sci 8:28. https://doi.org/10.1186/s41935-018-0060-z
Scholtz CH, Caveney S (1992) Daily biphasic behaviour in keratin-feeding desert trogid beetles in relation to climate. Ecol Entomol 17:155–159. https://doi.org/10.1111/j.1365-2311.1992.tb01173.x
Setyaningrum H, Al Dhafer HM (2014) The Calliphoridae the blow flies (Diptera: Oestroidea) of Kingdom of Saudi Arabia. Egypt Acad J Biol Sci 7:49–139
Shiravi A, Mostafavi R, Akbarzadeh K, Oshaghi MA (2011) Temperature requirements of some common forensically important blow and flesh flies (Diptera) under laboratory conditions. Iran J Arthropod-borne Dis 5:54
Stevens JR, Wallman JF (2006) The evolution of myiasis in humans and other animals in the Old and New Worlds (part I): phylogenetic analyses. Trends Parasitol 22:129–136
Thyssen PJ, de Souza CM, Shimamoto PM, de Britto ST, Moretti TC (2014) Rates of development of immatures of three species of Chrysomya (Diptera: Calliphoridae) reared in different types of animal tissues: implications for estimating the postmortem interval. Parasitol Res 113:3373–3380
Vasconcelos SD, Costa DL, Oliveira DL (2019) Entomological evidence in a case of a suicide victim by hanging: first collaboration between entomologists and forensic police in north-eastern Brazil. Aust J Forensic Sci 51:231–239
Vasconcelos SD, Cruz TM, Salgado RL, Thyssen PJ (2013) Dipterans associated with a decomposing animal carcass in a rainforest fragment in Brazil: notes on the early arrival and colonization by necrophagous species. J Insect Sci 13:145
Vélez MC, Wolff M (2008) Rearing five species of Diptera (Calliphoridae) of forensic importance in Colombia in semicontrolled field conditions. Papéis Avulsos de Zoologia 48:41–47
Yang Y et al (2016) Developmental times of Chrysomya megacephala (Fabricius) (Diptera: Calliphoridae) at constant temperatures and applications in forensic entomology. J Forensic Sci 61:1278–1284
Zhang Y, Wang Y, Sun J, Hu G, Wang M, Amendt J, Wang J (2019) Temperature-dependent development of the blow fly Chrysomya pinguis and its significance in estimating postmortem interval. R Soc Open Sci 6:190003
Acknowledgments
The author wishes to appreciate the Forensic Center in Jazan City, Animal Research Center of the Jazan University Medical Research Center (JUMRC), and Biology Department, Faculty of Science, Jazan University.
Funding
Not applicable (no funding).
Author information
Authors and Affiliations
Contributions
The author declares that this research was designed experimentally and procedurally and written by the author. The author has read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The Ethical approval applied and provided within submission Reference No.: REC-43/02/016.
Consent for publication
Not applicable.
Competing interests
The author declares that she has no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bosly, H.A.EK. Development of Chrysomya albiceps (Wiedemann, 1819) (Diptera: Calliphoridae) from the Jazan region of Southwest Saudi Arabia under different laboratory temperatures: applications in forensic entomology. Egypt J Forensic Sci 11, 30 (2021). https://doi.org/10.1186/s41935-021-00245-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41935-021-00245-3