Abstract
Background
Previous studies on magnetic resonance neurography (MRN) found different patterns of structural nerve damage in type 1 diabetes (T1D) and type 2 diabetes (T2D). Magnetization transfer ratio (MTR) is a quantitative technique to analyze the macromolecular tissue composition. We compared MTR values of the sciatic nerve in patients with T1D, T2D, and healthy controls (HC).
Methods
3-T MRN of the right sciatic nerve at thigh level was performed in 14 HC, 10 patients with T1D (3 with diabetic neuropathy), and 28 patients with T2D (10 with diabetic neuropathy). Results were subsequently correlated with clinical and electrophysiological data.
Results
The sciatic nerve’s MTR was lower in patients with T2D (0.211 ± 0.07, mean ± standard deviation) compared to patients with T1D (T1D 0.285 ± 0.03; p = 0.015) and HC (0.269 ± 0.05; p = 0.039). In patients with T1D, sciatic MTR correlated positively with tibial nerve conduction velocity (NCV; r = 0.71; p = 0.021) and negatively with hemoglobin A1c (r = − 0.63; p < 0.050). In patients with T2D, we found negative correlations of sciatic nerve’s MTR peroneal NCV (r = − 0.44; p = 0.031) which remained significant after partial correlation analysis controlled for age and body mass index (r = 0.51; p = 0.016).
Conclusions
Lower MTR values of the sciatic nerve in T2D compared to T1D and HC and diametrical correlations of MTR values with NCV in T1D and T2D indicate that there are different macromolecular changes and pathophysiological pathways underlying the development of neuropathic nerve damage in T1D and T2D.
Trial registration
https://classic.clinicaltrials.gov/ct2/show/NCT03022721. 16 January 2017.
Relevance statement
Magnetization transfer ratio imaging may serve as a non-invasive imaging method to monitor the diseases progress and to encode the pathophysiology of nerve damage in patients with type 1 and type 2 diabetes.
Key points
• Magnetization transfer imaging detects distinct macromolecular nerve lesion patterns in diabetes patients.
• Magnetization transfer ratio was lower in type 2 diabetes compared to type 1 diabetes.
• Different pathophysiological mechanisms drive nerve damage in type 1 and 2 diabetes.
Graphical Abstract

Similar content being viewed by others
Background
Distal symmetric neuropathy (DSN) is one of the most frequent complications of diabetes, affecting about half of the patients during the course of the disease [1]. Despite its economical and epidemiological impact, the pathophysiological mechanisms of DSN in diabetes mellitus type 1 (T1D) and diabetes mellitus type 2 (T2D) remain uncertain [2, 3]. However, the current body of literature provides evidence that distinct pathophysiological pathways are responsible for the development of DSN in patients with T1D and T2D [4, 5].
Since experimental animal models of diabetes mellitus fail to replicate all the complex pathophysiological mechanisms of human disease in one model [6], in vivo studies using magnetic resonance neurography (MRN) offer a promising technique to gain new insights into the pathophysiology of both entities. So far, diffusion-weighted and T2-weighted as well as dynamic contrast-enhanced sequences have been used to examine DSN [7,8,9,10,11,12,13]. In that regard, diffusion weighted imaging in terms of diffusion tensor imaging MRN embodies one of the main sequences in studies of peripheral nerve imaging in general [14, 15], and specifically in patients with T1 and T2D [9, 11, 16]. This approach, among others, enabled the detection of novel biomarkers of peripheral nerve damage in patients with T2D, such as high-sensitivity Troponin T [17].
Meanwhile, the application of T2-weighted MRN sequences demonstrated that the dominating type of fascicular nerve lesion in DSN patients differs between T1D and T2D [7]. While the amount of nerve lesions in T1D was associated with poor glycemic control, nerve lesions of T2D were associated with parameters of lipid metabolism [7], which is in line with the results of large clinical trials in patients with T1D and T2D [4, 5]. Since the exact molecular mechanisms behind the development of DSN in T1D and T2D remain unknown to date, the application of magnetization transfer ratio (MTR) imaging may offer new insights into the pathophysiology of DSN by assessing the nerves macromolecular composition in vivo. Due to the short T2 time of protons bound to macromolecules, these protons do not contribute to the signal of conventional MRI sequences [18].
However, MTR imaging enables analysis of the composition of macromolecules in a specific tissue by visualizing the concentration of protons bound to macromolecules like myelin [19]. MTR imaging uses two identical MR sequences, one with and one without an off-resonance saturation pulse, that are applied to selectively saturate protons bound to macromolecules. This can be achieved because the bound protons possess a broader bandwidth of Larmor frequencies compared to free-water protons [20]. After highly selective excitation of the protons bound to macromolecules, spin exchange processes between these protons and the free-water protons result in a decreased longitudinal magnetization, which can be measured and then used to calculate MTR as the quotient of the difference between signal intensities without and with off-resonance saturation and the signal intensity without off-resonance saturation [21].
Previous studies applied MTR imaging in several diseases of the central and peripheral nervous system, the former especially in patients with multiple sclerosis [22]. In patients with multiple sclerosis, a decline of white matter MTR is associated with increased pathomorphological and neurological damage. Meanwhile, in peripheral nerves, MTR imaging was not able to demonstrate an age associated decline of MTR [23] but also that higher levels of neurological deficits are accompanied by lower MTR values thereby demonstrating the value of MTR as a quantitative imaging biomarker in peripheral nerve disorders [24,25,26]. As MTR allows the quantitative assessment of cerebral and nerval structures, previous studies demonstrated the detection of subclinical pathomorphological alteration in the peripheral and central nervous system [22, 26].
MTR imaging of peripheral nerves has not been performed in T1D and T2D patients yet. The objective of this study was therefore to investigate potential differences of the sciatic nerve’s MTR and correlation with clinical and electrophysiological parameters in order to evaluate the contribution of potential risk factors to nerve damage in in both entities.
Methods
Study design and participants
This study was approved by the ethics committee of Heidelberg University Hospital (HEIST-DiC, clinicaltrials.gov identifier NCT03022721, local ethics number S-383/2016), and written informed consent was obtained from all participants. All participants were screened and recruited at the outpatient clinic of the Department of Endocrinology at Heidelberg University Hospital where all clinical, serological, and electrophysiological examinations took place. MRN and image processing was performed in the Department of Neuroradiology at Heidelberg University Hospital. Fifty-two study participants (14 healthy controls (HC), 10 T1D patients, and 28 T2D patients) were enrolled in this prospective single-center study between June 2018 and March 2020. HC were characterized by the absence of any kind of medical condition predisposing for peripheral neuropathy and the absence of any systemic or chronic diseases. Inclusion and exclusion criteria are summarized in Table 1.
In line with Gibbon’s criteria [27], diagnosis of DSN was given with a neuropathy disability score (NDS) ≥ 3. Accordingly, four of the T1D patients and ten of the T2D patients were diagnosed with DSN. To preclude differences of sciatic nerve MTR to be caused by typical confounding factors of nerve damage and changes of MTR, groups were matched for hemoglobin A1c (HbA1c), sex, body mass index (BMI), and age [28], so that there were no significant differences between the three groups regarding these parameters.
Clinical and electrophysiologic examination
A detailed medical history was taken for every participant including an examination of neuropathic symptoms comprising the NDS and the neuropathy severity scale (NSS) as issued by the German Diabetes Association [29]. Blood was drawn in fasting state followed by an immediate analysis by the central laboratory of Heidelberg University Hospital. All electrophysiological studies were conducted on the patients’ right leg by two trained medical technical assistants with more than 6 years of experience in electrophysiological assessments on patients with diabetes maintaining a skin temperature of 32 °C throughout the examination. The electrophysiological examination included the assessment of nerve conduction velocities (NCVs) of tibial, peroneal, and sural nerve, distal motor latencies (DMLs) of the tibial and peroneal nerve, compound muscle action potentials (CMAPs) of the tibial and peroneal nerve, and sensory nerve action potentials of the sural nerve.
MRI protocol
High-resolution 3-T MRN of the right thigh (Magnetom Tim TRIO, Siemens Healthineers, Erlangen, Germany) was performed at the Department of Neuroradiology at Heidelberg University Hospital using a 15-channel transmit-receive extremity coil. The applied sequences were centered to the sciatic nerve bifurcation at distal thigh level and applied according to the following protocol.
-
1.
One axial high resolution T2-weighted turbo spin echo two-dimensional sequence with spectral fat saturation of the right mid-thigh and the following parameters: repetition time 5,970 ms; echo time 55 ms; field of view 160 × 160 mm2; matrix size 512 × 512; slice thickness 4 mm; interslice gap 0.35 mm; voxel size 0.3 × 0.3 × 4.0 mm3, number of excitations: 3; images: 24.
-
2.
Two axial proton density-weighted gradient echo sequences, with and without an off-resonance saturation pulse (Gaussian envelop, duration 9,984 μs, frequency offset 1,200 Hz), applied with the exact same field of view and the exact same slice position and the following parameters: repetition time 46 ms; echo time 12.3 ms; flip angle 7°; field of view 200 × 176 mm2; matrix size 256 × 256; slice thickness 4 mm, bandwidth 370 Hz/pixel; distance factor 20%; voxel size 1.3 × 1.3 × 4.0 mm3; slices: 24, acquisition time 2:17 s:min.
Image analysis
Image pseudonymization was conducted before analysis and observers were blinded to all clinical data. To ensure precise anatomical segmentation of the sciatic nerve as the region of interest, manual segmentation was performed on the axial T2-weighted sequence by two trained neuroradiologists (C.M.M., F.T.K.) with 5 and 10 years of experience in MRN, respectively, using ImageJ [30]. A custom-written MATLAB code (MathWorks, Natick, MA, USA, R2020b) was applied to conduct semiautomatic coregistration of the created regions of interest to the MTR images with and without off-resonance saturation using affine transformations [31], and to calculate MTR of the sciatic nerve as:
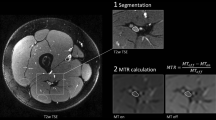
where S0 and S1 represent the signal intensity without and with off-resonance saturation pulse, respectively. MTR was first calculated separately for each image slice whereby only the ten central image slices of each image stack were included into analysis to avoid artifacts or inhomogeneities caused by the B1 field. Values were then averaged to obtain a mean value for each patient. The process of image coregistration is illustrated in Fig. 1.
Process of nerve segmentation and image coregistration. a Manual segmentation of the sciatic nerve was performed on an axial T2-weighted sequence with spectral fat-saturation (T2w) of the right thigh. Regions of interest were then coregistered with magnetization transfer ratio (MTR) sequences with (b) and without (c) off-resonance saturation pulse
Statistical analysis
MATLAB 7.14.0.0739 (R2012a) and GraphPad Prism 7 were used for all statistical analyses. To test for Gaussian normal distribution, the D’Agostino-Pearson omnibus normality test was applied. If a Gaussian normal distribution was given, t-tests were used for comparisons of two groups, one-way ANOVAs were used for comparisons of more than two groups, and Pearson correlation coefficients were used for correlation analysis. If data did not follow Gaussian distribution, the Mann–Whitney U test was used for comparisons of two groups, and the Kruskal–Wallis test with post hoc Dunn correction was used for multiple comparisons of more than two groups, and nonparametric Spearman correlation was used for correlation analysis. The level of significance was defined at p < 0.05 for all tests.
Results
Group comparisons of clinical and demographical data
Of the 52 subjects who took part in this study, 14 were HC (8 women, 6 men), 10 were T1D patients (5 women, 5 men), and 28 T2D patients (14 women, 14 men). Between the groups, there were no significant differences for age, sex, and BMI (p ≥ 0.086). In addition, no differences for NDS and NSS scores between HC and patients with T1D and T2D could be found. Disease duration was longer in T1D patients than in T2D patients (29.9 years ± 18.3 [mean ± standard deviation] versus 9.7 years ± 9.3, respectively; p = 0.002).
Group comparisons of serological and electrophysiological parameters
HbA1c was higher in T1D patients compared to T2D patients (58.6 mmol/mol ± 9.8 [mean ± standard deviation] versus 50.3 mmol/mol ± 8.7; p = 0.009), while both T1D and T2D patients had higher HbA1c values than HC (37.0 mmol/mol ± 6.0; p < 0.001 for both comparisons). Sural nerve conduction studies were incomplete in three T1D patients and in ten T2D patients due to severe DSN. The remaining measurements were complete. No differences could be found for sural NCV and SNAP, peroneal NCV, CMAP, and DML and for tibial NCV, CMAP, and DML (p ≥ 0.105).
Group comparison of MTR
T2D patients (0.211 ± 0.071 [mean ± standard deviation]) had significantly lower MTR values of the sciatic nerve than T1D patients (0.285 ± 0.027; p = 0.015) or HC (0.269 ± 0.053; p = 0.039). See Fig. 2 for graphic illustration of MTR values and Table 2 for a detailed summary of all group comparisons on demographic, electrophysiological, serological, and MTR data.
Group comparison of magnetization transfer ratio (MTR) of sciatic nerve in controls (MTR 0.269 ± 0.039, mean ± standard deviation) and patients with type 1 diabetes (T1D, MTR 0.285 ± 0.027) and type 2 diabetes (T2D, MTR 0.211 ± 0.071). MTR was lower in T2D patients compared to controls (p = 0.039) and to T1D (p = 0.015)
Correlation analysis of MTR values with clinical and demographical parameters
In T1D patients, no correlations could be found for age, duration of diabetes, or BMI. The negative correlation of MTR with NDS score did not reach statistical significance (r = -0.56; p = 0.094). In T2D patients, MTR correlated negatively with age (r = -0.38; p = 0.047). A negative correlation with BMI (r = -0.36; p = 0.061) did not reach statistical significance. In HC, a weak correlation was found between the sciatic nerve’s MTR with BMI (r = -0.52; p = 0.059), which did not reach statistical significance in HC. For HC, no further correlations for sciatic nerve’s MTR with the compiled demographical and clinical data were found. A detailed summary of the results of correlation analysis is given in Table 3.
Correlation analysis of MTR with serological and electrophysiological parameters
In patients with T1D, HbA1c correlated negatively with MTR (r = -0.63; p < 0.050; Fig. 3a). Furthermore, tibial NCV correlated with MTR (r = 0.71; p = 0.021; Fig. 3b), while a correlation for peroneal NCV did not reach statistical significance (r = 0.53; p = 0.118). In patients with T2D, sural (r = -0.50; p = 0.034), tibial NCV (r = -0.40; p < 0.050), and peroneal (r = -0.44; p = 0.031; Fig. 4a) correlated negatively with sciatic MTR. Partial correlation analysis controlled for age and BMI between the sciatic nerve’s MTR and the peroneal NCV (r = 0.51; p = 0.016) remained significant, while sural NCV (r = 0.49; p = 0.054) and tibial NCV (r = 0.34; p = 0.115) failed to reach statistical significance. No correlations could be found for HbA1c (Fig. 4b). There were no significant correlations of sciatic nerve’s MTR with the compiled data in HC (p ≥ 0.059).
Correlation analysis of magnetization transfer ratio (MTR) of sciatic nerve with serological and electrophysiological parameters in patients with type 1 diabetes (T1D). a Correlation of sciatic nerve’s MTR with HbA1c (r = -0.63; p < 0.050). b Correlation of sciatic nerve’s MTR with tibial nerve conduction velocity (NCV; r = -0.71; p = 0.021)
Correlation analysis of magnetization transfer ratio (MTR) of sciatic nerve with serological and electrophysiological parameters in patients with type 2 diabetes (T2D). a Correlation of peroneal nerve conduction velocity (NCV) with sciatic nerve’s MTR (r = -0.44; p = 0.031). b No correlations could be found between HbA1c with the sciatic nerve’s MTR (r = -0.17; p = 0.383)
Discussion
This study used 3-T MRN with MTR sequences to investigate potential differences of the sciatic nerve’s macromolecular composition between healthy controls and subjects with diabetes and to assess potential links of MTR parameters with clinical and electrophysiological parameters. The main findings were that (i) MTR of the sciatic nerve is lower in patients with T2D compared to patients with T1D and healthy controls; (ii) in T1D, MTR showed a positive correlation with tibial NCV, while, in T2D, MTR of the sciatic nerve correlated negatively with sural, peroneal, and tibial NCV; and (iii) in T1D, MTR correlated negatively with HbA1c, but not in T2D.
Our findings indicate that different pathophysiological mechanisms in T1D and T2D lead to distinct macromolecular changes in the peripheral nerves, which may be quantified with the use of MTR. In the context of different MRN lesion patterns in T1D and T2D DSN, with T2-weighted hyperintense nerve lesions linked to elevated glucose levels in T1D and T2-weighted hypointense lesions to lipid metabolism in T2D [7], this study supports the hypothesis that the underlying pathophysiological mechanisms of DSN differ between T1D and T2D [4, 5, 7]. In that regard, we only found HbA1c to be associated with the MTR of the sciatic nerve in patients with T1D, but not in T2D. We found HbA1c negatively and NCV of tibial nerve positively associated with MTR of sciatic nerve in patients with T1D, while NDS showed a trend of negative correlation with the sciatic nerve’s MTR (p = 0.094). These findings are in line with the fact that glycemic control is a key factor in preventing and treating DSN in T1D [4, 5] and that hyperglycemia is one of the main contributors to nerve damage in T1D [7].
Higher MTR values of the sciatic nerve were associated with structural nerve integrity in T1D patients. Meanwhile, in patients with T2D, we could not reproduce this finding, which agrees with the finding of previous studies that factors apart from hyperglycemia are the main contributors to nerve damage in patients with T2D [4, 5, 7]. However, the fact that fatty nerve lesions have been shown to predominate in T2D patients [7] may explain that MTR of the sciatic nerve is decreased in patients with T2D compared to patients with T1D and HC, as fatty tissues exhibit a low magnetization transfer [18, 32, 33]. It will be interesting to investigate which mechanisms are responsible for a similar sciatic MTR of HC and patients with T1D, especially because a previous study [26] was able to demonstrate that sciatic MTR was sensitive to discriminate between asymptomatic carriers of the mutant transthyretin gene causing hereditary transthyretin amyloidosis and HC.
The hypothesis, that nerve damage in T1D and T2D underlie different pathophysiological mechanisms, is further supported by the finding of opposed correlations of NCV with MTR of the sciatic nerve in T1D and T2D patients, respectively. The fact that MTR of the sciatic nerve showed correlations with NCV of the tibial and peroneal nerve indicates that MTR is a valuable marker of structural nerve integrity in T1D and T2D, as electrophysiological studies are considered to be the gold standard of assessing structural nerve integrity in vivo [34]. In T1D, MTR showed a positive correlation with tibial NCV, which agrees with the finding that lower MTR values were associated with a higher level of neurological deficits in patients with Charcot-Marie-Tooth disease, in hereditary transthyretin amyloidosis, and 5q spinal muscular atrophy [24,25,26]. As it is known that the lowering of NCV is generally an indicator of demyelinating nerve injury [35], one could hypothesize that a lowering of MTR in T1D might partly represent demyelinating nerve damage in T1D. Since this study found sciatic nerve MTR to be decreased in T2D compared to T1D and healthy controls, we hypothesize that the process of demyelination—one hallmark of DSN [36]—might be more pronounced in T2D. These assumptions are supported by previous studies on MTR of the central nervous system which proved a decrease of MTR to be a marker of demyelination [37, 38]. Conversely, the MTR of the sciatic nerve of amphibians [39] and the MTR of the optic nerve in patients with MS and optic neuritis [40] was associated not only with markers of demyelination but also of axonal nerve damage. These hypotheses remain to be demonstrated as we did not obtain specimens of peripheral nerves.
In contrast, our findings for T2D patients appear to be contradictory: while MTR is already lowered in T2D compared to HC, a further decrease would supposedly result in higher NCVs. One potential explanation for this may be that previous studies have shown that a low amount of structural nerve lesions in T2D patients does not cause changes that can be electrophysiologically detected, and that a higher amount of nerve lesions is required for patients to become symptomatic [41]. Thus, it is well possible that, in the T2D group, subclinical structural nerve damage is more present compared to the T1D group.
Since MTR has been shown to decrease with age and higher BMI in healthy subjects [23, 28], participants were matched for age and BMI to minimize the confounding impact of age. Also, no differences could be found between the T1D and T2D participants for NDS/NSS scores, HbA1c values, and electrophysiological parameters, rendering it unlikely that the observed differences between MTR in T1D and T2D are caused by a different severity of nerve damage or a difference in typical cofounding factors. The fact that T1D patients were diagnosed with disease a longer time ago than patients with T2D, and that MTR was only lower in T2D compared to T1D and HC, while T1D and HC did not differ, supports the hypothesis that different pathological pathways drive nerve damage in T1D and T2D. If nerve damage in T1D and T2D originated from the same mechanisms, we would expect MTR of T1D patients to be lower than that of T2D patients, since disease duration in T1D patients was significantly longer.
Our study is limited by several factors.
The cross-sectional design of this study does not allow a predictive assessment of the reported findings, which will be part of ongoing research. Moreover, future studies should correlate and compare T2-weighted hyperintense and hypointense nerve lesions with MTR and other MRN markers of DSN in T1D and T2D, to possibly gain more insights on the different nature of macromolecular changes and lesion types in both entities. It will especially be interesting to examine the outlined paradox of a lower MTR and its negative association with NCV in T2D.
The interpretation of the study results is also compromised by a lack of histological nerve samples. Subsequently, future research should focus on trying to correlate MRN findings with histological findings to explore these mechanisms and decode which histomorphological changes lead to alterations of proton spin density, T2 relaxation time, and T2-weighted hyperintense and hypointense nerve lesions as well as changes of MTR.
Another limitation represents the relatively small sample size of the cohort, which is why we cannot preclude all potential confounders through multivariate analysis. However, the patient groups did not show significant differences regarding important potential confounding factors such as age, BMI, and sex, and partial correlation analysis controlled for age and BMI, both potential confounders of MTR, was performed if needed. Also, the sample size did not allow conducting a comprehensive analysis for patients with and without DSN in T1D and T2D. Yet, patient groups did not differ regarding NSS, NDS, and parameters of nerve conductions studies, while it is also known that DSN represents a continuous process of accumulating nerve damage [41].
Another potential limitation is that only a relatively short segment of the sciatic nerve at the level of the distal thigh was examined. However, previous studies on MRN were able to demonstrate that nerve fiber damage predominates at the distal thigh and that MTR does not differ between proximal and distal thigh as well as lower leg [7, 23].
In summary, this study demonstrates that MTR imaging may provide a new imaging biomarker of structural damage/integrity of peripheral nerves in T1D and T2D. Our results underline that patterns of structural nerve damage and accompanied macromolecular changes differ in T1D and T2D patients. Consequently, these finding emphasize that different pathophysiological pathways drive nerve damage in T1D and T2D. Longitudinal studies applying MTR imaging are needed to decode these pathways in T1D and T2D.
Availability of data and materials
The datasets generated during and/or analyzed during the current study are not publicly available due to reasons of patient data protection but are available from the corresponding author upon reasonable request.
Abbreviations
- BMI:
-
Body mass index
- CMAP:
-
Compound motor action potential
- DML:
-
Distal motor latency
- DSN :
-
Distal symmetric neuropathy
- Hb1Ac:
-
Hemoglobin A1c
- HC:
-
Healthy control
- MRN:
-
Magnetic resonance neurography
- MTR:
-
Magnetization transfer ratio
- NCV:
-
Nerve conduction velocity
- NDS:
-
Neuropathy disability score
- NSS:
-
Neuropathy severity scale
- SNAP:
-
Sural nerve action potential
- T1D:
-
Type 1 diabetes mellitus
- T2D:
-
Type 2 diabetes mellitus
References
Hicks CW, Selvin E (2019) Epidemiology of peripheral neuropathy and lower extremity disease in diabetes. Curr Diab Rep 19:86. https://doi.org/10.1007/s11892-019-1212-8
Alleman CJ, Westerhout KY, Hensen M et al (2015) Humanistic and economic burden of painful diabetic peripheral neuropathy in Europe: a review of the literature. Diabetes Res Clin Pract 109:215–225. https://doi.org/10.1016/j.diabres.2015.04.031
Feldman EL, Callaghan BC, Pop-Busui R et al (2019) Diabetic neuropathy. Nat Rev Dis Primers 5:41. https://doi.org/10.1038/s41572-019-0092-1
Callaghan BC, Hur J, Feldman EL (2012) Diabetic neuropathy: one disease or two? Curr Opin Neurol 25:536–541. https://doi.org/10.1097/WCO.0b013e328357a797
Callaghan BC, Little AA, Feldman EL, Hughes RA (2012) Enhanced glucose control for preventing and treating diabetic neuropathy. Cochrane Database Syst Rev 6:CD007543. https://doi.org/10.1002/14651858.CD007543.pub2
King A, Bowe J (2016) Animal models for diabetes: understanding the pathogenesis and finding new treatments. Biochem Pharmacol 99:1–10. https://doi.org/10.1016/j.bcp.2015.08.108
Jende JME, Groener JB, Oikonomou D et al (2018) Diabetic neuropathy differs between type 1 and type 2 diabetes: insights from magnetic resonance neurography. Ann Neurol 83:588–598. https://doi.org/10.1002/ana.25182
Pham M, Oikonomou D, Baumer P et al (2011) Proximal neuropathic lesions in distal symmetric diabetic polyneuropathy: findings of high-resolution magnetic resonance neurography. Diabetes Care 34:721–723. https://doi.org/10.2337/dc10-1491
Vaeggemose M, Pham M, Ringgaard S et al (2017) Diffusion tensor imaging MR neurography for the detection of polyneuropathy in type 1 diabetes. J Magn Reson Imaging 45:1125–1134. https://doi.org/10.1002/jmri.25415
Jende JME, Mooshage C, Kender Z et al (2022) Sciatic nerve microvascular permeability in type 2 diabetes decreased in patients with neuropathy. Ann Clin Transl Neurol 9:83–0840. https://doi.org/10.1002/acn3.51563
Jende JME, Mooshage C, Kender Z et al (2021) Magnetic resonance neurography reveals smoking-associated decrease in sciatic nerve structural integrity in type 2 diabetes. Front Neurosci 15:811085. https://doi.org/10.3389/fnins.2021.811085
Mooshage CM, Schimpfle L, Kender Z et al (2023) Diametrical effects of glucose levels on microvascular permeability of peripheral nerves in patients with type 2 diabetes with and without diabetic neuropathy. Diabetes 72:290–298. https://doi.org/10.2337/db22-0548
Mooshage CM, Schimpfle L, Kender Z et al (2023) Association of small fiber function with microvascular perfusion of peripheral nerves in patients with type 2 diabetes: study using quantitative sensory testing and magnetic resonance neurography. Clin Neuroradiol. https://doi.org/10.1007/s00062-023-01328-5
Breckwoldt MO, Stock C, Xia A et al (2015) Diffusion tensor imaging adds diagnostic accuracy in magnetic resonance neurography. Invest Radiol 50:498–504. https://doi.org/10.1097/RLI.0000000000000156
Baumer P, Pham M, Ruetters M et al (2014) Peripheral neuropathy: detection with diffusion-tensor imaging. Radiology 273:185–193. https://doi.org/10.1148/radiol.14132837
Vaeggemose M, Haakma W, Pham M et al (2020) Diffusion tensor imaging MR neurography detects polyneuropathy in type 2 diabetes. J Diabetes Complications 34:107439. https://doi.org/10.1016/j.jdiacomp.2019.107439
Jende JME, Groener JB, Kender Z et al (2020) Troponin T parallels structural nerve damage in type 2 diabetes: a cross-sectional study using magnetic resonance neurography. Diabetes 69:713–723. https://doi.org/10.2337/db19-1094
Nunez-Peralta C, Montesinos P, Alonso-Jimenez A et al (2021) Magnetization transfer ratio in lower limbs of late onset pompe patients correlates with intramuscular fat fraction and muscle function Tests. Front Neurol 12:634766. https://doi.org/10.3389/fneur.2021.634766
Schmierer K, Scaravilli F, Altmann DR, Barker GJ, Miller DH (2004) Magnetization transfer ratio and myelin in postmortem multiple sclerosis brain. Ann Neurol 56:407–415. https://doi.org/10.1002/ana.20202
Wolff SD, Balaban RS (1989) Magnetization transfer contrast (MTC) and tissue water proton relaxation in vivo. Magn Reson Med 10:135–144. https://doi.org/10.1002/mrm.1910100113
Knutsson L, Xu J, Ahlgren A, van Zijl PCM (2018) CEST, ASL, and magnetization transfer contrast: how similar pulse sequences detect different phenomena. Magn Reson Med 80:1320–1340. https://doi.org/10.1002/mrm.27341
Horsfield MA (2005) Magnetization transfer imaging in multiple sclerosis. J Neuroimaging 15:58S-67S. https://doi.org/10.1177/1051228405282242
Kollmer J, Kastel T, Jende JME, Bendszus M, Heiland S (2018) Magnetization transfer ratio in peripheral nerve tissue: does it depend on age or location? Invest Radiol 53:397–402. https://doi.org/10.1097/RLI.0000000000000455
Dortch RD, Dethrage LM, Gore JC, Smith SA, Li J (2014) Proximal nerve magnetization transfer MRI relates to disability in Charcot-Marie-Tooth diseases. Neurology 83:1545–1553. https://doi.org/10.1212/WNL.0000000000000919
Kollmer J, Kessler T, Sam G et al (2021) Magnetization transfer ratio: a quantitative imaging biomarker for 5q spinal muscular atrophy. Eur J Neurol 28:331–340. https://doi.org/10.1111/ene.14528
Kollmer J, Hegenbart U, Kimmich C et al (2020) Magnetization transfer ratio quantifies polyneuropathy in hereditary transthyretin amyloidosis. Ann Clin Transl Neurol 7:799–807. https://doi.org/10.1002/acn3.51049
Gibbons CH, Freeman R, Veves A (2010) Diabetic neuropathy: a cross-sectional study of the relationships among tests of neurophysiology. Diabetes Care 33:2629–2634. https://doi.org/10.2337/dc10-0763
Fosleitner O, Schwehr V, Godel T et al (2021) Magnetization transfer ratio of peripheral nerve and skeletal muscle: correlation with demographic variables in healthy volunteers. Clin Neuroradiol. https://doi.org/10.1007/s00062-021-01067-5
Ziegler D, Keller J, Maier C, Pannek J, German Diabetes A (2014) Diabetic neuropathy. Exp Clin Endocrinol Diabetes 122:406–415. https://doi.org/10.1055/s-0034-1366435
Schindelin J, Arganda-Carreras I, Frise E et al (2012) Fiji: an open-source platform for biological-image analysis. Nat Methods 9:676–682. https://doi.org/10.1038/nmeth.2019
Mattes D, Haynor DR, Vesselle H, Lewellen TK, Eubank W (2003) PET-CT image registration in the chest using free-form deformations. IEEE Trans Med Imaging 22:120–128. https://doi.org/10.1109/TMI.2003.809072
Chen JH, Sambol EB, Decarolis P et al (2006) High-resolution MAS NMR spectroscopy detection of the spin magnetization exchange by cross-relaxation and chemical exchange in intact cell lines and human tissue specimens. Magn Reson Med 55:1246–1256. https://doi.org/10.1002/mrm.20889
Morrow JM, Sinclair CD, Fischmann A et al (2016) MRI biomarker assessment of neuromuscular disease progression: a prospective observational cohort study. Lancet Neurol 15:65–77. https://doi.org/10.1016/S1474-4422(15)00242-2
Tesfaye S, Boulton AJ, Dyck PJ et al (2010) Diabetic neuropathies: update on definitions, diagnostic criteria, estimation of severity, and treatments. Diabetes Care 33:2285–2293. https://doi.org/10.2337/dc10-1303
Chung T, Prasad K, Lloyd TE (2014) Peripheral neuropathy: clinical and electrophysiological considerations. Neuroimaging Clin N Am 24:49–65. https://doi.org/10.1016/j.nic.2013.03.023
Pasnoor M, Dimachkie MM, Kluding P, Barohn RJ (2013) Diabetic neuropathy part 1: overview and symmetric phenotypes. Neurol Clin 31:425–445. https://doi.org/10.1016/j.ncl.2013.02.004
Chen JT, Easley K, Schneider C et al (2013) Clinically feasible MTR is sensitive to cortical demyelination in MS. Neurology 80:246–252. https://doi.org/10.1212/WNL.0b013e31827deb99
Chen JT, Collins DL, Atkins HL, Freedman MS, Arnold DL, Canadian MS/BMT Study Group (2008) Magnetization transfer ratio evolution with demyelination and remyelination in multiple sclerosis lesions. Ann Neurol 63:254–262. https://doi.org/10.1002/ana.21302
Does MD, Beaulieu C, Allen PS, Snyder RE (1998) Multi-component T1 relaxation and magnetisation transfer in peripheral nerve. Magn Reson Imaging 16:1033–1041. https://doi.org/10.1016/s0730-725x(98)00139-8
Trip SA, Schlottmann PG, Jones SJ et al (2007) Optic nerve magnetization transfer imaging and measures of axonal loss and demyelination in optic neuritis. Mult Scler 13:875–879. https://doi.org/10.1177/1352458507076952
Groener JB, Jende JME, Kurz FT et al (2020) Understanding diabetic neuropathy-from subclinical nerve lesions to severe nerve fiber deficits: a cross-sectional study in patients with type 2 diabetes and healthy control subjects. Diabetes 69:436–447. https://doi.org/10.2337/db19-0197
Acknowledgements
The authors thank Dorothea Willich (Department of Neuroradiology, Heidelberg University Hospital) for ongoing support and excellent technical performance of all MRN examinations.
Large language models have not been used during the process of manuscript writing.
Funding
Open Access funding enabled and organized by Projekt DEAL. The German Research Council (DFG, SFB 1158) provided financial support for personnel expenditure, MRI costs, and costs for the technical equipment required for electrophysiologic and serologic analysis. The DFG had no influence on the study design, collection, and analysis of data or on the writing of the article.
Author information
Authors and Affiliations
Contributions
CM: collection of MR data, image segmentation, data analysis and interpretation, literature research, writing of manuscript, and arrangement of figures. LS: collection of clinical, electrophysiologic and serologic data, and organization of participants. DT: collection of clinical, electrophysiologic and serologic data, and organization of participants. ZK: collection of clinical, electrophysiologic and serologic data, and organization of participants. TA: collection of MR data, and data analysis. AH: literature research, data analysis and interpretation. JS: study design and coordination. PN: study design and coordination. VS: programming of image analysis tools, data analysis. SH: conception of MRN sequence protocol. MB: study design and coordination, development of MR sequence protocol, and writing of manuscript. SK: development of clinical and electrophysiologic study protocol, collection of clinical, electrophysiologic, and serologic data. JJ: conception of study, organization of participants, collection of MR data, literature research, writing of manuscript. FK: conception of study, organization of participants, collection of MR data, programming of image analysis tools, image segmentation, data analysis and interpretation, literature search, writing of manuscript, and arrangement of figures.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The Heidelberg University Hospital Ethics Committee approved this retrospective study (28.03.2017, S-383/2016), and all patients gave written informed consent.
Consent for publication
Not applicable.
Competing interests
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
ZK received grants from the German Center of Diabetes Research (DZD); AH received funding from the Medical Faculty of Heidelberg University (Rahel-Straus-Program), JS received grants from the DFG (SFB 1116; SFB 1118), DZD, and the Ministry of Science, Research and the Arts of the State of Baden-Württemberg, travel grants and personal fees from Boehringer Ingelheim Pharma, SHZ received grants from the DFG (TRR 205/2; TRR 333/1; SFB 1231/1; SFB 1118), Ministry of Science, Research and the Arts (DZHK Standortprojekt; DZHK VHB Säule B project; VIP + project MIRANDA), the Helmholtz Association (Helmholtz International Research School for Diabetes), Edith-Haberland-Wagner (EHW) Foundation, Else Kröner-Fresenius-Stiftung (EKFS), PN received grants from the (SFB 1118 and 1158) the DZD, and from Novo Nordisk, SHL received a grant from the Dietmar Hopp Foundation and the DFG (SFB 1118), MB received grants and personal fees from Codman, Guerbet, Bayer, and Novartis, personal fees from Roche, Teva, Springer, Boehringer, Grifols, Braun and grants from the European Union, Siemens, the Dietmar Hopp Foundation, Stryker and the DFG (SFB 1118 and 1158), JJ acknowledges support from the International Foundation for Research in paraplegia (IRP) and the EKFS, FK was supported by the German Research Foundation (KU 3555/1–1, ZH 902/3–1), the Hoffmann-Klose Foundation of Heidelberg University Hospital, and a research grant of Heidelberg University Hospital.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mooshage, C.M., Schimpfle, L., Tsilingiris, D. et al. Magnetization transfer ratio of the sciatic nerve differs between patients in type 1 and type 2 diabetes. Eur Radiol Exp 8, 6 (2024). https://doi.org/10.1186/s41747-023-00405-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41747-023-00405-1