Abstract
The incidence of brain diseases in humans is increasing as we experience a worldwide ageing of the population. Treatment for such diseases is still only symptomatic as there are almost no disease-modifying therapies available. Further, since treatment often starts when symptoms appear which is only at a late stage of pathology, we need treatments that will create new cells or restore function to still living cells. Cell transplant therapy, where neuronal progenitor cells derived from stem cells are transplanted to the brain, has seen experimental success. And though there has been some clinical progress, there is still no available therapy. While through the years brain research has focused on neurons, it is now shifting to the so-called support cells of the brain, glia. In neurodegenerative diseases and stroke, glia play roles in the pathogenesis of disease. Therefore, replacing them or enhancing their functions to ultimately save or restore neurons is a new avenue of research that has gained traction in recent years. In this review, we present the current state-of-the-art on transplantation of glia cells, feasibility of this as a therapy, and upcoming directions in the field.
Similar content being viewed by others
Background
“The world’s population is ageing” – these are the introductory words from a 2015 UN report, which states that the number of people aged over 80 years old is expected to triple by 2050, increasing to 434 million [1]. Ageing is known to be the major risk factor for brain diseases, including Alzheimer’s disease, Parkinson’s disease, and stroke. And while lifestyle changes can help in mitigation of dementia [2], it is likely that many of the population will develop a brain disease in their lifetime. Treatments currently in clinical trials for neurodegenerative diseases that aim to support remaining neurons or halt protein aggregation have potential in the earlier disease stages. But until we can reliably diagnose pre-symptomatic patients, successful treatments for those with the majority of cells already lost are greatly needed.
Cell transplant therapy to restore function to an area with dead cells is applicable to the human brain where neurons are post-mitotic cells, and except in a few niche areas [3], are not robustly regenerated after injury. In stroke for example, where the ischemic core is deprived of blood and oxygen, cells die rapidly and are unlikely to be regenerated. Therefore, this has been one brain condition where much work has been done to bring a cell transplant therapy to the clinic. Various stem cells have been transplanted to the penumbral area in human patients to try to both restore the neuronal network lost as well as reduce inflammation. This has been done with stereotaxic surgery directly to the brain [4, 5] and also via intravenous/arterial infusion [6, 7]. However, even with the implementation of guidelines such as STEPS for stem cell transplant therapy in stroke [8] to aid in standardizing the field, there has yet to be a successfully approved therapy of this kind. Indeed, several clinical trials for cell transplant therapy have taken place with many ongoing for neurodegenerative diseases. Alzheimer’s disease, Parkinson’s disease, and amyotrophic lateral sclerosis (ALS) have all had or are having human trials using stem cell transplants with different cell sources. Yet not one has successfully brought a treatment to the clinic. Researchers, doctors, and patients alike continue to ask why. Of course, there are practical issues beyond complicated surgery, immune reactions, tumorgenicity, and whether the cells survive and integrate to the host brain. There are also issues of scalability, manufacturing time, and quality control between cell batches that might prevent transplant therapy from becoming a widely available treatment. Unresolved issues are also how to select cell source and the patient that has the best likelihood of a positive outcome.
As mentioned, ischemic stroke has been one of the most explored brain diseases in terms of stem cell transplants. In 2020, already 52 clinical trials using stem cells in ischemic stroke have been completed [9]. Cell source, route of administration, and administration time after stroke onset varied between studies. The most effective method of delivery, optimal cell dose, and type of cells used has yet to be determined, and further this may depend on the patient’s status. On the plus side, majority of stem cell transplants were shown to be safe. Peripheral transplant, intravenously or intraarterially, was more often used if the patient was administered treatment soon after the ischemic event in order to alleviate secondary inflammatory damage. Recently published results from a Phase 2/3 trial transplanting allogeneic bone-marrow derived stem cell progenitors intravenously to patients 18–36 h after ischemic stroke onset showed safety but no significant efficacy in the short term [10]. Whereas overall intracranial administration was favoured if the patient was post-stroke for an extended period of time. To that end, another recent trial transplanted autologous mesenchymal stromal cells to the peri-infarct region of patients 47–64 days after ischemic stroke [11]. The cells were tracked using MRI and found that they migrated and engrafted around the ischemic borders. Importantly, the patients showed neurological recovery up to one year post-transplant. While this is promising, it is clear that further testing and research is needed for an undeniably successful treatment of stem cell transplant for ischemic stroke to be available.
Transplantation of neurons derived from stem cells has also seen some success in Parkinson’s disease for example and continues to be explored [12]. Perhaps because when it is diagnosed most of the dopamine neurons have already been lost [13], but if started early enough, survival and integration of a small number of cells is sufficient to show some functional recovery in patients. There is an upcoming trial where the FDA has cleared Aspen Neuroscience (co-founded by Jeanne Loring of the Scripps Research Institute) to proceed with their stem cell therapy, ANPD001 [14]. Notably, a study was published in 2020 [15] where a Parkinson’s patient received an autologous transplant of dopamine precursor cells, where they survived and seem to produce some clinical benefit, at least in the short-term. This gives promise for the upcoming trials. Additionally, one trial using embryonic stem cells (ESCs) is recruiting patients in Lund, Sweden, in partner with doctors at the University Hospital in Cambridge, UK (STEM-PD [16]; ClinicalTrials.gov ID: NCT05635409). Of course, transplantation of neurons derived from stem cells should continue to be explored if it results in patient benefit.
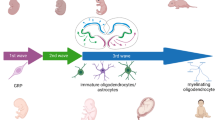
However, answering all of the above problems depends on the nature of the disease itself and also the patient, as well as what we would consider a successful cell transplant outcome. In general, the field of neurodegenerative disease has often focused on neurons. But now researchers have begun to focus on other cells of the central nervous system (CNS): glia. These are abundant support cells of the brain that have a variety of necessary functions. Research has shown that glia are key players in the pathophysiology of several brain diseases. Can we then transplant glial cell progenitors to the patient brain in order to save neurons and preserve cellular functioning (Fig. 1)?
Glia and glial cell sources
While clinical trials are ongoing to transplant neurons derived from stem cells for major brain diseases, an upcoming direction of the field is the transplantation of glial cells. The rationale behind the transplantation of glial cells for the cure of brain diseases is based on the non-cell autonomous concept for neurodegeneration [17]. Essentially it implies that the neuronal (and white matter) pathology can be cured by targeting glial cells in order to correct their functions or boost their positive functions. Thus, transplantation of glia poses an appealing treatment for several chronic brain disorders such as Parkinson’s disease, Alzheimer’s disease, ALS, acute injuries like stroke and traumatic brain injury, as well as autoimmune disorder multiple sclerosis. On the other hand, each disease may have different aetiology, comprising the sporadic form and inherited forms with various genetic backgrounds. But here, targeting of glial cells can serve as the common approach regardless of the disease cause (Table 1).
Glial cells inhabiting the CNS play an important role in maintaining brain homeostasis and responding to injury or disease. Microglia, the innate immune cells residing in the CNS, make up about 10% of the cell population in the brain. In the adult human brain, microglia are not distributed uniformly. Gray matter has lower densities compared with the white matter, and there are markedly lower densities in the cerebellar cortex compared to the substantia nigra [18]. Even though they are not uniformly distributed, they exhibit a high level of adaptability. Microglia play crucial roles in surveillance and maintaining brain homeostasis by regulating synaptic formation and pruning, phagocytosing cells and debris, and releasing inflammatory modulators [19]. Through their close interactions with neurons, as well as other glial cells astrocytes and oligodendrocytes, microglia contribute to the development of various CNS-related injuries and pathologies associated with neurodegenerative diseases as well as being a strong contributor to inflammation in stroke.
Astrocytes are the most abundant glial cell in the brain, constituting approximately 30% of the cell population in the CNS. They form complex networks which interact with other brain cell populations of neurons, microglia, and oligodendrocytes. They are part of the tripartite synapse and neurovascular unit. The functions of astrocytes are thought to play a particularly important role in the human brain [20, 21] where they support brain metabolic homeostasis and are involved in neuroinflammatory responses [22]. Malfunction of astrocytes has been demonstrated in many brain disorders [23]; accordingly, transplantation of healthy astrocytes is expected to provide a therapeutic benefit [24].
Oligodendrocytes are also an abundant glia population, particularly in white matter [25]. Their main function is production of myelin, leading to myelination of axons and providing trophic support for said axon, along with facilitating propagation of action potentials. One of the main diseases involving oligodendrocyte cell loss is multiple sclerosis, as well as white matter injury in stroke. Replacing damaged or dysfunctional oligodendrocytes with healthy ones could assist in remyelination processes and therefore rescue axons as well.
Glial cells used for transplantation can be generated from various sources. The choice of source depends on several factors, including the type of glial cell needed, the purpose of transplantation, and the specific research or clinical context. In addition to ESCs, neural stem cells (NSCs), and induced pluripotent stem cells (iPSCs) that can be differentiated into glial cells, also glial progenitor cells. Glial progenitor cells are intermediate cells with the potential to differentiate into specific types of glial cells. They can be isolated from embryonic or fetal brain tissue or derived from pluripotent stem cells through specific differentiation protocols. An interesting line of research also shows it is possible to directly convert one type of cell into another through a process called transdifferentiation. For instance, fibroblasts (connective tissue cells) can be directly reprogrammed into glial cells using specific transcription factors or gene editing techniques. Currently, the primary sources for glial cell transplantation are either ESCs or iPSCs. In both cases these cells undergo further differentiation to glial progenitor cells and selection in order to obtain an optimal phenotype for transplantation [26].
Experimental glial cell transplantation
Microglia transplantation for brain diseases
Microglia transplantation is a type of cell therapy that involves transplanting microglia cells into the brain or spinal cord of a recipient [27]. The cells are typically harvested from a donor animal or generated in vitro from iPSCs and then transplanted, where they are expected to integrate into the recipient's CNS and assume their normal functions, such as immune surveillance and phagocytosis [28, 29]. Microglia transplantation is being explored as a potential therapeutic approach for various neurological disorders, such as Alzheimer's disease, Parkinson's disease, and multiple sclerosis [30, 31]. There have been several preclinical studies that have shown promising results for microglia transplantation in models of neurodegenerative diseases (Table 2). As microglia are the primary immune cells of the CNS, they exert innate immunity and may become activated from an insult resulting in a pro-inflammatory phenotype, however they also possess anti-inflammatory abilities. Recently, an effective method for ex vivo induction of anti-inflammatory microglia has been established, and the cells were used for transplantation in a mouse spinal cord injury model. In contrast to pro-inflammatory microglia, they promoted recovery of motor function and retrograde axonal transport [32].
Microglia transplantation in Alzheimer’s disease
There is ample evidence for microglia involvement in Alzheimer’s disease pathology and pathogenesis [38]. Several risk genes for the disease are highly or only expressed in microglia [39] and dysfunctional microglia will exacerbate disease by reducing clearance of amyloid-β and increase engulfment of synapses [40], as well as potentiating harmful inflammatory cascades that further damage neurons (also in concert with astrocytes [41]). Therefore, microglia transplantation may also be used in combination with NSCs, to replace lost neurons and support their survival and function in Alzheimer’s disease. In a recent study, the treatment of a mouse model of Alzheimer’s disease by transplantation of genetically engineered NSCs expressing the choline acetyltransferase and human microglial cells expressing the neprilysin and scavenger receptor A was carried out [34]. Significant restoration of learning and memory function, decrease in amyloid burden, recovery of acetylcholine level, and attenuation of neuroinflammation was demonstrated.
Microglia transplantation in Parkinson’s disease
Microglia cells are involved in the immune response in the brain and can contribute to the neuroinflammation and neurodegeneration that occurs in Parkinson's disease. Therefore, transplanting healthy microglia cells into the brain may help to mitigate the immune response and inflammation, and thus protect remaining neurons from further damage. Several preclinical studies have shown promising results with microglia transplantation in animal models of Parkinson's disease. However, the safety and efficacy of this approach in humans is still under investigation, and more research is needed to determine if it can be used as a viable treatment option for Parkinson's disease. In the situation where neurotrophic factor delivery is chosen as the treatment strategy, genetically engineered cell transplantation can be considered. Recently developed approach to deliver GDNF (glial cell-derived neurotrophic factor) to a neurotoxin induced Parkinson’s disease animal model by GDNF-expressing hematopoietic stem cell (HSC)-derived macrophages. The transplanted cells integrated into the mouse brains and improved their motor function, reduced inflammation, and protected against further neuron loss [33], providing a rationale for neuroprotective macrophage/microglia transplantation in Parkinson’s disease patients.
Astrocyte transplantation in amyotrophic lateral sclerosis
Reactive astrocytes are a hallmark of ALS. Their supportive homeostatic function is lost and interactions with neurons suffers [42], leading to increased motor neuron vulnerability. Recent research has demonstrated beneficial effects of intrathecal transplantation of human ESC-derived astrocytes into transgenic hSOD1 G93A mice model of ALS [35]. These investigations have yielded a major breakthrough phase I/IIa clinical trial, where allogeneic healthy and functional human astrocytes, derived from ESCs (brand name AstroRx®) and intrathecally injected to ALS patients, resulted in a beneficial clinical effect. This was observed for the three months following cell injection without adverse events [43].
Oligodendrocytes for multiple sclerosis treatment
In addition to microglia and astrocytes, oligodendrocytes are also important glial cells. Demyelination occurs in multiple sclerosis, as well as in spinal cord injuries and WMS. Further, the loss of function or death of oligodendrocytes has recently been evidenced in neurodegenerative disorders Parkinson’s, Alzheimer’s, and ALS. Therefore, they also represent a potential candidate for transplant in various diseases. However, creating human oligodendrocytes to use to study disease mechanisms has proved difficult and most research comes from rodent models. This has been due to the fact that differentiation protocols for oligodendrocytes, although constantly being refined and improved, takes on the order of several months to derive mature human cells void of other glia and neurons [44], discussed further below. Already in the early 2000s, oligodendrocyte progenitor cells derived from human ESCs were transplanted to shiverer mice where they survived and gave rise to mature oligodendrocytes [45]. Further studies in rodents have been performed with varying levels of success. However, since ESCs have the potential to cause an immune reaction on their own as well as their limited availability, iPSCs have therefore been tried as well. In 2013, Wang and colleagues [46] transplanted oligodendrocyte progenitor cells to neonatal shiverer mice, where 79% became oligodendrocytes and the remaining became astrocytes. The transplanted mice lived significantly longer than control mice and it was also noted that the remyelination efficiency was greater than using fetal transplants. Though the cells were transplanted to newborn mice, this study gave a promising proof-of-concept going forward. Another group showed they were able to reprogram human iPSC neural progenitor cells to generate O4+ oligodendrocytes and transplant them to adult shiverer mice where they sufficiently myelinated the mouse axons [37]. Here, 56% of the cells co-expressed OLIG2 and CC1, while there was 3% expression of NeuN + neurons and 17% GFAP + astrocytes. While these studies have shown potential in animal models, the issue of lengthy protocols remains. Additionally, reprogramming neural progenitor cells may not result in the most original oligodendrocytes and caution must be taken when using these for therapeutic purposes.
Glial cell transplantation in white matter stroke
White matter stroke (WMS) is characterized by damage primarily to astrocytes, axons, oligodendrocytes, and myelin leading to cognitive and sensorimotor impairments [47]. Llorente et al. [36] differentiated glial enriched progenitor cells (GEPs) from human iPSCs as a possible treatment option for WMS. Human iPSC-GEPs were transplanted into the brain in the subacute period in mice with WMS and were shown to migrate widely and to mature into astrocytes, and in general to alleviate white matter pathology. More interestingly, human iPSC-GEP transplantation enhanced cognitive and motor recovery in WMS mice. This approach might be effective for treating not only WMS, but also traumatic brain injury and spinal cord injury.
While these studies show promise for glial cell transplantation as a potential therapeutic approach for neurodegenerative diseases and brain injuries in humans, it is important to note that more research is needed to determine the safety and efficacy of this.
Optimizing clinical translation for transplantation of glia
Clinical trials have demonstrated that the transplantation of neurons derived from stem cells is safe and feasible. To improve translational success, the stroke community has produced recommendations that could be applicable and adaptable to other diseases and in the context of glial cells [8, 48]. First, maximizing comparability between preclinical and clinical studies is crucial to facilitate the translation of specific cell products from bench to clinic. Second, a stronger focus on safety, rather than solely on confirming efficacy in early preclinical research, followed by early, safety-oriented clinical research, has the potential to accelerate translational research without sacrificing quality. Third, sharing preclinical and clinical data will enable the community to tackle more complex research questions, such as the impact of comorbidities on efficacy or safety. Lastly, confirmatory multicenter preclinical trials are a valuable research format, but larger research consortia, including industry joint ventures, are required for successful implementation.
Selecting cell source for transplantation
Various sources for stem cells have their advantages and disadvantages. As mentioned above, ESCs, NSCs, and iPSCs can all be used to derive glial cells (Table 3).
In terms of an immune rejection, ESCs are the most likely to cause a reaction. In addition to the ethical concerns associated with ESCs, along with their limited availability, they also have the possibility to generate tumours due to their origin [49]. While using NSCs somewhat reduces tumorgenicity and immune rejection, it is difficult to obtain enough tissue to produce the number of cells needed. IPSCs are differentiated into the progenitor stage and can be autologously transplanted to the patient, mitigating detrimental phenotypes due to aging and reducing graft rejection, however these cells can also cause tumour formation. Major hurdles for iPSCs as a therapy are currently the manufacturing time, replicability, quality control, and scalability. While no cell source is perfect, ESCs and iPSCs are both renewable and offer more flexibility since one can differentiate all three glial cell types from them: microglia, astrocytes, and oligodendrocytes. And although ESCs have a high capacity to expand, obtaining them has ethical limitations. In contrast, iPSCs can be created from a biopsy – paving the way for personalized medicine. However, batch differences may be high, making replicability difficult to achieve.
Development of glial cell differentiation protocols
Differentiation of cells for transplant is a serious undertaking as the material to inject should be as free of contaminating cell types as possible. With iPSCs for example, some experience is needed from the user to optimize cell quality and number, as well as the ability to achieve consistent outcomes. Naturally, a pipeline for differentiation of glial cells would be needed for successful clinical translation. Each cell type also has its own challenges.
Microglia
In recent years, protocols to differentiate microglia from ESCs and iPSCs have been developed [50, 51], and have subsequently been transplanted to immunosuppressed mice [52,53,54]. Importantly, the transplanted human microglia were demonstrated to resemble microglia from the human brain at the transcriptional level [53, 55], indicating that this could also occur were the cells to be transplanted to humans. In addition to producing cells that more closely resemble the microglia found in the human brain, the differentiation protocols have been optimized to be faster, more straightforward, and to produce a reasonably high yield of cells [56].
Astrocytes
Astrocytes have a plethora of functions in the brain, and in certain areas they are significantly abundant. Therefore, finding a differentiation protocol that would both produce a high number of cells for transplant and one that would yield cells that most closely recapitulate astrocytes in the living human brain is necessary. Several protocols have been developed to produce astrocytes from stem cells [57], but may only result in a low yield of cells. For transplantation, direct differentiation of astrocytes from ESCs and iPSCs was demonstrated in [58, 59]. The protocol differentiates glial progenitor cells from neuroepithelial cells, and the glial progenitor cells are expanded to yield a large number of cells with high purity. The astrocytes are functional, and when transplanted to the mouse brain, maintain their human identity. The authors note that the maturation phase of the protocol is fairly extended, but for the purposes of transplantation, a high, pure yield of cells is what is needed.
Oligodendrocytes
As discussed above, transplantation of oligodendrocyte precursors from iPSCs has already been performed successfully. However, the culture time was between 120–150 days, making this a rather time-consuming protocol [46]. Another protocol allows the differentiation of O4+ oligodendrocyte progenitor cells in 75 days [60]. The progenitor cells can be cell sorted and used for transplantation. Importantly, the authors note that these can be frozen and thawed immediately before transplant, which is extremely useful when considering the clinical situation.
Site of delivery for glial cells
While the least invasive method for delivery of any drug is most preferred, more extensive research and testing will need to be performed for peripheral delivery of drugs that aim to reach the brain. For example, while delivery of stem cells intravenously or intraarterially in stroke has seen some success [6, 7], it still ends up with few cells reaching the target area due to first pass metabolism, a possible instantaneous immune reaction, and ability to cross the blood–brain barrier. Therefore, brain surgery is likely still needed at this stage. Proposing the optimal target site(s) for glial cell transplantation is also not trivial since brain diseases may have many affected areas. Additionally, with different glial cells being involved in different aspects of disease progression, the situation becomes exponentially more complicated. Since neurodegenerative diseases have a buildup of aberrant protein that may cause overactivation and subsequent neurotoxic functions of microglia and astrocytes, being able to visualize where this is occurring in the living patient is also necessary. Along these lines, if the glia cells are dysfunctional and unable to properly process aggregated proteins, replacing them at the site of the most pathology would also be the best option.
Alzheimer’s disease
In Alzheimer’s disease, in addition to potential neurotoxic functions of overreactive microglia, these cells are found around amyloid plaques [61]. How microglial cells contribute to disease is of course changing throughout disease progression, since these cells are normally serving protective functions. Therefore, the current status and most affected location of microglia-amyloid interaction needs to be visualized as clearly as possible in order to inform site of delivery for replacement microglia. This can be done via positron emission tomography (PET) imaging since there are available radioligands for amyloid and microglia.
Parkinson’s disease
There is the advantage in Parkinson’s disease research that it is already known that dopamine neurons die in the substantia nigra pars compacta, preceded by axon loss in the striatum [13]. Dopamine neuron progenitors have mainly been transplanted to the striatum, but exploration of transplantation into both sites is being pursued [62]. Interestingly, there are more microglia in striatum and substantia nigra, and therefore transplantation of cells into one or both of these areas is the most logical in Parkinson’s disease when the aim is to also restore dysfunctional dopamine neurons. Activated microglia may bring about a neurotoxic phenotype in astrocytes [41], and astrocytes are also involved in Parkinson’s, including in genetic forms of the disease [63]. Therefore, simultaneous transplant of microglia and astrocytes together to the striatum and substantia nigra could be useful. Particularly since it has also been demonstrated in vitro that these cells work in concert to clear away the fibrillar form of α-synuclein [64].
ALS
Cell replacement for ALS has been explored extensively due to the devastating nature of the disease. The aim is to replace lost motor neurons using stem cell-derived progenitors. Several delivery routes have been tried in clinical trials: intraspinal, intrathecal, intramuscular, intravenous, and intracranially to the motor cortex. For replacement of microglia and astrocytes involved in ALS pathogenesis, the spinal cord and motor cortex would be the prime areas. Microglia have been shown to be activated in both areas in ALS [65, 66] and astrocytes have been shown to cause cell death to spinal motor neurons [67, 68] as well as being present throughout the brain. Although when delivery methods have improved, transplant via the blood or thecal space should also be explored.
Multiple sclerosis and WMS
Loss of myelination in multiple sclerosis leads to lesions in white and grey matter in several brain areas [69], making selection of the optimal site of transplant difficult. However, experimental studies in mice have shown that the oligodendrocyte progenitors will migrate and remyelinate axons rostrally and caudally from the site of transplantation [70]. This is a promising result and further, as with other brain diseases, multiple sites could be selected based on imaging results showing the most demyelinated areas. In WMS, transplantation of oligodendrocyte progenitors and astrocytes to the site of infarct would be the most obvious place to start.
Upcoming directions and practical challenges in glial cell transplantation
Though glia transplant studies are still experimental, due to the clinical failure of transplanted neurons in the brains of diseased patients, there is evidence that this line of research is worth pursuing.
A recent study showed proof-of-concept that diseased or even aged glia in the brain could be replaced by healthy cells [71]. The authors first created a chimeric mouse model using human glial progenitor cells differentiated from ESCs of donors with Huntington’s disease. After 36 weeks, they transplanted healthy human glial progenitor cells and found that these proliferated and displaced the diseased cells. The authors also performed a similar experiment to determine whether this was related to disease or the fact that younger cells were being transplanted to compete against aged cells: they first transplanted healthy human glial progenitor cells to the neonatal mouse and then again 36 weeks later performed another round of transplantation of healthy cells. This again resulted in replacement, where the younger cells proliferated, and the older cells died via apoptosis. This is an exciting prospect in terms of treatment of brain diseases, particularly since these are primarily diseases of ageing. While this still needs a lot of research, including in future to see if this could have a functional impact in a model of Huntington’s disease [72] and how well these cells are able to differentiate in the aged brain to, for example, astrocytes and oligodendrocytes.
An appealing prospect in stem cell therapy, particularly in terms of glial cell transplantation, is to transplant multiple cell types to the brain. This leverages two aspects of transplant therapy: that one would be able to replace both lost neurons and glial cells, and at the same time the replaced glial cells would simultaneously support the neuronal cells. It is known that microglia and astrocytes work in concert in several neurodegenerative diseases to bring about the potential for increased neurotoxicity [41]. Therefore, replacing both cell types could result in increased protection.
Except for the study mentioned above [34], there are no published studies to date testing preclinical application of combination cell therapy to the brain. Interestingly, two clinical studies have transplanted different cell types using intracranial as well as intravenous and intrathecal delivery routes, one in stroke [73] and another in multiple system atrophy [74], both from the same group in China. Both studies used four cell types (olfactory ensheathing cells, Schwann cells, neural progenitor cells, and umbilical cord mesenchymal stromal cells) from fetuses. While both cases demonstrated safety and some benefit for patients, there are ethical and practical considerations that would make this unfeasible on a larger scale. Specifically, the use of cells from a fetal source may not be easy to obtain and further, transplantation via different routes in the clinic for aged patients could outweigh potential benefits. However, basic and preclinical testing of the general idea should be pursued. In neurodegenerative diseases, transplanting multiple cell types to a single area or areas in the brain, rather than giving the cells systemically, might be the place to start. Since neurodegenerative diseases are complex disorders that encompass a variety of symptoms linked to different brain areas and cell types. As with any cell therapy, researchers must ensure that the cells are of sufficient purity and mature into the cell type of interest. Luckily, cells would not have to be grown together and could rather be combined just prior to injection, avoiding any issues with different needs for factors, media etc. In our hands, thorough testing of combination transplantation of three different cell types in adult immunosuppressed mouse brain without any further manipulation has proven difficult from a technical perspective. While the progenitors reach the target areas, survive, and mature to the cell type of interest, getting enough cells to the brain area is less trivial. Particularly since we do not currently know how many cells are needed to replace glia or support neurons, this would need to be tested if there is any hope to scale this to the human brain. Since the number of glial cells differs between brain areas according to counting studies, learning approximate cell numbers in the area of interest would be useful. Additionally, the size of the mouse brain and availability of extracellular space likely play a factor when attempting to put human cells there. That being said, protocol refinement, combined with a model of neurodegeneration where cells are lost and therefore brain space is potentially freed up, would result in further success.
The transplantation of different cell types obtained from two-dimensional culture naturally brings up the idea of accomplishing this another way: by using brain organoids. Transplantation of human brain organoids to mouse brain has already been done. In the first case [75], the mouse brain was used as a vector for the organoid to incorporate host vasculature and microglia, two things missing from human brain organoid models grown on their own due to the nature of their derivation [76]. The results indicated that in addition to the organoid gaining host microglia and vasculature, the human brain organoids also integrated neuronally with the host mouse brain. This is a strong proof-of-concept for such studies that could eventually serve as a therapy. However, these organoids were transplanted on the surface of the cortex, which would not be applicable to all neurodegenerative diseases as cell replacement. As discussed above, the physical space is an issue here as well. Putting a small piece of tissue that can be seen by eye to deeper into the intact mouse brain faces equal technical issues. But again, these could certainly be overcome. Additionally, the aforementioned study has incorporated mouse microglia. A better way to accomplish this would be to first integrate human microglia to the brain organoid and then transplant it. This has also been done in mice, where the authors first incorporated human microglia derived from human ESCs or iPSCs to a forebrain organoid, then transplanted it to the cortex area just above the hippocampus [77]. They showed similar success to their previous above study and demonstrated that this may be a more human-relevant model to study microglia in disease. Since both studies showed integration of the brain organoid containing human cells to the host brain after xenotransplantation, this may be a possible way to successfully replace lost or dysfunctional cells in neurodegenerative diseases in the future.
One way to circumvent the use of multiple cell types was to use human iPSC-derived long-term neuroepithelial stem (lt-NES) line [78]. In this recent study, lt-NES cells were shown to give rise to neurons and oligodendrocytes and can repair both injured neural circuitries and demyelinated axons. This is an attractive option since one could have different cell types present in the brain without having to transplant them simultaneously.
An exciting prospect in the field of stem cell transplantation is to use iPSC stocks for research and transplantation. While the promise of personalized medicine using autologous iPSCs is appealing, the timeline of going from skin biopsy to GMP-grade cells for transplantation is easily about one year [79]. On the other hand, creating a bank of clinical grade lines would be a way to save a substantial amount of time and therefore put the field a step closer to making cell transplantation a reality for patients. Japan has already funded, and researchers developed such a bank called iPSC Stock Project [80]. Here, they aim to have a stock of human leukocyte antigen (HLA) homozygous iPSCs that can be compatible with almost the entire Japanese population. These can be used for research with the idea to use them for transplantation. Therefore, this could be a prime starting point for differentiation into different brain cell types to be transplanted to a patient.
Conclusions
The potential of glial cell transplant to save neurons is an appealing prospect. Considering that in brain diseases there are common mechanisms of neuron loss, such as mitochondrial stress, inflammation, endoplasmic reticulum stress, and disruption of proteostasis, particularly in neurodegenerative diseases with aggregating proteins. Since all three types of glial cells have been found to be involved in the processing of aggregated proteins [64, 81], restoring this function could be beneficial in Alzheimer’s disease, Parkinson’s disease, and ALS. Overall, replacement of dysfunctional glia or enhancement of glial cell functions is a promising avenue of research into therapies for a variety of brain diseases (Fig. 2). Although several practical hurdles still exist, including transferring preclinical studies to humans. But with more research, especially carefully controlled studies that can be compared easily between each other, we can find a new way to treat these debilitating and increasingly common disorders.
Illustration of potential mechanisms of how each glial cell type would give rise to neuroprotection in several brain diseases. Corresponding lines to each cell type connect to various brain diseases (microglia = blue; astrocytes = green; oligodendrocytes = dark pink). Next to each line are molecules released/absorbed, receptors activated/blocked, or processes increased/decreased for each cell type that have been demonstrated to be protective or restorative in each disease (data from cells, animals, and/or patients). Each brain disease has the corresponding area and neurons affected. Abbreviations: Aβ = amyloid beta; αSyn = alpha-synuclein; CB2 = cannabinoid receptor 2; CXCL12 = C-X-C motif chemokine 12; GSH = glutathione stimulating hormone; HPC = hippocampus; IGF-1 = insulin growth factor 1; IL-4 = interleukin 4; IL-10 = interleukin 10; LCN2 = Lipocalin-2; LINGO-1 = Leucine rich repeat and Immunoglobin-like domain-containing protein 1; MC = motor cortex; Nrf2 = NF-E2-related factor 2; OPCs = oligodendrocyte progenitor cells; SC = spinal cord; SN = substantia nigra; Sox17 = SRY-box transcription factor 17; STR = striatum; TGF-β1 = transforming growth factor beta-1. Figure created using Biorender
Availability of data and materials
Not applicable.
Abbreviations
- ALS:
-
Amyotrophic lateral sclerosis
- CNS:
-
Central nervous system
- ESC:
-
Embryonic stem cell
- GDNF:
-
Glial cell-derived neurotrophic factor
- GEP:
-
Glial enriched progenitor cells
- GFAP:
-
Glial fibrillary acidic protein
- GMP:
-
Good manufacturing practice
- HLA:
-
Human leukocyte antigen
- HSC:
-
Hematopoietic stem cell
- iPSC:
-
Induced pluripotent stem cell
- lt-NES:
-
Long-term neuroepithelial stem
- MRI:
-
Magnetic resonance imaging
- NSC:
-
Neural stem cell
- OLIG2:
-
Oligodendrocyte transcription factor 2
- PET:
-
Positron emission tomography
- WMS:
-
White matter stroke
References
United Nations. United nations department of economic and social affairs, population division. world population ageing 2015. 2015. Available from: https://www.un.org/en/development/desa/population/publications/pdf/ageing/WPA2015_Report.pdf. Cited 2023 Jun 14.
Livingston G, Huntley J, Sommerlad A, Ames D, Ballard C, Banerjee S, et al. Dementia prevention, intervention, and care: 2020 report of the lancet commission. The Lancet. 2020;396(10248):413–46.
Moreno-Jiménez EP, Terreros-Roncal J, Flor-García M, Rábano A, Llorens-Martín M. Evidences for adult hippocampal neurogenesis in humans. J Neurosci. 2021;41(12):2541–53.
Muir KW, Bulters D, Willmot M, Sprigg N, Dixit A, Ward N, et al. Intracerebral implantation of human neural stem cells and motor recovery after stroke: multicentre prospective single-arm study (PISCES-2). J Neurol Neurosurg Psychiatry. 2020;91(4):396–401.
Steinberg GK, Kondziolka D, Wechsler LR, Lunsford LD, Coburn ML, Billigen JB, et al. Clinical outcomes of transplanted modified bone marrow-derived mesenchymal stem cells in stroke: a phase 1/2a study. Stroke. 2016;47(7):1817–24.
Savitz SI, Yavagal D, Rappard G, Likosky W, Rutledge N, Graffagnino C, et al. A phase 2 randomized, sham-controlled trial of internal carotid artery infusion of autologous bone marrow-derived ALD-401 cells in patients with Recent Stable Ischemic Stroke (RECOVER-Stroke). Circulation. 2019;139(2):192–205.
Jaillard A, Hommel M, Moisan A, Zeffiro TA, Favre-Wiki IM, Barbieux-Guillot M, et al. Autologous mesenchymal stem cells improve motor recovery in subacute ischemic stroke: a randomized clinical trial. Transl Stroke Res. 2020;11(5):910–23.
Boltze J, Modo MM, Mays RW, Taguchi A, Jolkkonen J, Savitz SI, et al. Stem cells as an emerging paradigm in stroke 4. Stroke. 2019;50(11):3299–306.
Kawabori M, Shichinohe H, Kuroda S, Houkin K. Clinical Trials of stem cell therapy for cerebral ischemic stroke. Int J Mol Sci. 2020;21(19): 7380.
Houkin K, Osanai T, Uchiyama S, Minematsu K, Taguchi A, Maruichi K, et al. Allogeneic stem cell therapy for acute ischemic stroke: the phase 2/3 TREASURE randomized clinical trial. JAMA Neurol. 2024;81(2):154–62.
Kawabori M, Kuroda S, Shichinohe H, Kahata K, Shiratori S, Ikeda S, et al. Intracerebral transplantation of MRI-trackable autologous bone marrow stromal cells for patients with subacute ischemic stroke. Med. 2024;5(5):432–444.e4.
Wang F, Sun Z, Peng D, Gianchandani S, Le W, Boltze J, et al. Cell-therapy for Parkinson’s disease: a systematic review and meta-analysis. J Transl Med. 2023;21(1):601.
Cheng HC, Ulane CM, Burke RE. Clinical progression in Parkinson disease and the neurobiology of axons. Ann Neurol. 2010;67(6):715–25.
Aspen neuroscience announces FDA clearance of investigational new drug application for ANPD001, autologous cell therapy for the treatment of Parkinson’s disease. 2023. Available from: https://aspenneuroscience.com/aspen-neuroscience-announces-fda-clearance-of-investigational-new-drug-application-for-anpd001-autologous-cell-therapy-for-the-treatment-of-parkinsons-disease/. Cited 2023 Oct 6.
Schweitzer JS, Song B, Herrington TM, Park TY, Lee N, Ko S, et al. Personalized iPSC-derived dopamine progenitor cells for Parkinson’s disease. N Engl J Med. 2020;382(20):1926–32.
Kirkeby A, Nelander J, Hoban DB, Rogelius N, Bjartmarz H, Novo Nordisk Cell Therapy RandD, et al. Preclinical quality, safety, and efficacy of a human embryonic stem cell-derived product for the treatment of Parkinson’s disease, STEM-PD. Cell Stem Cell. 2023;30(10):1299–1314.e9.
Van Harten ACM, Phatnani H, Przedborski S. Non-cell-autonomous pathogenic mechanisms in amyotrophic lateral sclerosis. Trends Neurosci. 2021;44(8):658–68.
Mittelbronn M, Dietz K, Schluesener HJ, Meyermann R. Local distribution of microglia in the normal adult human central nervous system differs by up to one order of magnitude. Acta Neuropathol. 2001;101(3):249–55.
Priller J, Prinz M. Targeting microglia in brain disorders. Science. 2019;364:32–3 Association for the Advancement of Science.
Oberheim NA, Takano T, Han X, He W, Lin JHC, Wang F, et al. Uniquely hominid features of adult human astrocytes. J Neurosci. 2009;29(10):3276–87.
Oberheim NA, Wang X, Goldman S, Nedergaard M. Astrocytic complexity distinguishes the human brain. Trends Neurosci. 2006;29(10):547–53.
Oksanen M, Lehtonen S, Jaronen M, Goldsteins G, Hämäläinen RH, Koistinaho J. Astrocyte alterations in neurodegenerative pathologies and their modeling in human induced pluripotent stem cell platforms. Cell Mol Life Sci. 2019;76(14):2739–60.
Verkhratsky A, Zorec R, Parpura V. Stratification of astrocytes in healthy and diseased brain. Brain Pathol. 2017;27(5):629–44.
Trujillo-Estrada L, Gomez-Arboledas A, Forner S, Martini AC, Gutierrez A, Baglietto-Vargas D, et al. Astrocytes: from the physiology to the disease. Curr Alzheimer Res. 2019;16(8):675–98.
von Bartheld CS, Bahney J, Herculano-Houzel S. The search for true numbers of neurons and glial cells in the human brain: a review of 150 years of cell counting. J Comparat Neurol. 2016;524(18):3865–95.
Goldman SA, Goldman SA. Progenitor cell-based treatment of glial disease. Prog Brain Res. 2017;231:165.
Xu Z, Rao Y, Huang Y, Zhou T, Feng R, Xiong S, et al. Efficient strategies for microglia replacement in the central nervous system. Cell Reports. 2020;32(6). Available from: https://www.cell.com/cell-reports/abstract/S2211-1247(20)31026-3. Cited 2023 Aug 14.
Deczkowska A, Keren-Shaul H, Weiner A, Colonna M, Schwartz M, Amit I. Disease-associated microglia: a universal immune sensor of neurodegeneration. Cell Cell Press. 2018;173:1073–81.
Hashemiaghdam A, Mroczek M. Microglia heterogeneity and neurodegeneration: The emerging paradigm of the role of immunity in Alzheimer’s disease. J Neuroimmunol. 2020;341:577185 Elsevier B.V.
Lloyd AF, Davies CL, Holloway RK, Labrak Y, Ireland G, Carradori D, et al. Central nervous system regeneration is driven by microglia necroptosis and repopulation. Nat Neurosci. 2019;22(7):1046–52.
Sosna J, Philipp S, Albay RI, Reyes-Ruiz JM, Baglietto-Vargas D, LaFerla FM, et al. Early long-term administration of the CSF1R inhibitor PLX3397 ablates microglia and reduces accumulation of intraneuronal amyloid, neuritic plaque deposition and pre-fibrillar oligomers in 5XFAD mouse model of Alzheimer’s disease. Mol Neurodegener. 2018;13(1):11.
Kobashi S, Terashima T, Katagi M, Nakae Y, Okano J, Suzuki Y, et al. Transplantation of M2-deviated microglia promotes recovery of motor function after spinal cord injury in mice. Mol Ther. 2020;28(1):254–65.
Chen C, Guderyon MJ, Li Y, Ge G, Bhattacharjee A, Ballard C, et al. Non-toxic HSC transplantation-based macrophage/microglia-mediated GDNF delivery for parkinson’s disease. Mol Ther Methods Clin Dev. 2020;17:83.
Ban YH, Park D, Choi EK, Kim TM, Joo SS, Kim YB. Effectiveness of combinational treatments for alzheimer’s disease with human neural stem cells and microglial cells over-expressing functional genes. Int J Mol Sci. 2023;24(11): 9561.
Izrael M, Slutsky SG, Admoni T, Cohen L, Granit A, Hasson A, et al. Safety and efficacy of human embryonic stem cell-derived astrocytes following intrathecal transplantation in SOD1 G93A and NSG animal models. Stem Cell Res Ther. 2018;9(1):152.
Llorente IL, Xie Y, Mazzitelli JA, Hatanaka EA, Cinkornpumin J, Miller DR, et al. Patient-derived glial enriched progenitors repair functional deficits due to white matter stroke and vascular dementia in rodents. Sci Transl Med. 2021;13(590):eaaz6747.
Ehrlich M, Mozafari S, Glatza M, Starost L, Velychko S, Hallmann AL, et al. Rapid and efficient generation of oligodendrocytes from human induced pluripotent stem cells using transcription factors. Proc Natl Acad Sci U S A. 2017;114(11):E2243–52.
Hansen DV, Hanson JE, Sheng M. Microglia in Alzheimer’s disease. J Cell Biol. 2018;217:459–72 Rockefeller University Press.
Srinivasan K, Friedman BA, Etxeberria A, Huntley MA, van der Brug MP, Foreman O, et al. Alzheimer’s patient microglia exhibit enhanced aging and unique transcriptional activation. Cell Rep. 2020;31(13): 107843.
P Y, C C, Cd K, Y W, Td B, Sm P, et al. TREM2 Haplodeficiency in Mice and Humans Impairs the Microglia Barrier Function Leading to Decreased Amyloid Compaction and Severe Axonal Dystrophy. Neuron. 2016;90(4). Available from: https://pubmed.ncbi.nlm.nih.gov/27196974/. Cited 2024 Jan 17.
Liddelow SA, Guttenplan KA, Clarke LE, Bennett FC, Bohlen CJ, Schirmer L, et al. Neurotoxic reactive astrocytes are induced by activated microglia. Nature. 2017;541(7638):481–7.
Yamanaka K, Komine O. The multi-dimensional roles of astrocytes in ALS. Neurosci Res. 2018;126:31–8.
Gotkine M, Caraco Y, Lerner Y, Blotnick S, Wanounou M, Slutsky SG, et al. Safety and efficacy of first-in-man intrathecal injection of human astrocytes (AstroRx®) in ALS patients: phase I/IIa clinical trial results. J Transl Med. 2023;21(1):122.
McCaughey-Chapman A, Connor B. Cell reprogramming for oligodendrocytes: a review of protocols and their applications to disease modeling and cell-based remyelination therapies. J Neurosci Res. 2023;101(6):1000–28.
Nistor GI, Totoiu MO, Haque N, Carpenter MK, Keirstead HS. Human embryonic stem cells differentiate into oligodendrocytes in high purity and myelinate after spinal cord transplantation. Glia. 2005;49(3):385–96.
Wang S, Bates J, Li X, Schanz S, Chandler-Militello D, Levine C, et al. Human iPSC-derived oligodendrocyte progenitor cells can myelinate and rescue a mouse model of congenital hypomyelination. Cell Stem Cell. 2013;12(2):252–64.
Iadecola C. The pathobiology of vascular dementia. Neuron. 2013;80(4):844–66.
Cui LL, Golubczyk D, Tolppanen AM, Boltze J, Jolkkonen J. Cell therapy for ischemic stroke: are differences in preclinical and clinical study design responsible for the translational loss of efficacy? Annals Neurol. 2019;86(1):5–16.
Blum B, Benvenisty N. The tumorigenicity of human embryonic stem cells. Adv Cancer Res. 2008;100:133–58.
McQuade A, Coburn M, Tu CH, Hasselmann J, Davtyan H, Blurton-Jones M. Development and validation of a simplified method to generate human microglia from pluripotent stem cells. Mol Neurodegener. 2018;13. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6303871/. Cited 2020 Aug 12.
Muffat J, Li Y, Yuan B, Mitalipova M, Omer A, Corcoran S, et al. Efficient derivation of microglia-like cells from human pluripotent stem cells. Nat Med. 2016;22(11):1358–67.
Abud EM, Ramirez RN, Martinez ES, Healy LM, Nguyen CHH, Newman SA, et al. iPSC-derived human microglia-like cells to study neurological diseases. Neuron. 2017;94(2):278–293.e9.
Hasselmann J, Coburn MA, England W, Velez DXF, Shabestari SK, Tu CH, et al. Development of a chimeric model to study and manipulate human microglia in vivo. Neuron. 2019;103(6):1016–1033.e10.
Fattorelli N, Martinez-Muriana A, Wolfs L, Geric I, De Strooper B, Mancuso R. Stem-cell-derived human microglia transplanted into mouse brain to study human disease. Nat Protoc. 2021;16(2):1013–33.
Mancuso R, Van Den Daele J, Fattorelli N, Wolfs L, Balusu S, Burton O, et al. Stem-cell-derived human microglia transplanted in mouse brain to study human disease. Nat Neurosci. 2019;22(12):2111–6.
Haenseler W, Rajendran L. Concise review: modeling neurodegenerative diseases with human pluripotent stem cell-derived microglia. Stem Cells. 2019;37(6):724–30.
de Majo M, Koontz M, Rowitch D, Ullian EM. An update on human astrocytes and their role in development and disease. Glia. 2020;68(4):685–704.
Krencik R, Zhang SC. Directed differentiation of functional astroglial subtypes from human pluripotent stem cells. Nat Protoc. 2011;6(11):1710–7.
Krencik R, Weick JP, Liu Y, Zhang ZJ, Zhang SC. Specification of transplantable astroglial subtypes from human pluripotent stem cells. Nat Biotechnol. 2011;29(6):528–34.
Douvaras P, Fossati V. Generation and isolation of oligodendrocyte progenitor cells from human pluripotent stem cells. Nat Protoc. 2015;10(8):1143–54.
Fruhwürth S, Zetterberg H, Paludan SR. Microglia and amyloid plaque formation in Alzheimer’s disease – evidence, possible mechanisms, and future challenges. J Neuroimmunol. 2024;15(390):578342.
Björklund A, Parmar M. Dopamine Cell Therapy: From Cell Replacement to Circuitry Repair. J Parkinsons Dis. 2021;11(Suppl 2):S159–65.
Kim S, Pajarillo E, Nyarko-Danquah I, Aschner M, Lee E. Role of astrocytes in Parkinson’s disease associated with genetic mutations and neurotoxicants. Cells. 2023;12(4): 622.
Rostami J, Mothes T, Kolahdouzan M, Eriksson O, Moslem M, Bergström J, et al. Crosstalk between astrocytes and microglia results in increased degradation of α-synuclein and amyloid-β aggregates. J Neuroinflammation. 2021;18(1):124.
Dols-Icardo O, Montal V, Sirisi S, López-Pernas G, Cervera-Carles L, Querol-Vilaseca M, et al. Motor cortex transcriptome reveals microglial key events in amyotrophic lateral sclerosis. Neurology Neuroimmunology & Neuroinflammation. 2020;7(5): e829.
D’Erchia AM, Gallo A, Manzari C, Raho S, Horner DS, Chiara M, et al. Massive transcriptome sequencing of human spinal cord tissues provides new insights into motor neuron degeneration in ALS. Sci Rep. 2017;7(1):10046.
Nagai M, Re DB, Nagata T, Chalazonitis A, Jessell TM, Wichterle H, et al. Astrocytes expressing ALS-linked mutated SOD1 release factors selectively toxic to motor neurons. Nat Neurosci. 2007;10(5):615–22.
Haidet-Phillips AM, Hester ME, Miranda CJ, Meyer K, Braun L, Frakes A, et al. Astrocytes from familial and sporadic ALS patients are toxic to motor neurons. Nat Biotechnol. 2011;29(9):824–8.
Lassmann H. Multiple Sclerosis Pathology. Cold Spring Harb Perspect Med. 2018;8(3):a028936.
Mangale V, McIntyre LL, Walsh CM, Loring JF, Lane TE. Promoting remyelination through cell transplantation therapies in a model of viral-induced neurodegenerative disease. Dev Dyn. 2019;248(1):43–52.
Vieira R, Mariani JN, Huynh NPT, Stephensen HJT, Solly R, Tate A, et al. Young glial progenitor cells competitively replace aged and diseased human glia in the adult chimeric mouse brain. Nat Biotechnol. 2024;42(5):719–30.
Moser VA, Svendsen CN. Survival of the fittest glia. Nat Biotechnol. 2024;42(5):700–2.
Chen L, Xi H, Huang H, Zhang F, Liu Y, Chen D, et al. Multiple cell transplantation based on an intraparenchymal approach for patients with chronic phase stroke. Cell Transplant. 2013;22(Suppl 1):S83–91.
Xi H, Chen L, Huang H, Zhang F, Liu Y, Chen D, et al. Preliminary report of multiple cell therapy for patients with multiple system atrophy. Cell Transplant. 2013;22(Suppl 1):S93–99.
Mansour AA, Gonçalves JT, Bloyd CW, Li H, Fernandes S, Quang D, et al. An in vivo model of functional and vascularized human brain organoids. Nat Biotechnol. 2018;36(5):432–41.
Lancaster MA, Renner M, Martin CA, Wenzel D, Bicknell LS, Hurles ME, et al. Cerebral organoids model human brain development and microcephaly. Nature. 2013;501(7467):373–9.
Schafer ST, Mansour AA, Schlachetzki JCM, Pena M, Ghassemzadeh S, Mitchell L, et al. An in vivo neuroimmune organoid model to study human microglia phenotypes. Cell. 2023;186(10):2111–2126.e20.
Martinez-Curiel R, Jansson L, Tsupykov O, Avaliani N, Aretio-Medina C, Hidalgo I, et al. Oligodendrocytes in human induced pluripotent stem cell-derived cortical grafts remyelinate adult rat and human cortical neurons. Stem Cell Reports. 2023;18(8):1643–56.
Madrid M, Sumen C, Aivio S, Saklayen N. Autologous induced pluripotent stem cell-based cell therapies: promise, progress, and challenges. Current Protocols. 2021;1(3): e88.
Hanatani T, Takasu N. CiRA iPSC seed stocks (CiRA’s iPSC Stock Project). Stem Cell Res. 2021;1(50):102033.
Peng C, Gathagan RJ, Covell DJ, Medellin C, Stieber A, Robinson JL, et al. Cellular milieu imparts distinct pathological α-synuclein strains in α-synucleinopathies. Nature. 2018;557(7706):558–63.
Acknowledgements
Not applicable.
Funding
The authors have received funding from the Jane and Aatos Erkko Foundation (Š.L.), Sigrid Juselius Foundation (Š.L.), Päivikki and Sakari Sohlberg Foundation (Š.L., K.A.), Yrjö Jahnsson Foundation (Š.L.), Orion Foundation (K.A.), Young Universities for the Future of Europe (YUFE) (K.A.), and Doctoral Programme for Molecular Medicine (S.K.).
Author information
Authors and Affiliations
Contributions
K.A. and G.G. wrote the manuscript, J.J. and Š.L. contributed and gave critical comments, S.K. made Fig. 1.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Albert, K., Goldsteins, G., Kälvälä, S. et al. Glial cell transplant for brain diseases: the supportive saviours?. transl med commun 9, 22 (2024). https://doi.org/10.1186/s41231-024-00182-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41231-024-00182-y