Abstract
Background
Bloodstream infections (BSIs) are among the most lethal complications of liver transplantation (LT). Natural killer (NK) cells are an important component of innate immunity and play an essential role in infection and cancer. Adoptive transfer of activated NK cells has the potential to decrease post-LT infections, including BSIs.
Methods
In this prospective, single-center, interventional, single-arm, historical control, phase I/II study, 37 LT recipients will enroll. The patient will receive a single infusion of donor liver-derived NK cells 3−5 days after LT.
Discussion
The primary endpoint is the incidence of BSIs during the first month after LT. Secondary endpoints include overall survival, adverse events, immunological responses, hepatocellular or de novo malignancy, and incidence of infectious disease.
Trial registration
This study was prospectively registered with UMIN000019183 (https://center6.umin.ac.jp/cgi-open-bin/ctr/ctr_view.cgi?recptno=R000022074) on November 1, 2015 and jRCTa060190036 on February 27, 2020.
Similar content being viewed by others
Background
Although perioperative management and surgical techniques have advanced in living donor liver transplantation (LDLT), infectious complications are the most common cause of death shortly after LDLT [1]. Bloodstream infections (BSIs) are still mainly associated with morbidity and mortality and the incidence of BSIs has been reported to be approximately 10−40% [2,3,4,5]. Therefore, effective prevention and treatment are required.
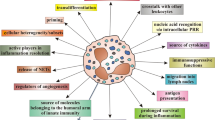
Natural killer (NK) cells play a crucial role in the first line of defense against infections and tumors [6]. The liver contains many NK cells that exhibit vigorous cytotoxicity against hepatoma cells and produce large amounts of cytokines in humans and mice [7, 8]. We have previously reported the safety and efficacy of clinical immunotherapy using liver-derived NK cells to prevent hepatocellular (HCC) after LT [9, 10]. In a subgroup analysis of this clinical trial, the NK therapy group showed significantly lower BSIs [11]. We reported a single nucleotide polymorphism of FcgRIIIa involved in bloodstream infection after liver transplantation [12]. We also identified ADCC (antibody dependent cellular cytotoxicity)-binding vulnerability of NK cells as a contributing factor and the possibility that this vulnerability can be overcome by NK cell therapy [13].
Therefore, we conducted a clinical trial of an adoptive immunotherapy approach using lymphocytes extracted from a liver allograft perfusate to prevent BSIs after LT.
Methods/design
Study design
This single-center, open-label, phase I/II study was designed to gain evidence for living donor liver-derived NK cell immunotherapy to prevent BSI after LDLT. This study is being conducted at Hiroshima University Hospital, Hiroshima, Japan. Participants who meet the eligibility criteria will be enrolled in the study. The study design is illustrated in Fig. 1.
Thirty-seven patients will be enrolled in the study. Eligible patients received standard LT and will be infused with enriched donor liver NK cells 3−5 days after LT. The enrolled patients are divided into two groups according to the following cell doses: the low-dose group (10−100 ⋅ 106 cells); and the high-dose group (100−1,000 ⋅ 106 cells/body), according to the results of our preliminary study [14]. This study aims to evaluate the safety of intravenous administration of donor liver NK cells after LT. The possible clinical efficacy of this immunotherapy will also be evaluated. Immunosuppression consists of a 3−6 month course of tapering corticosteroids and a calcineurin inhibitor, usually tacrolimus, to be maintained with or without mofetil mycophenolate. Prophylactic antibacterial agents given perioperatively and immunosuppressive agents given postoperatively are collected and analyzed. The primary endpoint is the incidence of BSI during the first month after LT. The secondary endpoints are the following:
-
1.
Overall survival (6 months, 1, and 3 years postoperatively).
-
2.
Adverse events: Type and severity of adverse events that occurred in this clinical study, frequency of occurrence, duration of occurrence, and causal relationship.
-
3.
Effect on immune response: Evaluation of donor-specific immunoreactivity by CFSE-MLR (1, 2, 3, and 4 weeks postoperatively), detection of donor-specific antibody (annual screening), incidence of rejection, and evaluation of NK cell activity in recipient peripheral blood.
-
4.
Presence and timing of hepatocellular carcinoma recurrence (hepatocellular carcinoma cases only) and de novo carcinogenesis.
-
5.
Incidence of infectious diseases (bacterial infections, cytomegalovirus infections, fungal infections, and analysis of genetic polymorphisms).
Inclusion criteria
Selection criteria for recipients (recipients of regenerative medicine) are the following:
-
1.
Patients undergoing partial LT for the treatment of refractory uncompensated liver cirrhosis with medical therapy.
-
2.
Patients aged ≥20 years at the time of consent.
-
3.
Patients who have obtained written consent from the patient or a substitute.
Selection criteria for cell donors (donors) are as follows:
-
1.
Patients who met the criteria of the LT Study Group’s “Guidelines for Living Donor Surgery” and undergo living donor surgery as liver donors.
-
2.
The patient must be ≥20 years of age at the time of obtaining consent.
-
3.
Patients who have obtained written consent from the donor or surrogate to prepare liver-derived NK cells from the perfusate of the donor liver graft and administer them to the recipient.
Exclusion criteria
The exclusion criteria for recipients are as follows:
-
1.
Patients undergoing reLT.
-
2.
Patients undergoing deceased donor LT.
-
3.
Other patients were judged by the principal investigator or subinvestigator to be inappropriate to participate in this clinical research.
The exclusion criteria for cell donors (donors) are as follows:
-
1.
Patients undergoing LDLT for reLT.
-
2.
Patients were judged by the principal investigator or subinvestigator to be inappropriate to participate in this clinical research.
Sample size calculation
In a previous study comparing the incidence of BSIs 1 month after LDLT in 21 patients in the NK therapy group and 21 patients in the matched non-NK therapy group for 114 living donor liver transplants performed between January 2004 and December 2009, the incidence of BSI in the NK therapy group was 10% compared with 30% in the non-NK therapy group [11]. Based on these results, the expected incidence of BSIs in NK therapy 1 month after LDLT was set at 10%, and the number of cases required to examine the threshold of 30% incidence in non-NK therapy at the 5% significance level (both sides) and the power of 80% was calculated at 34. Considering the dropout cases at the time of enrollment, we set the target number of cases to 37.
Treatment
Cell preparation is performed as previously described [14]. Briefly, after organ recovery, the liver is perfused through the portal vein with 2 L of the University of Wisconsin solution on the back table. Liver mononuclear cells are collected using Ficoll-Hypaque density-gradient centrifugation and cultured with 1000 U/mL of human recombinant interleukin (IL)-2 (Teceleukin, Shionogi, Japan) in X-VIVO 15 medium (LONZA, Walkersville, MD) supplemented with 100 µg/ml of gentamycin (APP Pharmaceuticals, Schaumburg, IL), 10% human AB serum (Valley Biomedical, Winchester, VA) for 3−5 days to prime NK cells with enhanced antitumor properties. To prevent graft-versus-host disease (GVHD), i.e., to inactivate CD3+ alloreactive T cells, an anti-CD3 monoclonal antibody (mAb) (Miltenyi, Germany), is added to the culture medium (100 µg/mL) 1 day before cell harvest. A minimum of 1 × 107 cells with cell viability of > 80% is required to release the NK cell product for infusion. After verification of quality control, including Gram staining, culture, endotoxin, and mycoplasma tests, all unfractionated cells, processed as described above and defined as the final NK cell products, are infused into the patients via intravenous administration on the day of cell harvesting (Fig. 2). Periodic blood tests before and after cell administration, various culture tests, x-rays, and measurement of NK cell phenotype and cytotoxic activity in peripheral blood are performed.
Follow-up and assessment of efficacy
Within the first month after LT, patients undergo weekly blood culture and fever tests. Patients undergo survival checks, adverse event checks, and infection and malignancy checks up to 3 years after surgery.
Immunological assessment
All flow cytometry (FCM) analyses are performed on a BD LSR Fortessa, FACS Canto II Cytometer, and FACSCalibur flow cytometer (BD Biosciences, San Jose, CA). To detect the surface phenotype, leukocytes are stained with monoclonal antibodies: against CD3 (clone HIT3a; BD Biosciences – Pharmingen), the tumor necrosis factor-related apoptosis-inducing ligand (clone RIK-2; eBioscience), NKG2D (clone 1D11; BD Biosciences – Pharmingen), CD69 (clone FN50; BD Biosciences – Pharmingen), CD226 (clone 11A8; BioLegend), and CD56 (clone B159; BD Biosciences – Pharmingen). Data are analyzed using FlowJo software (Tree Star, Inc. Ashland, OR). A 51Cr-release assay was performed as previously described [7], using HepG2 tumor cells (Japanese Cancer Research Resources Bank) as targets. Briefly, 51Cr-labeled target tumor cells were added to effector cells in round-bottomed 96-well microtiter plates (BD Biosciences - Discovery Labware) for 4 h at 37 °C. The percentage of specific 51Cr released was calculated as follows: % cytotoxicity = [(cpm of experimental release – cpm of spontaneous release)/(cpm of maximum release – cpm of spontaneous release)] × 100. All assays are performed in triplicate.
Safe evaluation and reporting of adverse effects
After cell administration, the investigator will record all events, whether cell administration is related or not, including complications after LT. In the event of serious complications related to prolonged hospitalization or death, the investigator will promptly report to the Committee for Regenerative Medicine and the Ministry of Health, Labor and Welfare.
Discussion
BSI is one of the most serious complications after LT and affects the prognosis. To address this high medical need, we introduce immunotherapy using donor liver NK cells to evaluate safety and efficacy in patients after LT.
Sixty to eighty percent of liver transplant recipients experience some type of infection within the first year of surgery. Most of these infections are curable; however, some are fatal [15, 16]. BSI has been reported to be responsible for 30% of posttransplant deaths. In particular, the frequency of infection is highest within the first month after transplantation [17].
Immunotherapy with donor liver-derived NK cells was originally applied to LDLT at Hiroshima University, Japan, since 2006 [10]. This NK therapy involves the risk of GVHD and acute rejection because of the administration of allo-cells. Anti-CD3 antibodies are used to eliminate alloreactive T cells [14]. In this trial, 24 LT recipients safely received NK cell infusion with acceptable outcomes, i.e., without severe adverse events, including GVHD, acute rejection and recurrence-free satisfactory overall survival [9]. We analyzed the incidence of early postoperative BSIs in 114 LDLT cases performed at Hiroshima University Hospital from 2004 to 2009. The frequency of BSIs was significantly lower in the NK cell infusion group than in patients who did not receive NK cell infusion, adjusted for background factors using the propensity score matching method [11]. The argument that immunosuppression increases the risk of infection is accepted. In contrast, immunosurveillance against microbes is exerted by orchestrating innate and acquired immunity, and the immunosuppressive drugs currently used after LT preferentially suppress acquired components such as T and B cells [18]. Therefore, innate immunity, including NK cells, plays a major role in prophylactic activity in the LT setting. We have previously demonstrated that polymorphisms in genes encoding FcγRIIIa, which is expressed by NK cells and mediates natural antibody-directed activity in innate immunity, can be a predisposing factor to severe bacterial infections and predict mortality after LT [12]. Despite the important role of NK cells, functional impairment and decreased number of NK cells have been observed in patients with end-stage liver disease that requires LT [7, 19, 20]. In LT recipients, who might be in such an immunological predicament, augmentation of functionally activated liver NK cells may be a promising approach. However, the only technique to harvest liver-derived NK cells is liver transplantation in which the liver can be flushed. We are currently investigating the technology to generate liver-derived NK cells from peripheral blood or bone marrow CD34-positive stem cells for application to diseases other than liver transplantation.
Availability of data and materials
Trial and data collection are ongoing. Clinical datasets are available from the corresponding author.
Abbreviations
- BSI:
-
Bloodstream infection
- FCM:
-
Flow cytometry
- GVHD:
-
Graft-versus-host disease
- LDLT:
-
Living donor liver transplantation
- LT:
-
Liver transplantation
- NK:
-
Natural killer
References
Baganate F, Beal EW, Tumin D, Azoulay D, Mumtaz K, Black SM, et al. Early mortality after liver transplantation: Defining the course and the cause. Surgery. 2018;164(4):694–704.
Santos CA, Hotchkiss RS, Chapman WC, Olsen MA. Epidemiology of Bloodstream Infections in a Multicenter Retrospective Cohort of Liver Transplant Recipients. Transplantation direct. 2016;2(3):e67.
Park J, Kim BW, Choi HJ, Hong SH, Park CS, Choi JH, et al. Risk stratification for early bacteremia after living donor liver transplantation: a retrospective observational cohort study. BMC Surg. 2020;20(1):2.
Iida T, Kaido T, Yagi S, Yoshizawa A, Hata K, Mizumoto M, et al. Posttransplant bacteremia in adult living donor liver transplant recipients. Liver Transpl. 2010;16(12):1379–85.
Ikegami T, Shirabe K, Yoshiya S, Yoshizumi T, Ninomiya M, Uchiyama H, et al. Bacterial sepsis after living donor liver transplantation: the impact of early enteral nutrition. J Am Coll Surg. 2012;214(3):288–95.
Vivier E, Tomasello E, Baratin M, Walzer T, Ugolini S. Functions of natural killer cells. Nat Immunol. 2008;9(5):503–10.
Ishiyama K, Ohdan H, Ohira M, Mitsuta H, Arihiro K, Asahara T. Difference in cytotoxicity against hepatocellular carcinoma between liver and periphery natural killer cells in humans. Hepatology. 2006;43(2):362–72.
Ohira M, Ohdan H, Mitsuta H, Ishiyama K, Tanaka Y, Igarashi Y, et al. Adoptive transfer of TRAIL-expressing natural killer cells prevents recurrence of hepatocellular carcinoma after partial hepatectomy. Transplantation. 2006;82(12):1712–9.
Ohira M, Hotta R, Tanaka Y, Matsuura T, Tekin A, Selvaggi G, et al. Pilot study to determine the safety and feasibility of deceased donor liver natural killer cell infusion to liver transplant recipients with hepatocellular carcinoma. Cancer Immunol Immunother. 2022;71(3):589–99.
Ohira M, Ishiyama K, Tanaka Y, Doskali M, Igarashi Y, Tashiro H, et al. Adoptive immunotherapy with liver allograft-derived lymphocytes induces anti-HCV activity after liver transplantation in humans and humanized mice. J Clin Invest. 2009;119(11):3226–35.
Tashiro H, Ishiyama K, Ohira M, Igarashi Y, Tahara H, Ide K, et al. Impact of adjuvant immunotherapy using liver allograft-derived lymphocytes on bacteremia in living-donor liver transplantation. Transplantation. 2011;92(5):575–80.
Shimizu S, Tanaka Y, Tazawa H, Verma S, Onoe T, Ishiyama K, et al. Fc-Gamma Receptor Polymorphisms Predispose Patients to Infectious Complications After Liver Transplantation. Am J Transplant. 2016;16(2):625–33.
Shimizu S, Ohira M, Tanaka Y, Ide K, Tahara H, Kuroda S, et al. Adoptive immunotherapy overcomes genetic susceptibility to bloodstream infections due to fc-gamma receptor polymorphisms after liver transplantation. Am J Transplant. 2022. (in press)
Ohira M, Nishida S, Tryphonopoulos P, Tekin A, Selvaggi G, Moon J, et al. Clinical-scale isolation of interleukin-2-stimulated liver natural killer cells for treatment of liver transplantation with hepatocellular carcinoma. Cell Transpl. 2012;21(7):1397–406.
Colonna JO 2nd, Winston DJ, Brill JE, Goldstein LI, Hoff MP, Hiatt JR, et al. Infectious complications in liver transplantation. Arch Surg. 1988;123(3):360–4.
Kawecki D, Chmura A, Pacholczyk M, Lagiewska B, Adadynski L, Wasiak D, et al. Bacterial infections in the early period after liver transplantation: etiological agents and their susceptibility. Med Sci monitor: Int Med J experimental Clin Res. 2009;15(12):CR628–37.
Fishman JA, Rubin RH. Infection in organ-transplant recipients. N Engl J Med. 1998;338(24):1741–51.
Harada N, Shimada M, Okano S, Suehiro T, Soejima Y, Tomita Y, et al. IL-12 gene therapy is an effective therapeutic strategy for hepatocellular carcinoma in immunosuppressed mice. J Immunol. 2004;173(11):6635–44.
Balch CM, Tilden AB, Dougherty PA, Cloud GA, Abo T. Heterogeneity of natural killer lymphocyte abnormalities in colon cancer patients. Surgery. 1984;95(1):63–70.
Cai L, Zhang Z, Zhou L, Wang H, Fu J, Zhang S, et al. Functional impairment in circulating and intrahepatic NK cells and relative mechanism in hepatocellular carcinoma patients. Clin Immunol. 2008;129(3):428–37.
Acknowledgements
We would like to thank Editage.com for proofreading of this manuscript.
Funding
This study was supported by AMED under Grant Number JP21fk0210051 (Hideki Ohdan), and JSPS KAKENHI under Grant Number JP20K09104 (Masahiro Ohira).
Author information
Authors and Affiliations
Contributions
MO participated in the trial design, performed the NK therapy, and prepared the manuscript. YI, KS, KI, RN and NT prepared the study protocol, and performed the NK therapy. HT, KI, and TK assisted in preparing the study protocol and performed surgical procedures. YT assisted in the preparation of the study protocol and conducted the immunological analysis. HO designed the trial, prepared the study protocol, and conducted correspondence. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This phase I clinical trial was approved by the Special Committee for Regenerative Medicine, Hiroshima University, and registered with the Japan Registry of Clinical Trials (jRCTa060190036). This trial was designed and conducted following the Declaration of Helsinki. A written informed consent was obtained from all patients before enrollment in the study.
Consent for publication
Not applicable.
Competing interests
The authors of this manuscript have no conflict of interest to disclose.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ohira, M., Imaoka, Y., Sato, K. et al. A phase I/II study of adoptive immunotherapy using donor liver graft-derived natural killer cells to prevent bloodstream infection after liver transplantation: a study protocol. transl med commun 7, 19 (2022). https://doi.org/10.1186/s41231-022-00126-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41231-022-00126-4