Abstract
The study objective was to assess the frequency of the kdr-L995F and ace-1 G280S genetic mutations in Anopheles gambiae s.l. mosquitoes and examine their ability to transmit Plasmodium falciparum in areas where indoor residual spraying (IRS) was implemented with Clothianidin 50 WG. The study was conducted in six communes in the Alibori and Donga departments of which four were IRS-treated and two were untreated and served as control. Post-IRS monthly samples of adult mosquitoes were collected in study communes using human landing catches (HLC). An. gambiae s.l. specimens were processed to detect kdr-L995F and ace-1 G280S mutations via PCR as well as Plasmodium falciparum infectivity through CSP ELISA. Our data revealed a high and similar allelic frequency for the kdr-L995F mutation in both treated and control communes (79% vs. 77%, p = 0.14) whilst allelic frequency of the ace-1 G280S mutation was lower across the study area (2–3%, p = 0.58). The sporozoite rate was 2.6% and 2.4% respectively in treated and untreated communes (p = 0.751). No association was found between Plasmodium falciparum infection in Anopheles gambiae s.l. vectors and carriage of kdr-L995F and ace-1 G280S mutations regardless of genotypes. The study findings underline the need for an integrated approach to malaria control, combining different control methods to effectively target transmission. Regular monitoring of insecticide resistance and genetic mutations is essential to guide control strategies.
Similar content being viewed by others
Background
Malaria is a vector-borne disease transmitted by mosquitoes of the Anopheles genus [1]. In 2021, 95% of malaria cases (234 million/247 million) and 96% of deaths (593,000/619,000) were reported in the WHO African Region. Approximately 80% of all malaria-related deaths in the region involve children under 5-year old. This rate due to malaria deaths in children under 5-year old has remained constant in the region since 2015 [2]. The causative agent is a protozoan of the Plasmodium genus transmitted to humans through the bite of female mosquitoes [3]. In Benin, Plasmodium falciparum alone was responsible for 2,876,368 malaria cases recorded in 2021 and 2956 deaths with an increase 620 deaths compared to 2020 (2336) [2, 4]. The primary vector of this parasite in West Africa [5] and Benin [6] is Anopheles gambiae s.l. In the northern departments of Benin, malaria remains a significant public health issue [6].
Indoor residual spraying (IRS) is commonly used in Benin to complement the use of pyrethroid-impregnated mosquito nets to reduce malaria transmission in high-transmission areas (PMI, 2021). These interventions have been effective in reducing malaria transmission [7, 8]. Unfortunately, the expansion of vector resistance to pyrethroids (used in agriculture [9, 10], for impregnation of mosquito nets [11, 12] and for other domestic uses), carbamates and organophosphates (used for indoor residual spraying in Benin from 2008 to 2019 [13, 14]), poses a serious threat to progress in malaria control.
In 2021, Benin’s national malaria control program (NMCP) has opted to select Sumishield 50 WG, a dispersible granule formulation of clothianidin for large-scale IRS campaigns in the Djougou–Copargo–Ouake and Kandi–Gogounou–Segbana health zones [15]. This decision stems from the insecticide’s ability to act differently from previously used insecticides, particularly on vectors carrying the kdr-L995F and ace-1 G280S mutations [16]. Clothianidin, as a neonicotinoid insecticide, acts as an agonist of nicotinic acetylcholine receptors, causing paralysis and insect death [17]. In Benin, clothianidin-based insecticides have a long residual efficacy of 8 to 10 months in large-scale in community trials on mud and cement walls [18]. Its introduction into public health aims to optimize IRS by reducing infectivity through the elimination of malaria vectors, particularly those with the kdr L995F and ace-1 G280S resistance mechanisms. The kdr-L995F mutation in the voltage-dependent sodium channel (Vgsc), a target of pyrethroids and DDT, alters the interaction with insecticide molecules, resulting in knock-down resistance [19, 20]. The presence of this mutation at position 1014 in house flies (Musca domestica) was first documented [21]. In Anopheles gambiae s.l. mosquitoes, knock-down resistance mutations are found at position 995 [21,22,23]. They result from a leucine to phenylalanine substitution (L995F) in West Africa [24, 25] and a leucine to serine substitution (L995S) in Central and East Africa [26]. The ace-1 G280S mutation in acetylcholinesterase (AChE) makes resistant individuals less sensitive to the inhibitory action of organophosphates and carbamates [27]. Acetylcholinesterase (AChE) is an enzyme that terminates synaptic transmission by catalyzing the hydrolysis of the neurotransmitter acetylcholine. It has been demonstrated that the most common ace-1 gene mutation in Anopheles gambiae s.l. results in the replacement of glycine (GGC) with serine (AGC) at codon 280 (G280S) [28]. This mutation of the acetylcholinesterase enzyme is also found at codon 119 in a partial crystal structure of the electric ray Torpedo californica [27, 29, 30]. AChE in resistant individuals is less sensitive to the inhibitory action of organophosphates and carbamates compared to that in susceptible individuals [31, 32]. In Benin, the resistance allele 280S has been consistently found at a low proportion in field populations of An. gambiae and An. coluzzii [13, 14]. These mutations are crucial as they are associated with insecticide resistance, particularly in Anopheles gambiae s.l. The frequency of these mutations in mosquito populations has a direct impact on the effectiveness of IRS programs [26, 33].
The need to assess the ability of resistant genotypes to transmit Plasmodium falciparum in communes under IRS with clothianidin 50 WG is essential to understand whether the use of clothianidin maintains significant efficacy against malaria vectors, despite the mutations observed.
Methods
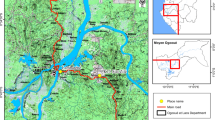
The study was conducted in the health zones of Djougou–Copargo–Ouaké and Kandi–Gogounou–Segbana located respectively in Northwestern and Northeastern Benin where Clothianidin IRS has been implemented. Entomological monitoring and evaluation (M&E) data were collected in a total of 6 communes of which two treated communes and one neighbouring untreated commune to serve as control per department. Overall Copargo and Djougou in the Donga department and Kandi and Gogounou in Alibori were the treated communes surveyed whilst Bassila and Bembèrèkè were the control communes identified nearby (Fig. 1). Each commune is characterized by two seasons: a rainy season and a dry season. The population’s main activities are farming, livestock breeding and fishing [34,35,36].
Sampling of adult mosquitoes
Post-IRS mosquito sampling was carried out on monthly basis between April and December in 2021 using the human landing catch (HLC). In both treated and control communes, mosquitoes were collected in two selected villages and four houses per village. Adult mosquitoes were captured of each visit by local volunteers. One collector inside and one collector outside were stationed at a household for hourly collections of mosquitoes at the level of each household.
Malaria vector mosquitoes collected were morphologically identified using taxonomic keys [37, 38]. The head and thorax were kept to process ELISA-CSP. The rest of the body (abdomen, wings, and legs) was used for genomic DNA extraction and molecular characterization.
Screening of P. falciparum infection in An. gambiae s.l.
The heads-thoraxes of identified An. gambiae s.l. specimens were analysed using the CSP ELISA method following the protocol described by Wirtz et al. [39] to detect the circumsporozoite antigen (CSP), indicative of the presence of Plasmodium falciparum.
DNA extraction of mosquito
Abdomens, wings, and legs were crushed in 200 μL of 2% cetyltrimethylammonium bromide (CTAB). After 5 min of water bath at 65 °C, 200 μL of chloroform was added to the mixture and centrifuged at 12,000 rpm for 5 min. The top portion was gently collected in another tube and supplemented with 200 μL of isopropanol, homogenized, and centrifuged again at 12,000 rpm for 15 min. The liquid in the tube was carefully inverted so as not to lose the pellet at the bottom. 200 µL of 70% ethanol were added to the pellet for precipitation. After 5 min of centrifugation, the contents of the tube were finely inverted again. The pellet was then drained for at least 3 h on the benchtop. The extracted DNA was reconstituted with 20 μL of sterile water and left in suspension on the benchtop overnight [40].
Screening of kdr-L995F mutation in An. gambiae s.l.
Studies on insect species have revealed various substitutions of the Vgsc gene inducing a resistance phenotype [20, 41]. The PCR for detecting the kdr mutation employs four primers with distinct sequences: D1. ATAGATTCCCCGACCATG; D2. AGACAAGGATGATGAACC; D3. AATTTGCATTACTTACGACA; D4. CTGTAGTGATAGGAAATTTA [23].
The amplification program is composed of 40 cycles. Each cycle includes initial denaturation at 94 °C for 1 min, hybridization at 48 °C for 2 min, and elongation at 72 °C for 2 min. Finally, this PCR ends with a final elongation at 72 °C for 10 min [42].
Screening of ace-1 G280S mutation in An. gambiae s.l.
The PCR for the G280S mutation utilizes two specific primers with the following base sequences: Ex3AGdir GATCGTGGACACCGTGTTCG and Ex3AGrev AGGATGGCCCGCTGGAACAG, according to the protocol by Weill et al. [41]. The amplification program was as follows: 30 cycles and each cycle included denaturation at 94 °C for 30 s, hybridization at 52 °C for 30 s, and elongation at 72 °C for 1 min. PCR products were digested with AluI restriction enzyme according to the manufacturer’s instructions before migration onto a 2% agarose gel.
Clothianidin 50 WG
Insects possess nicotinic acetylcholine receptors (nAChR), which are the target of clothianidin in their nervous system [43]. These receptors are responsible for the transmission of nerve signals between nerve cells. Clothianidin acts by binding specifically to these nAChR receptors. When it binds to nAChR receptors, it activates them for an extended duration compared to acetylcholine, which is a natural neurotransmitter. This results in excessive stimulation of nerve cells and prolonged excitation of the insect’s nervous system [44]. Consequently, the insect becomes paralyzed because its nervous system remains constantly excited and can no longer function properly. Eventually, this leads to the insect’s death [43,44,45].
Clothianidin 50 WG was introduced for public health use in Benin in 2021 as part of large-scale community-based indoor residual spraying (IRS) campaigns. This choice aimed to optimize IRS by reducing infectivity through the elimination of malaria vectors, particularly those with resistance mechanisms such as kdr-L995F and ace-1 G280S, with the new chemical mode of action of clothianidin.
Statistical analysis
Insecticide resistance mutations and P. falciparum infection data were analysed under R statistical software (version 4.1.0). The chi-square test for comparison of proportions was performed to verify the relationship between IRS-treated areas and untreated areas (control). The odds ratio was also evaluated using R software to assess the malaria transmission ability of different genotypes for kdr-L995F and ace-1 G280S mutations. Frequencies of the mutant allele kdr L995F and ace-1 G280S were calculated using the formula:
where F(R) is the frequency of resistance, n the number of mosquitoes of a given genotype, RR the homozygous resistant genotype, RS the heterozygous resistant genotype, and SS the susceptible genotype [14]. A multivariable logistic regression was done in RStudio version 1.3.959 and R statistical software version 4.21 to determine the association (odds ratio) between the independent parameters, (1) different genotypes for kdr-L995F and ace-1 G280S mutations (RR, RS, and SS), (2) location (Kandi, Gogounou, Bembèrèkè and Djougou, Copargo, Bassila).
Results
Overall, 10,091 An. gambiae s.l. females, including 3585 and 6506 respectively from the two control communes and four IRS-treated communes, were processed for ELISA. However, a sub-sample of 2432 specimens of An. gambiae s.l. representing 24% of the total collected was analysed for insecticide resistance mutations.
Frequency of kdr-L995F and ace-1 G280S mutations in Anopheles gambiae s.l.
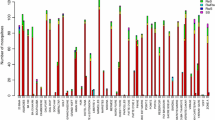
Tables 1 and 2 present genotypic and allelic frequency variations of respectively kdr-L995F and ace-1 G280S mutations in mosquitoes of the Anopheles gambiae complex across the study communes. Pooled allelic frequency in IRS communes (79%) was higher than in control communes (77%, p = 0.14). At department level, kdr-L995F mutation showed generally high frequency in all the study communes, particularly in the communes of Donga department where a significantly lower frequency was found in the control commune Bassila (80%) compared to the two treated communes of Copargo (85%, p = 0.01) and Djougou (88%, p < 0.001) (Table 1). Meanwhile, the Alibori department showed significantly higher allelic frequency of kdr-L995F mutation in the control commune (Bembèrèkè) with similar frequency trend in Gogounou (72%, p = 0.67) unlike Kandi (68%, p = 0.03) (Table 1). Across all the study communes, homozygous resistant (RR) specimens for the kdr-L995F mutation were most prevalent, fluctuating between 70 and 83% in the Donga department and 54% to 59% in the Alibori department. Conversely, homozygous susceptible (SS) individuals were the least represented (Table 1).
The allelic frequency of the ace-1 G280S mutation in An. gambiae complex mosquitoes was generally low (2–3%) in all communes and no significant difference was observed between control and IRS communes in both Alibori and Donga departments (Table 2). Susceptible homozygous specimens for the ace-1 G280S mutation were the most represented (94–96%). No resistant homozygous (RR) specimens were found in the study area (Table 2).
Infectivity to Plasmodium falciparum in An. gambiae s.l.
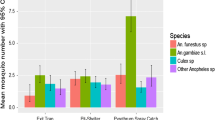
The assessment of infectivity to Plasmodium falciparum using the enzyme-linked immunosorbent assay (ELISA) targeting the circumsporozoite protein (CSP) was conducted on 10,091 An. gambiae s.l. Table 3 and Fig. 2 show variation of sporozoite rates in the study area. Pooled data showed no difference between untreated and treated communes across the study area (2.4% vs. 2.6%, p = 0.751). The trend was similar in the Alibori department with similar sporozoite rates in both untreated (1.3%) and treated communes (0.8–1.3%, p > 0.05) whilst in the Donga department, the treated Copargo commune provided a significantly higher sporozoite rate as compared to the control (5.8% vs. 4.1%, p = 0.049) unlike in Djougou where the sporozoite rate was lower than in the control (2.6% vs. 4,1%, p = 0.015).
Ability of P. falciparum transmission by An. gambiae s.l. in presence of kdr-L995F and ace-1 G280S mutations
Tables 4 and 5 present logistic regression analysis outcomes of the infectivity to P. falciparum in different genotypes for respectively the kdr-L995F and ace-1 G280S mutations across the study communes. Regarding the kdr-L995F mutation, the odds ratios (OR) showed similar P. falciparum infection in resistant homozygous (RR) and heterozygous (RS) specimens of An. gambiae s.l. in Donga regardless of the commune and study arm (OR: 1.26 with p = 0.52 in Bassila; OR: 1.37 with p = 0.41 in Copargo; OR: 1.9 with p = 0.13 in Djougou) (Table 4). In contrast, homozygous susceptible (SS) An. gambiae s.l. mosquitoes from Donga exhibited significantly higher likelihood for P. falciparum transmission compared to specimens of homozygous resistant (RR) genotype in all study communes (OR: 3.49 with p < 0.001 in Bassila; OR: 21.53 with p < 0.0001 in Copargo; OR: 5.67 with p < 0.0001 in Djougou) (Table 4). The same observation was made for all IRS communes compared with control communes. From the kdr-L995F mutation data collected in the Alibori department, the probability of P. falciparum infection in An. gambiae s.l. was generally similar irrespectively of the genotypes (RR, RS, SS) (p > 0.05) in Bembèrèkè, Gogounou and Kandi (Table 4).
Regarding the ace-1 G280S mutation, whilst the communes of Alibori showed similar P. falciparum infection in hybrid (RS) and homozygous susceptible (SS) genotypes (OR: 0.48 with p = 0.28 in Gogounou; OR: 0.59 with p = 0.48 in Kandi), it was observed a significantly higher probability of P. falciparum infection in homozygous susceptible (SS) and heterozygous (RS) genotypes in the communes of Donga (p = 0.02 in Copargo; OR: 0.12 with p < 0.001 in Djougou) (Table 5).
Discussion
The present study aimed to investigate the ability of Anopheles gambiae s.l. to transmit P. falciparum in the presence of kdr-L995F and ace-1 G280S mutations in Northern Benin. This study was conducted in six communes of which four communes were subject to IRS intervention and two communes were untreated. Our data revealed a relatively high allelic frequency of the kdr-L995F mutation across all study communes. These findings indicate the level of resistance of malaria vector Anopheles gambiae s.l. to the pyrethroids and organochlorine dichlorodiphenyltrichloroethane (DDT) within the study area. This aligns with the recent studies which demonstrated high allelic frequencies of the kdr-L995F mutation in various localities in Benin [12, 46, 47] and across different malaria-endemic African countries [48,49,50,51]. The widespread pyrethroid resistance is a consequence of the heavy selection pressure of intensified deployment of insecticide-treated bed nets in recent years [52]. In addition, the observed resistance situation in the surveyed sites is also linked to the extensive pyrethroid use in agriculture for crop protection, as reported by Yadouleton et al. [53, 54]. The allelic frequency of the kdr-L995F mutation within the Vgsc gene was significantly higher in the IRS communes than in the Donga department, unlike in the Alibori communes. This could be explained by the heavy use of pyrethroids in agriculture in the commune of Bembèrèkè and by IRS intervention in Copargo and Djougou. Indeed, although neonicotinoids act primarily on the insect nervous system by targeting nicotinic acetylcholine receptors, there is evidence that exposure to clothianidin can be associated with genetic mutations and metabolic resistance alleles in An. gambiae [55, 56].
Regarding the frequency of the G280S mutation within the ace-1 gene, it remained very low across all communes, not exceeding 4%. However, it was observed an allelic frequency slightly lower in Bassila (control commune) than in Copargo and Djougou (IRS communes). Overall, the allelic frequencies of IRS communes (3%) and control communes (2%) were similar (p = 0.58), confirming the lesser targeting of the ace-1 gene in vector control interventions in Benin.
The infectivity of An. gambiae s.l. to Plasmodium falciparum mosquitoes significantly varied across communes and departments, regardless of the evolving frequencies of the kdr-L995F Vgsc and G280S ace-1 genes mutation. Copargo commune showed significantly higher sporozoite rates (p = 0.049) compared to the other communes (Bassila and Djougou). The potential for increased malaria transmission observed in Copargo (treated zone) compared to the control commune (Bassila) could be attributed to the inherently higher malaria transmission in this area, as observed by Yadouleton et al. [57]. In fact, Copargo is a bordering commune of the Atacora department, where malaria incidence is amongst the highest nationally [6, 58].
The ability of P. falciparum transmission in An. gambiae s.l. carrying the kdr-L995F and ace-1 G280S mutations was investigated across all study communes. Our data found no association between P. falciparum infection and kdr-L995F mutation carriage in An. gambiae s.l. Thus, whether the mosquito is homozygous (RR), homozygous susceptible (SS), or heterozygous (RS), it does not impact its ability transmit P. falciparum. A similar trend was observed with the G280S mutation in the ace-1 gene in the presence of which a comparable P. falciparum transmission was recorded irrespective of mosquito genotype. These findings are consistent with Ossè et al. [59] study demonstrating no significant difference in allelic frequency between P. falciparum-infected and non-infected An. gambiae s.l. female mosquitoes with all genotypes for the kdr-L995F Vgsc gene and G280S ace-1 gene mutations. Similarly, Mitri et al. [60] reported linkage of genetic variation influencing Plasmodium infection to a natural 3-megabase haplotype on chromosome 2L carrying the kdr allele of the gene, which does not directly influence susceptibility to the parasite.
Conclusion
The results of the investigations revealed that the kdr-L995F gene mutation is very frequent in the study area whilst the ace-1 mutation was less predominant in An. gambiae s.l. malaria vector within Donga and Alibori departments. Although malaria transmission was detected at variable levels across IRS and control communes, the results showed that the ace-1 G280S and kdr-L995F genes mutations have no impact on the transmission of P. falciparum by Anopheles gambiae complex members. This finding underscores the importance of adopting an integrated approach to malaria control, combining various control strategies to effectively target the vectors.
Availability of data and materials
The data used and/or analysed in this study are available from the corresponding author on reasonable request.
Abbreviations
- IRS:
-
Indoor residual spraying
- WHO:
-
World Health Organization
- SR:
-
Sporozoite rate
- HLC:
-
Human landing catch
- NMCP:
-
National malaria control program
References
Vector-borne diseases. 2020. https://www.who.int/news-room/fact-sheets/detail/vector-borne-diseases.
World Health Organization. Global messaging briefing kit, world malaria report 2022. https://cdn.who.int/media/docs/default-source/malaria/world-malaria-reports/world-malaria-report-2022-global-briefing-kit-eng.pdf?sfvrsn=5ec7ec5c_6.
Crutcher JM, Hoffman SL. Malaria. In: Baron S, editor. Medical microbiology. 4th ed. Galveston: University of Texas Medical Branch at Galveston; 1996.
World malaria report. Med. Malar. Venture. 2022. https://www.mmv.org/newsroom/news-resources-search/world-malaria-report-2022.
Bayoh MN, Thomas CJ, Lindsay SW. Mapping distributions of chromosomal forms of Anopheles gambiae in West Africa using climate data. Med Vet Entomol. 2001;15:267–74.
PMI. U.S. president’s malaria initiative Benin malaria operational plan FY 2022. 2022.
Aïkpon R, Sèzonlin M, Tokponon F, Okè M, Oussou O, Oké-Agbo F, et al. Good performances but short lasting efficacy of actellic 50 EC indoor residual spraying (IRS) on malaria transmission in Benin, West Africa. Parasit Vectors. 2014;7:256.
Salako AS, Dagnon F, Sovi A, Padonou GG, Aïkpon R, Ahogni I, et al. Efficacy of actellic 300 CS-based indoor residual spraying on key entomological indicators of malaria transmission in Alibori and Donga, two regions of northern Benin. Parasit Vectors. 2019;12:612.
Fassinou AJYH, Koukpo CZ, Ossè RA, Agossa FR, Azondékon R, Sominahouin A, et al. Pesticides and the evolution of the genetic structure of Anopheles coluzzii populations in some localities in Benin (West Africa). Malar J. 2019;18:407.
Nkya TE, Poupardin R, Laporte F, Akhouayri I, Mosha F, Magesa S, et al. Impact of agriculture on the selection of insecticide resistance in the malaria vector Anopheles gambiae: a multigenerational study in controlled conditions. Parasit Vectors. 2014;7:480.
Ochomo EO, Bayoh NM, Walker ED, Abongo BO, Ombok MO, Ouma C, et al. The efficacy of long-lasting nets with declining physical integrity may be compromised in areas with high levels of pyrethroid resistance. Malar J. 2013;12:368.
Sagbohan HW, Kpanou CD, Sovi A, Osse R, Sidick A, Adoha C, et al. Pyrethroid resistance intensity in Anopheles gambiae s.l. from different agricultural production zones in Benin, West Africa. Vector Borne Zoonotic Dis. 2022;22:39–47.
Aïkpon R, Agossa F, Ossè R, Oussou O, Aïzoun N, Oké-Agbo F, et al. Bendiocarb resistance in Anopheles gambiae s.l. populations from Atacora department in Benin, West Africa: a threat for malaria vector control. Parasit Vectors. 2013;6:192.
Salako AS, Ahogni I, Aïkpon R, Sidick A, Dagnon F, Sovi A, et al. Insecticide resistance status, frequency of L1014F Kdr and G119S Ace-1 mutations, and expression of detoxification enzymes in Anopheles gambiae (s.l.) in two regions of northern Benin in preparation for indoor residual spraying. Parasit Vectors. 2018;11:618.
PMI, Vectorlink Benin. The PMI Vectorlink Benin 2021 end of spray report (EOSR). 2021. https://d1u4sg1s9ptc4z.cloudfront.net/uploads/2022/02/End-of-Spray-Report-Benin-2021.pdf.
Tomizawa M, Casida JE. Neonicotinoid insecticide toxicology: mechanisms of selective action. Annu Rev Pharmacol Toxicol. 2005;45:247–68.
Ullah F, Gul H, Tariq K, Desneux N, Gao X, Song D. Fitness costs in clothianidin-resistant population of the melon aphid, Aphis gossypii. PLoS ONE. 2020;15: e0238707.
Odjo EM, Salako AS, Padonou GG, Yovogan B, Adoha CJ, Adjottin B, et al. What can be learned from the residual efficacy of three formulations of insecticides (pirimiphos-methyl, clothianidin and deltamethrin mixture, and clothianidin alone) in large-scale in community trial in North Benin, West Africa? Malar J. 2023;22:150.
Dong K, Du Y, Rinkevich F, Nomura Y, Xu P, Wang L, et al. Molecular biology of insect sodium channels and pyrethroid resistance. Insect Biochem Mol Biol. 2014;50:1–17.
Williamson MS, Martinez-Torres D, Hick CA, Devonshire AL. Identification of mutations in the housefly para-type sodium channel gene associated with knockdown resistance (kdr) to pyrethroid insecticides. Mol Gen Genet. 1996;252:51–60.
Clarkson CS, Miles A, Harding NJ, O’Reilly AO, Weetman D, Kwiatkowski D, et al. The genetic architecture of target-site resistance to pyrethroid insecticides in the African malaria vectors Anopheles gambiae and Anopheles coluzzii. Mol Ecol. 2021;30:5303–17.
Etang J, Mandeng SE, Nwane P, Awono-Ambene HP, Bigoga JD, Ekoko WE, et al. Patterns of Kdr-L995F allele emergence alongside detoxifying enzymes associated with deltamethrin resistance in Anopheles gambiae s.l. from North Cameroon. Pathogens. 2022;11:253.
Martinez-Torres D, Chandre F, Williamson MS, Darriet F, Bergé JB, Devonshire AL, et al. Molecular characterization of pyrethroid knockdown resistance (kdr) in the major malaria vector Anopheles gambiae s.s. Insect Mol Biol. 1998;7:179–84.
Ranson H, Jensen B, Vulule JM, Wang X, Hemingway J, Collins FH. Identification of a point mutation in the voltage-gated sodium channel gene of Kenyan Anopheles gambiae associated with resistance to DDT and pyrethroids. Insect Mol Biol. 2000;9:491–7.
Santolamazza F, Mancini E, Simard F, Qi Y, Tu Z, della Torre A. Insertion polymorphisms of SINE200 retrotransposons within speciation islands of Anopheles gambiae molecular forms. Malar J. 2008;7:163.
Reimer L, Fondjo E, Patchoké S, Diallo B, Lee Y, Ng A, et al. Relationship between kdr mutation and resistance to pyrethroid and DDT insecticides in natural populations of Anopheles gambiae. J Med Entomol. 2008;45:260–6.
Grau-Bové X, Lucas E, Pipini D, Rippon E, van’t Hof AE, Constant E, et al. Resistance to pirimiphos-methyl in West African Anopheles is spreading via duplication and introgression of the Ace1 locus. PLOS Genet. 2021;17: e1009253.
Feyereisen R, Dermauw W, Van Leeuwen T. Genotype to phenotype, the molecular and physiological dimensions of resistance in arthropods. Pestic Biochem Physiol. 2015;121:61–77.
Greenblatt HM, Guillou C, Guénard D, Argaman A, Botti S, Badet B, et al. The complex of a bivalent derivative of galanthamine with torpedo acetylcholinesterase displays drastic deformation of the active-site gorge: implications for structure-based drug design. J Am Chem Soc. 2004;126:15405–11.
Oppenoorth FJ. Biochemistry and genetics of insecticide resistance. Compr Insect Physiol Biochem Pharmacol Control. 1985;12:731–73.
Djogbénou L, Pasteur N, Akogbéto M, Weill M, Chandre F. Insecticide resistance in the Anopheles gambiae complex in Benin: a nationwide survey. Med Vet Entomol. 2011;25:256–67.
Soderlund DM, Bloomquist JR. Molecular mechanisms of insecticide resistance. In: Pesticide resistance in arthropods. Boston: Springer; 1990. p. 58–96.
Davies TGE, Field LM, Usherwood PNR, Williamson MS. A comparative study of voltage-gated sodium channels in the Insecta: implications for pyrethroid resistance in Anopheline and other Neopteran species. Insect Mol Biol. 2007;16:361–75.
RGPH-4. Cahier des villages et quartiers de ville du département de la Donga. Institut National de la Statistique et de l’Analyse Economique (INSAE); 2013. p. 24.
RGPH-4. Cahier des villages et quartiers de ville du département du Borgou. Institut National de la Statistique et de l’Analyse Economique (INSAE); 2013. p. 31.
RGPH-4. Cahier des villages et quartiers de ville du département de l’Alibori. Institut National de la Statistique et de l’Analyse Economique (INSAE); 2013. p. 26.
Gillies MT, Coetzee M. A supplement to the Anophelinae of Africa south of the Sahara (Afrotropical Region). Johannesburg: South African Institute for Medical Research; 1987.
Gillies MT, De Meillon B. The Anophelinae of Africa south of the Sahara (Ethiopian zoogeographical region). 2nd ed. Johannesburg: South African Institute for Medical Research; 1968.
Wirtz RA, Burkot TR, Andre RG, Rosenberg R, Collins WE, Roberts DR. Identification of Plasmodium vivax sporozoites in mosquitoes using an enzyme-linked immunosorbent assay. Am J Trop Med Hyg. 1985;34:1048–54.
Myriam et Cécile. Protocoles de biologie moléculaire en usage au lin. Institut de recherche pour le développement IRD. 2003.
Weill M, Lutfalla G, Mogensen K, Chandre F, Berthomieu A, Berticat C, et al. Comparative genomics: insecticide resistance in mosquito vectors. Nature. 2003;423:136–7.
Scott JA, Brogdon WG, Collins FH. Identification of single specimens of the Anopheles gambiae complex by the polymerase chain reaction. Am J Trop Med Hyg. 1993;49:520–9.
Lu W, Liu Z, Fan X, Zhang X, Qiao X, Huang J. Nicotinic acetylcholine receptor modulator insecticides act on diverse receptor subtypes with distinct subunit compositions. PLoS Genet. 2022;18: e1009920.
Costas-Ferreira C, Faro LRF. Neurotoxic effects of neonicotinoids on mammals: what is there beyond the activation of nicotinic acetylcholine receptors?—A systematic review. Int J Mol Sci. 2021;22:8413.
Elamathi N, Barik TK, Verma V, Velamuri PS, Bhatt RM, Sharma SK, et al. Standardization of a bottle assay—an indigenous method for laboratory and field monitoring of insecticide resistance and comparison with WHO adult susceptibility test. Parasitol Res. 2014;113:3859–66.
Ngufor C, N’Guessan R, Fagbohoun J, Subramaniam K, Odjo A, Fongnikin A, et al. Insecticide resistance profile of Anopheles gambiae from a phase II field station in Cové, southern Benin: implications for the evaluation of novel vector control products. Malar J. 2015;14:464.
Yahouédo GA, Cornelie S, Djègbè I, Ahlonsou J, Aboubakar S, Soares C, et al. Dynamics of pyrethroid resistance in malaria vectors in southern Benin following a large scale implementation of vector control interventions. Parasit Vectors. 2016;9:385.
Awolola TS, Oduola OA, Strode C, Koekemoer LL, Brooke B, Ranson H. Evidence of multiple pyrethroid resistance mechanisms in the malaria vector Anopheles gambiae sensu stricto from Nigeria. Trans R Soc Trop Med Hyg. 2009;103:1139–45.
Casimiro S, Coleman M, Hemingway J, Sharp B. Insecticide resistance in Anopheles arabiensis and Anopheles gambiae from Mozambique. J Med Entomol. 2006;43:276–82.
Nwane P, Etang J, Chouaibou M, Toto JC, Kerah-Hinzoumbé C, Mimpfoundi R, et al. Trends in DDT and pyrethroid resistance in Anopheles gambiae s.s. populations from urban and agro-industrial settings in southern Cameroon. BMC Infect Dis. 2009;9:163.
Ochomo E, Bayoh MN, Brogdon WG, Gimnig JE, Ouma C, Vulule JM, et al. Pyrethroid resistance in Anopheles gambiae s.s. and Anopheles arabiensis in western Kenya: phenotypic, metabolic and target site characterizations of three populations. Med Vet Entomol. 2013;27:156–64.
Williams J, Ingham VA, Morris M, Toé KH, Hien AS, Morgan JC, et al. Sympatric populations of the Anopheles gambiae complex in Southwest Burkina Faso evolve multiple diverse resistance mechanisms in response to intense selection pressure with pyrethroids. Insects. 2022;13:247.
Yadouleton AW, Padonou G, Asidi A, Moiroux N, Bio-Banganna S, Corbel V, et al. Insecticide resistance status in Anopheles gambiae in southern Benin. Malar J. 2010;9:83.
Yadouleton AWM, Asidi A, Djouaka RF, Braïma J, Agossou CD, Akogbeto MC. Development of vegetable farming: a cause of the emergence of insecticide resistance in populations of Anopheles gambiae in urban areas of Benin. Malar J. 2009;8:103.
Tchouakui M, Assatse T, Mugenzi LMJ, Menze BD, Nguiffo-Nguete D, Tchapga W, et al. Comparative study of the effect of solvents on the efficacy of neonicotinoid insecticides against malaria vector populations across Africa. Infect Dis Poverty. 2022;11:35.
Zoh MG, Bonneville J-M, Tutagana J, Laporte F, Fodjo BK, Mouhamadou CS, et al. Neonicotinoid and pyrethroid combination: a tool to manage insecticide resistance in malaria vectors? Insights from experimental evolution. bioRxiv. 2021. https://doi.org/10.1101/2021.06.09.447494.
Yadouleton A, Aikpon R, Houndeton G, Aboubacar S, Ursins F, Tchibçozo C, et al. Données entomologiques préliminaires pour la mise en place d’une pulvérisation intra-domiciliaire à grande échelle dans la commune de Corpargo au Nord-Est du Bénin. Int J Biol Chem Sci. 2018;12:1993–2003.
Hounnakan AC, Ayadji APV, Vodungbo V, Hounkpe E, Gbekan P, Wadochedohoun R, et al. Annuaire des statistiques sanitaires. 2019.
Ossè R, Gnanguenon V, Sèzonlin M, Aïkpon R, Padonou G, Yadouléton A, et al. Relationship between the presence of kdr and Ace-1 mutations and the infection with Plasmodium falciparum in Anopheles gambiae s.s. in Benin. Parasitol Vector Biol. 2012;4(3):31–9.
Mitri C, Markianos K, Guelbeogo WM, Bischoff E, Gneme A, Eiglmeier K, et al. The kdr-bearing haplotype and susceptibility to Plasmodium falciparum in Anopheles gambiae: genetic correlation and functional testing. Malar J. 2015;14:391.
Acknowledgements
We would like to thank the President’s Malaria Initiative which supported financially this study. We also thank the police and administrative authorities of the communes of Djougou, Copargo, Bassila, Kandi, Gogounou and Bembèrèkè who facilitated the collection of adult mosquitoes before, during and after the IRS implementation malaria in their localities. We acknowledge the entire team at the molecular laboratory of the Centre de Recherche Entomologique de Cotonou (CREC) for their assistance.
Funding
This study was supported by the U.S. President’s Malaria Initiative (PMI). The IRS activities were funded through the PMI Africa Indoor Residual Spraying (AIRS) Project, The PMI VectorLink Project, and the PMI Evolve Project.
Author information
Authors and Affiliations
Contributions
EMO and MCA designed the study. EMO, AD, GGP, RG, AAM, FTT, CA and MCA critically revised the manuscript. EMO, MT, ZCK and JMA carried out the field activities and the laboratory analysis. EMO and BA analysed the data. EMO drafted the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The Institutional Ethical Committee of CREC (IECC) studied and approved the protocol before the study began (Grant No. IORG005698). Local mosquito-collectors volunteers gave their consent to take part in the study. They were then vaccinated against yellow fever. They periodically took part in health check-ups organized by the attending physician, and were systematically treated in the event of confirmed malaria.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Odjo, E.M., Tognidro, M., Govoetchan, R. et al. Malaria transmission potential of Anopheles gambiae s.l. in indoor residual spraying areas with clothianidin 50 WG in northern Benin. Trop Med Health 52, 18 (2024). https://doi.org/10.1186/s41182-024-00582-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41182-024-00582-8