Abstract
Background
Peritoneal dialysis (PD)-associated peritonitis is a serious complication that can lead to PD discontinuation and mortality. Tsukamurella species are uncommon opportunistic pathogens that can cause peritonitis, often necessitating catheter removal.
Case presentation
A 46-year-old woman with a 1.5-year history of PD presented with fever and lower abdominal pain, along with cloudy peritoneal effluent showing an elevated cell count. Empiric antibiotic therapy with ciprofloxacin, cefazolin, and ceftazidime was started for PD-associated peritonitis. The peritoneal effluent culture yielded a gram-positive rod with weak acid-fast staining. A rebound in the cell count necessitated a switch of antibiotics to meropenem and vancomycin. This led to improvement, and the treatment was switched to oral levofloxacin on day 30 and the patient was discharged on day 35. Subsequently, 16S rRNA gene sequencing confirmed the isolate as Tsukamurella inchonensis.
Conclusion
This case highlights the challenges in identifying and treating Tsukamurella peritonitis. Successful treatment without catheter removal in this case suggests that early detection and appropriate antibiotics may enable catheter salvage in this rare infection. However, further research is needed to establish optimal treatment strategies.
Similar content being viewed by others
Introduction
Tsukamurella species are aerobic, gram-positive, weakly acid-fast bacilli found in various environmental sources and are increasingly recognized as opportunistic pathogens in humans [1, 2]. They can cause a wide range of infections, including pulmonary [3, 4], cutaneous [5], ocular [6], bloodstream [7, 8], central nervous system [9], and peritoneal infections [10]. However, accurate identification of these species remains challenging in many clinical microbiology laboratories due to phenotypic and biochemical similarities to other actinomycetes, such as Corynebacterium, Rhodococcus, Nocardia, and non-tuberculous mycobacteria [1].
One of the most significant complications of peritoneal dialysis (PD) is PD-associated peritonitis, which is a leading cause of PD discontinuation and mortality [11]. Although prompt empiric antibiotic treatment with broad-spectrum coverage of both gram-positive and gram-negative organisms is crucial, the emergence of less common and drug-resistant pathogens presents an obstacle to successful treatment. Reports of PD-associated peritonitis caused by Tsukamurella spp. are limited, and catheter removal is frequently required for resolution [10, 12]. Recent advances in molecular diagnostic methods, particularly matrix-assisted desorption ionization time-of-flight mass spectrometry (MALDI–TOF MS) and 16S rRNA gene sequencing, have significantly improved the identification of Tsukamurella infections.
We report a rare case of PD-associated peritonitis caused by Tsukamurella inchonensis that was successfully treated without catheter removal, highlighting the potential for catheter salvage treatment strategies.
Case presentation
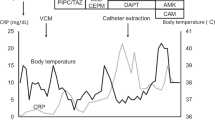
A 46-year-old Japanese woman with a 1.5-year history of automated PD due to autosomal dominant polycystic kidney disease presented with a 3-day history of fever and lower abdominal pain. She had no prior history of PD-associated peritonitis. She had body temperature of 38.9℃, blood pressure of 124/94 mmHg, and heart rate of 131 beats per minute. The PD effluent was cloudy with a cell count of 1000 cells/μL. There was no sign of exit-site infection, history of PD tube troubles, or touch contamination. She was a homemaker and did PD exchange manually without use of a PD connection assist device. She had no pets or hobbies such as gardening. She was admitted with the diagnosis of PD-associated peritonitis. Laboratory findings on admission are presented in Table 1. After collection of blood, urine, and peritoneal effluent culture samples, she was started on oral ciprofloxacin (CPFX), intraperitoneal (IP) cefazolin (CEZ), and IP ceftazidime (CAZ) daily. The IP antibiotics were administered continuously (every 6 h). The blood and urine cultures were all negative. The aerobic culture bottle of the peritoneal effluent was positive, and gram-positive rod bacteria with weak acid-fast staining were isolated (Fig. 1a, b). The bacterial isolate could not be identified by MALDI–TOF MS (Bruker MALDI Biotyper®). Our facility was not able to identify the species, but we suspected Tsukamurella spp. due to its yellowish cream-colored colonies and its gram-staining and other staining characteristics (Fig. 1c–e). We sent the specimen to the Medical Mycology Research Center of Chiba University for molecular analysis. The peritoneal effluent cell count initially decreased after treatment initiation. However, on day 11, a rebound in the cell count to 2770/μL prompted discontinuation of oral CPFX and IP CEZ, and addition of intravenous (IV) meropenem and IP vancomycin. This treatment modification resulted in a decrease in cell count to 70/μL by day 21. By day 30, the count remained consistently below 10/μL, allowing for a switch to oral levofloxacin (LVFX). On day 34, 16S rRNA gene sequencing identified the bacterial isolate as Tsukamurella inchonensis (antibiogram in Table 2) [10, 13, 14]. The following day, the patient was discharged with a 1-week prescription of LVFX.
Colony appearance and cell morphology for Tsukamurella Inchonensis. a Isolates were grown on TSA II 5% sheep blood agar MⓇ (BD, Tokyo, Japan) aerobic culture for 2 days. b Close-up image. Small, dry, round, yellowish cream-colored were observed. c Gram staining. d Kinyoun staining. e Ziehl–Neelsen staining
Two months later, the patient developed peritonitis caused by Micrococcus luteus and Moraxella osloensis, which resolved with a 3-week course of antibiotics. Although this episode was unrelated to the prior Tsukamurella infection, its occurrence within 2 months of discharge, coupled with the involvement of environmental bacteria, highlighted the need for improved hygiene practices, particularly in areas prone to bacterial growth, such as the kitchen and bathroom. Therefore, although we did not perform environmental culturing, we recommended improving the patient’s home environment, including the bathroom and shower, and thoroughly wiping off any water droplets that may have adhered to the catheter after bathing. There have been no signs of peritonitis for 6 months since then.
Discussion
Here, we presented a rare case of PD-associated peritonitis caused by Tsukamurella inchonensis, an emerging opportunistic pathogen. To our knowledge, only a few cases of PD-associated peritonitis due to Tsukamurella have been reported. Although our MALDI–TOF MS analysis of the bacterial isolate failed to provide definitive identification, the isolate’s colony morphology and Gram-staining characteristics suggested the possibility of Tsukamurella infection. This prompted further analyses such as 16S rRNA gene sequencing at another institution because of the lack of specialized equipment for Tsukamurella identification at our facility. Tsukamurella colonies are typically small and dry, with convex elevation, and appear white, cream-colored, or orange [1]. Only peritoneal effluent culture collected in an aerobic bottle yielded positive results, and the bacterial isolate from this case displayed slow growth, forming small, dry, round, yellowish cream-colored colonies.
According to an in vitro study, Tsukamurella spp. are susceptible to quinolones, trimethoprim/sulfamethoxazole, amikacin, minocycline, linezolid, and tigecycline [15]. In that study, Tsukamurella isolates were also susceptible to the third- and fourth-generation cephalosporins. Cases of PD-associated peritonitis caused by Tsukamurella spp. are limited, and the optimal management strategy has not yet been determined (Table 3) [10, 12,13,14]. Caution may be needed regarding the use of quinolone since the mechanism conferring quinolone resistance in Tsukamurella tyrosinosolvens has been reported [15], which might be one of the reasons for the difficulty we encountered using CPFX to treat Tsukamurella inchonensis. Another reason was the discrepancy between the isolate’s specific antibiogram, which showed “intermediate” susceptibility against CPFX, and our facility’s standard antibiogram, which indicated it was “susceptible.” This discrepancy could be due to the lack of species-specific data for Tsukamurella in our standard antibiogram.
For treatment of catheter-related bloodstream infections caused by Tsukamurella spp., the combination of catheter removal and appropriate antibiotic treatment is considered essential [8, 15]. In some previous reports, successful treatment of Tsukamurella PD-associated peritonitis required catheter removal [10, 12, 13]. However, a case from China [14] and our case demonstrated successful treatment without catheter removal. These reports are summarized in Table 3. Although we were unable to identify the route of infection, PD connection troubles [13] and diarrhea [14] prior to peritonitis have been reported as infection routes. The necessary treatment duration for Tsukamurella PD-associated peritonitis remains unclear. The case report from China described an 18-day treatment course [14]. Our case required 42 days of treatment including the duration of oral antibiotics administered after discharge. Also, repeat peritonitis caused by Tsukamurella has not been reported. Treatment recommendations for Gordonia peritonitis, a genus phenotypically similar to Tsukamurella, suggest at least 3 weeks of carbapenem–aminoglycoside combination therapy [16]. While evidence supporting successful treatment of Tsukamurella peritonitis without catheter removal is limited, our case demonstrates the potential for successful treatment with an antimicrobial therapy course exceeding 3 weeks, with subsequent close follow-up. Accurate identification of Tsukamurella can be achieved through newer molecular biological techniques, thus contributing to the appropriate selection of definitive therapy for infections caused by Tsukamurella [17]. Prompt therapy and understanding of the clinical, microbiological, and molecular characteristics of Tsukamurella spp. might prevent catheter removal in PD-associated peritonitis caused by these organisms.
Conclusion
Our experience with PD-associated peritonitis caused by Tsukamurella inchonensis suggests the potential for successful treatment without catheter removal. Accurate identification of Tsukamurella might contribute to the selection of appropriate therapy and infectious resolution.
Availability of data and materials
The data used in this study are available from the corresponding author.
Abbreviations
- CAZ:
-
Ceftazidime
- CEZ:
-
Cefazolin
- CPFX:
-
Ciprofloxacin
- IP:
-
Intraperitoneal
- LVFX:
-
Levofloxacin
- MALDI-TOF MS:
-
Matrix-assisted desorption ionization-time of flight mass spectrometry
- MEPM:
-
Meropenem
- PD:
-
Peritoneal dialysis
References
Safaei S, Fatahi-Bafghi M, Pouresmaeil O. Role of Tsukamurella species in human infections: first literature review. New Microbes New Infect. 2018;22:6–12.
Teng JLL, Tang Y, Wong SSY, Fong JYH, Zhao Z, Wong C-P, et al. MALDI-TOF MS for identification of Tsukamurella species: Tsukamurella tyrosinosolvens as the predominant species associated with ocular infections. Emerg Microbes Infect. 2018;7:80.
Mehta YB, Simonelli P, Goswami R, Bhanot N, Mehta Z. Tsukamurella infection: a rare cause of community-acquired pneumonia. Am J Med Sci. 2011;341:500–3.
Chen C-H, Lee C-T, Chang T-C. Tsukamurella tyrosinosolvens bacteremia with coinfection of Mycobacterium bovis pneumonia: case report and literature review. Springerplus. 2016;5:2033.
Granel F, Lozniewski A, Barbaud A, Lion C, Dailloux M, Weber M, et al. Cutaneous infection caused by Tsukamurella paurometabolum. Clin Infect Dis. 1996;23:839–40.
Kechker P, Senderovich Y, Ken-Dror S, Laviad-Shitrit S, Halpern M. Tsukamurella pulmonis conjunctivitis in patients with an underlying nasolacrimal duct obstruction—report of two cases. Access Microbiol. 2021;3:000185.
Del Molino P, Bernal IC, Cano ME, de la Fuente CG, Martínez-Martínez L, López M, Fernández-Mazarrasa C, et al. Tsukamurella pulmonis bloodstream infection identified by secA1 gene sequencing. J Clin Microbiol. 2015;53:743–5.
Suzuki J, Sasahara T, Toshima M, Morisawa Y. Peripherally inserted central catheter-related bloodstream infection due to Tsukamurella pulmonis: a case report and literature review. BMC Infect Dis. 2017;17:677.
Prinz G, Bán E, Fekete S, Szabó Z. Meningitis caused by Gordona aurantiaca (Rhodococcus aurantiacus). J Clin Microbiol. 1985;22:472–4.
Shaer AJ, Gadegbeku CA. Tsukamurella peritonitis associated with continuous ambulatory peritoneal dialysis. Clin Nephrol. 2001;56:241–6.
Kawanishi H. Historical overview and current practice of peritoneal dialysis in Japan. Ren Replace Ther. 2022;8:48.
Ismayilov R, Duran ZC, Hazirolan G, Inkaya AÇ. Tsukamurella paurometabola peritonitis in a patient on automated peritoneal dialysis. Enferm Infecc Microbiol Clin (Engl Ed). 2021;39:422.
Nagamatsu M, Takagi T, Ohyanagi T, Kurosawa M, Tsumita N, Takemura H, et al. A case of Tsukamurella pulmonis peritonitis associated with peritoneal dialysis. J Jap Soc Clin Microbiol. 2012;22:154–60 (in Japanese).
Tang L, Huang Y, Li T, Li Y, Xu Y. Bacterial peritonitis caused by Tsukamurella inchonensis in a patient undergoing continuous ambulatory peritoneal dialysis. Infect Drug Resist. 2022;15:2475–80.
Li PK-T, Chow KM, Cho Y, Fan S, Figueiredo AE, Harris T, et al. ISPD peritonitis guideline recommendations: 2022 update on prevention and treatment. Perit Dial Int. 2022;2022(42):110–53.
Yu S, Ding X, Hua K, Zhu H, Zhang Q, Song X, et al. Systematic investigation of the emerging pathogen of Tsukamurella species in a Chinese tertiary teaching hospital. Microbiol Spectr. 2023;11:e0164423.
Usuda D, Tanaka R, Suzuki M, Shimozawa S, Takano H, Hotchi Y, et al. Obligate aerobic, gram-positive, weak acid-fast, nonmotile bacilli, Tsukamurella tyrosinosolvens: Minireview of a rare opportunistic pathogen. World J Clin Cases. 2022;10:8443–9.
Acknowledgements
We gratefully acknowledge Yumiko Tanimichi, a microbiological technologist, for her expertise in analyzing the bacterial isolates and suggesting the possibility of Tsukamurella. We thank Dr. Takashi Yaguchi, Division of Bio-Resources, Medical Mycology Research Center, Chiba University, Japan, for the analysis of 16S rRNA gene sequencing to identify the bacterial isolates.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
R.Y. wrote the manuscript. R.Y., M.K., K.I., Y.K., T.M., S.H., and M.A. contributed of management for the case. M.A. revised the manuscript. R.Y., H.T., T.M., and M.A. discussed the results and contributed to the final manuscript. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Written informed consent was obtained from the patient for publication of this case report and accompanying images.
Competing interests
M.A. is the deputy editor of Renal Replacement Therapy. The other authors declare that they have no other relevant financial interests. The publication of this report was not supported by any grants. No financial support was provided for this study.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Yamaguchi, R., Kashiwagi, M., Ichijo, K. et al. Peritoneal dialysis-associated peritonitis caused by Tsukamurella inchonensis: a case report and literature review. Ren Replace Ther 10, 48 (2024). https://doi.org/10.1186/s41100-024-00564-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41100-024-00564-w