Abstract
Background
Progressive multifocal leukoencephalopathy is a rare disease, but the prognosis is very poor, especially in the immunosuppressed state with a non-HIV background, and there is no established treatment.
Case presentations
A 49-year-old patient who had undergone a renal transplant and was receiving prednisolone and mycophenolate mofetil treatment was admitted for peritoneal dialysis initiation. While hospitalized, he experienced aphasia and other percutaneous symptoms. Magnetic resonance imaging of the brain revealed a subcortical demyelinating lesion. JC virus DNA was identified in cerebrospinal fluid, and he was diagnosed with progressive multifocal leukoencephalopathy. Immunosuppressant was ceased, and he was treated with mefloquine and mirtazapine. The patient subsequently underwent a head MRI scan, confirming lesion reduction, improved activities of daily life, and survival.
Conclusions
Progressive multifocal leukoencephalopathy is commonly observed in patients with compromised immune systems, which was the case for this patient due to long-standing immunosuppressive medication usage and end-stage renal failure necessitating dialysis.
Similar content being viewed by others
Background
Progressive multifocal leukoencephalopathy (PML) is a demyelinating disease of the central nervous system caused by the reactivation of JC virus (JCV) in individuals with impaired cellular immunity. Various risk factors have been associated with PML, such as human immunodeficiency virus (HIV) infection, hematologic malignancies, autoimmune diseases, and organ transplantation with immunosuppressive therapy. The typical clinical course of PML involves a subacute progression of neurological symptoms leading to a spontaneous rash within a few months [1]. Unfortunately, there is currently no established treatment for this condition, and non-HIV PML patients have a median survival of only 3 months with a very poor prognosis [2, 3].
In this context, we report a case of a patient who suffered from PML and required peritoneal dialysis due to impaired transplant renal function. However, the patient achieved a favorable outcome following the discontinuation of immunosuppressive drugs and a combination therapy with mefloquine and mirtazapine.
Case presentation
The patient is a 49-year-old man who had undergone kidney transplantation with his mother as a donor 30 years ago because of renal failure caused by chronic urinary tract infection due to vesicoureteral reflux disease. He was most recently treated with 5 mg prednisolone and 500 mg mycophenolate mofetil (MMF) per day. However, the patient’s renal function gradually declined. Simultaneously, mild memory loss began to appear; however, it did not interfere with his daily life. He was admitted to the hospital for induction of peritoneal dialysis, which was started on the same day. He complained of difficulty speaking and was found to have higher brain dysfunction, mainly aphasia, approximately one week after hospitalization (day 0 of diagnosis).
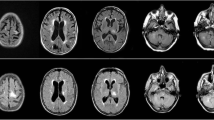
At the time of diagnosis, his body temperature was 35.8 °C, blood pressure was 120/89 mmHg, pulse rate was 85/min, and oxygen saturation was 99% on room air. Neurological examination revealed disorientation and aphasia, with dysgraphia, dyslexia, and dysarthria. The laboratory data at the time of diagnosis are presented in Table 1. Blood tests showed renal dysfunction due to end-stage renal failure and hypogammaglobulinemia (IgG, 599 mg/dL; normal range: 870–1,700 mg/dL), and the HIV antibody test was negative. Cerebrospinal fluid (CSF) examination showed normal cell count, protein, and glucose levels. Brain magnetic resonance imaging (MRI) showed irregular high-intensity lesions mainly in the subcortical white matter from the left frontal lobe to the temporal lobe in fluid-attenuated inversion recovery (FLAIR) images (Fig. 1A). PML was suspected based on the clinical course and imaging findings. Later, polymerase chain reaction (PCR) assay for JC virus (JCV) in the CSF yielded positive results (8825 copies/mL), which confirmed the diagnosis of PML.
We discontinued MMF on the day after diagnosis. On the 22nd day after diagnosis, the prednisolone dose was reduced to 4 mg/day. On the 41st day after diagnosis, seizures and right hemiplegia were observed. Brain MRI in FLAIR images showed an edematous expansile lesion (Fig. 1B). On the 46th day after diagnosis, we prescribed mirtazapine (15 mg daily). On the 82nd day after diagnosis, we also prescribed mefloquine (275 mg daily for 3 days, followed by 275 mg once a week), after approval by the hospital committee (approval number FR20210001, date of approval: August 16, 2021). On the 97th day after diagnosis, his CSF JCV DNA PCR was negative, and on the 176th day after diagnosis, brain MRI showed that the lesion had stopped enlarging and was shrinking (Fig. 1C). His activities of daily living were temporarily reduced to the point of requiring wheelchair mobility (Barthel Index 60 out of 100, Lawton Instrumental Activities of Daily Living Scale 1 out of 5). Following treatment, his activities of daily living improved sufficiently for him to walk with a cane (Barthel Index 100 out of 100, Lawton Instrumental Activities of Daily Living Scale 2 out of 5), and his aphasia also improved.
Discussion and conclusions
This report details the case of a patient who developed PML after undergoing renal transplantation and being on peritoneal dialysis. The patient ceased taking immunosuppressive agents and was successfully treated with a combination therapy of mefloquine and mirtazapine. Two significant factors emerged from this case. Firstly, the patient developed PML while experiencing a decline in the transplanted kidney’s renal function, necessitating the need for renal replacement therapy. Secondly, following the initiation of mefloquine and mirtazapine treatment and a decrease in the dosage of immunosuppressive drugs, the JCV PCR test of the spinal fluid turned negative, and both the clinical and imaging findings improved.
In this case, both chronic renal failure during the induction phase of dialysis and the use of post-transplant immunosuppressive medications could have contributed to the patient’s immunosuppressive state. It is known that renal insufficiency and uremia can impair humoral and cellular immune functions [4]. While PML cases resulting solely from chronic renal failure are uncommon, they have been observed in the past [5, 6]. The incidence of PML in post-transplantation cases is also rare, with a reported occurrence of only 0.027% in patients with kidney transplantation [7].
Blood transfusion frequency before transplant, panel reactive antibody levels exceeding 20%, and the use of anti-rejection medications during the first year are demonstrated as risk factors for PML [7]. In the present case, the transplant took place 30 years ago, and it remains unclear whether these risk factors were present due to the unavailability of his medical records at the time of transplantation.
BK virus nephropathy can cause transplant renal dysfunction and leads to loss of transplant renal function at a high rate. Reactivation of BK virus in urine has been observed in 33–49% of renal transplant recipients, independent of the duration of transplantation [8]. This case was not tested for the presence of BK virus in the urine; therefore, it is unclear whether there was an association between the graft, BK virus, and graft loss. Additionally, the patient’s condition at the time prevented a graft kidney biopsy. However, since patient’s urine output was relatively well maintained, peritoneal dialysis could be initiated. The treatment for PML consisted of reducing steroids reduction and discontinuing MMF. This approach aligns with the treatment for BK virus nephropathy, making it plausible that successful outcomes would have been achieved even if the patient had BK virus nephropathy.
Over the last few years, kidney transplants have become increasingly prevalent, with over 20,000 of them being conducted annually in the USA alone. The median graft survival for donated kidney transplants is 11.7 years, while living donor kidney transplants have a median survival rate of 19.2 years. These procedures are typically conducted on patients between the ages of 34 and 54 [9, 10]. As the number of transplants continues to increase worldwide, this case of PML may provide an important insight into the diagnosis of diseases other than uremia and cerebrovascular disease when the patient presents with unexpected neurological symptoms during the induction of dialysis at the time of graft loss.
Presently, immune reconstitution is the only treatment considered effective for non-HIV-PML, and no established therapy exists for this disease. In cases of drug-related PML caused by the use of biological agents or immunosuppressive drugs, reducing or discontinuing the drug and considering plasma exchange are recommended. Non-HIV-PML patients often have favorable outcomes when immunosuppression is reduced [11]. In this case, our patient had a positive outcome, which we attribute to the discontinuation of immunosuppressive drugs.
The inhibitory effects of mefloquine and mirtazapine on JCV are promising, and further studies are needed to confirm their effectiveness as a treatment for PML [12, 13]. In addition, it is important to note that these drugs have potential adverse effects and may interact with other medications, which should be taken into consideration when prescribing them to PML patients. Close monitoring and careful consideration of the risk–benefit ratio are necessary when using these drugs for the treatment of PML.
To the best of our knowledge, 60 cases in 52 studies have been reported in English papers using both mefloquine and mirtazapine for non-HIV-PML (Additional file 1: Table S1) [14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65]. In summary, as for background diseases, 15 cases of multiple sclerosis, 12 cases of hematologic malignancies such as lymphoma, 8 cases of autoimmune diseases such as systemic lupus erythematosus and rheumatoid arthritis, 7 cases of sarcoidosis, and 7 cases of immune deficiency have been reported mainly. There was one case of a dialysis patient [15]. It should be noted that patients treated with natalizumab or ocrelizumab for multiple sclerosis have different symptoms and course at the onset of the disease and adjuvant treatment from other background diseases because of the medical behavior of monitoring JCV during the course of the disease [14, 25, 27, 32, 35, 37, 38, 42, 49, 60]. The range of onset age was 19–85 years. Regarding the time from the onset of background disease to the diagnosis of PML, the longest period was 40 years in a case of Takayasu arteritis; however, many reports do not specify this time [22]. There have been reports of a wide variety of initial symptoms, including asymptomatic cases with neurological disease in the background and incidentally detected cases as well as cases with motor disturbance, headache, visual disturbance, and amnesia as the initial symptoms. In most cases, immunosuppressive drugs and other drugs used for the background disease were discontinued. Dose of mefloquine and mirtazapine were prescribed in the range of 250–375 mg/week and 15–60 mg/day, respectively. Most reports did not report or reported no side effects, but pancytopenia was reported in one case and hepatotoxicity in one case [38, 44]. Regarding prognosis, 37 patients reported improvement or survival of more than one year and 13 reported mortalities of less than one year. Considering that the reported median survival for PML with non-HIV background disease is three months, these medications may be effective, but the possibility of publication bias in reporting cannot be denied; therefore, it is possible that early deaths were not reported.
We reported a case study of a patient who developed PML after experiencing graft loss and requiring peritoneal dialysis. Thankfully, the discontinuation of immunosuppressive drugs and a combination therapy of mefloquine and mirtazapine resulted in long-term survival for the patient. As renal transplant recipients will inevitably experience graft loss, it is crucial to remain vigilant for the development of PML during the course of the disease and when dialysis is required again. Treatment, including the use of mefloquine and mirtazapine, should be initiated promptly upon the onset of PML.
Availability of data and materials
The datasets generated during the current study are available from the corresponding author on reasonable request.
Abbreviations
- DNA:
-
Deoxyribonucleic acid
- MRI:
-
Magnetic resonance imaging
- PML:
-
Progressive multifocal leukoencephalopathy
- JCV:
-
JC virus
- HIV:
-
Human immunodeficiency virus
- MMF:
-
Mycophenolate mofetil
- CSF:
-
Cerebrospinal fluid
- PCR:
-
Polymerase chain reaction
References
Nakamichi K, Mizusawa H, Yamada M, et al. Characteristics of progressive multifocal leukoencephalopathy clarified through internet-assisted laboratory surveillance in Japan. BMC Neurol. 2012;12:121.
Tan CS, Chen Y, Viscidi RP, Kinkel RP, Stein MC, Koralnik IJ. Discrepant findings in immune responses to JC virus in patients receiving natalizumab. Lancet Neurol. 2010;9:565–7.
Anand P, Hotan GC, Vogel A, Venna N, Mateen FJ. Progressive multifocal leukoencephalopathy: a 25-year retrospective cohort study. Neurol Neuroimmunol Neuroinflamm. 2019;6:e618.
Kato S, Chmielewski M, Honda H, et al. Aspects of immune dysfunction in end-stage renal disease. Clin J Am Soc Nephrol. 2008;3:1526–33.
Matsuda H, Hayashi K, Meguro M, Saruta T. A case report of progressive multifocal leukoencephalopathy in a human T-cell lymphotropic virus type 1-infected hemodialytic patient. Ther Apher Dial. 2006;10:291–5.
Irie T, Kasai M, Abe N, et al. Cerebellar form of progressive multifocal leukoencephalopathy in a patient with chronic renal failure. Intern Med. 1992;31:218–23.
Neff RT, Hurst FP, Falta EM, et al. Progressive multifocal leukoencephalopathy and use of mycophenolate mofetil after kidney transplantation. Transplantation. 2008;86:1474–8.
Funahashi Y, Kato M, Fujita T, Takai S, Kimura Y, Gotoh M. Prevalence of polyomavirus positivity in urine after renal transplantation. Transpl Proc. 2014;46:564–6.
Organ Procurement &Transplantation Network. https://optn.transplant.hrsa.gov/.
Poggio E, Augustine JJ, Arrigain S, et al. Long-term kidney transplant graft survival-Making progress when most needed. Am J Transplant. 2021;21(8):2824–32.
Dumortier J, Guillaud O, Bosch A, et al. Progressive multifocal leukoencephalopathy after liver transplantation can have favorable or unfavorable outcome. Transpl Infect Dis. 2016;18:606–10.
Brickelmaier M, Lugovskoy A, Kartikeyan R, et al. Identification and characterization of mefloquine efficacy against JC virus in vitro. Antimicrob Agents Chemother. 2009;53:1840–9.
Elphick GF, Querbes W, Jordan JA, et al. The human polyomavirus, JCV, uses serotonin receptors to infect cells. Science. 2004;306:1380–3.
Bianchi A, Ragonese P, Banco MA, et al. Four cases of progressive multifocal leukoencephalopathy in iatrogenic immunocompromised patients. eNeurologicalSci. 2020;19:100243.
Ohnuki E, Asayama S, Asayama T, Nakamichi K, Saijo M, Kosaka S. A case of progressive multifocal leukoencephalopathy with chronic renal failure, whose JC virus in cerebrospinal fluid disappeared after mefloquine-mirtazapine dual therapy. Rinsho Shinkeigaku. 2016;56:705–8.
Ishikawa Y, Kasuya T, Ishikawa J, Fujiwara M, Kita Y. A case of developing progressive multifocal leukoencephalopathy while using rituximab and mycophenolate mofetil in refractory systemic lupus erythematosus. Ther Clin Risk Manag. 2018;14:1149–53.
Epperla N, Medina-Flores R, Mazza JJ, Yale SH. Mirtazapine and mefloquine therapy for non-AIDS-related progressive multifocal leukoencephalopathy. WMJ. 2014;113:242–5.
Ikegawa S, Fujii N, Tadokoro K, Sato K, Iwamoto M, Matsuda M, et al. Progressive multifocal leukoencephalopathy after T-cell replete HLA-haploidentical transplantation with post-transplantation cyclophosphamide graft-versus-host disease prophylaxis. Transpl Infect Dis. 2018;20: e12850.
Dohrn MF, Ellrichmann G, Pjontek R, Lukas C, Panse J, Gold R, et al. Progressive multifocal leukoencephalopathy and immune reconstitution inflammatory syndrome in seven patients with sarcoidosis: a critical discussion of treatment and prognosis. Ther Adv Neurol Disord. 2021;14:17562864211035544.
Okazaki T, Kodama D, Yamadera M, Sugiyama Y, Tsuji H, Nishida F, et al. Progressive multifocal leukoencephalopathy in a patient with rheumatoid arthritis under salazosulfapyridine treatment. Rinsho Shinkeigaku. 2021;61(12):833–8.
Akagawa Y, Ueno A, Ikeda J, Ishii W, Shishido-Hara Y, Sekijima Y. Two patients with progressive multifocal leukoencephalopathy with immune response against JC virus showing good long-term outcome by combination therapy of mefloquine, mirtazapine, and risperidone. Rinsho Shinkeigaku. 2018;58(5):324–31.
Fukumoto S, Shiraishi H, Nakamichi K, Nakajima H, Saijyo M, Tsujino A. A case of progressive multifocal leukoencephalopathy with Takayasu arteritis and indolent adult T-cell lymphoma/leukemia. Rinsho Shinkeigaku. 2016;56(2):82–7.
Ito D, Yasui K, Hasegawa Y, Nakamichi K, Katsuno M, Takahashi A. Progressive multifocal leukoencephalopathy with bilateral middle cerebellar peduncle lesions confirmed by repeated CSF-JC virus tests and coexistence of JC virus granule cell neuronopathy. Report of a case Rinsho Shinkeigaku. 2016;56(7):481–5.
Pallin M, O’Sullivan C, Dodd JD, McCreery K, Brett F, Farrell M, et al. A case of progressive multifocal leukoencephalopathy in a patient with sarcoidosis. QJM. 2012;105(10):1011–6.
Toorop AA, van Lierop ZYG, Strijbis EEM, Teunissen CE, Petzold A, Wattjes MP, et al. Mild progressive multifocal leukoencephalopathy after switching from natalizumab to ocrelizumab. Neurol Neuroimmunol Neuroinflamm. 2020;8(1):e904.
Nambirajan A, Suri V, Kataria V, Sharma MC, Goyal V. Progressive multifocal leukoencephalopathy in a 44-year old male with idiopathic CD4+ T-lymphocytopenia treated with mirtazapine and mefloquine. Neurol India. 2017;65(5):1061–4.
Hervás JV, Presas-Rodríguez S, Crespo-Cuevas AM, Canento T, Lozano-Sánchez M, Massuet-Vilamajó A, et al. Progressive multifocal leukoencephalopathy associated to natalizumab extended dosing regimen. Neurodegener Dis Manag. 2015;5(5):399–402.
Peña M, Presas-Rodríguez S, Ribera JM. Efficacy of mefloquine and mirtazapine on progressive multifocal leukoencephalopathy in a patient with peripheral T-cell lymphoma. Med Clin. 2019;153(9):e47–8.
Christakis PG, Okin D, Huttner AJ, Baehring JM. Progressive multifocal leukoencephalopathy in an immunocompetent patient. J Neurol Sci. 2013;326(1–2):107–10.
Soleimani-Meigooni DN, Schwetye KE, Angeles MR, Ryschkewitsch CF, Major EO, Dang X, et al. JC virus granule cell neuronopathy in the setting of chronic lymphopenia treated with recombinant interleukin-7. J Neurovirol. 2017;23(1):141–6.
Di Pauli F, Berger T, Walder A, Maier H, Rhomberg P, Uprimny C, et al. Progressive multifocal leukoencephalopathy complicating untreated chronic lymphatic leukemia: case report and review of the literature. J Clin Virol. 2014;60(4):424–7.
Fabis-Pedrini MJ, Xu W, Burton J, Carroll WM, Kermode AG. Asymptomatic progressive multifocal leukoencephalopathy during natalizumab therapy with treatment. J Clin Neurosci. 2016;25:145–7.
McGuire JL, Fridman V, Wüthrich C, Koralnik IJ, Jacobs D. Progressive multifocal leukoencephalopathy associated with isolated CD8+ T-lymphocyte deficiency mimicking tumefactive MS. J Neurovirol. 2011;17(5):500–3.
Simopoulou T, Tsimourtou V, Katsiari C, Vlychou M, Bogdanos DP, Sakkas LI. Progressive multifocal leukoencephalopathy in a patient with systemic sclerosis treated with methotrexate: a case report and literature review. J Scleroderma Relat Disord. 2020;5(3):6.
Wüthrich C, Popescu BFG, Gheuens S, Marvi M, Ziman R, Denq SP, et al. Natalizumab-associated progressive multifocal leukoencephalopathy in a patient with multiple sclerosis: a postmortem study. J Neuropathol Exp Neurol. 2013;72(11):1043–51.
Motte J, Kneiphof J, Straßburger-Krogias K, Klasing A, Adams O, Haghikia A, et al. Detection of JC virus archetype in cerebrospinal fluid in a MS patient with dimethylfumarate treatment without lymphopenia or signs of PML. J Neurol. 2018;265(8):1880–2.
Zhang Y, Wright C, Flores A. Asymptomatic progressive multifocal leukoencephalopathy: a case report and review of the literature. J Med Case Rep. 2018;12(1):187.
Mitsikostas DD, Mastorodemos V, Tsagournizakis M, Kodounis A, Tsagkaropoulos A, Konitsiotis S, et al. Natalizumab-related progressive multifocal leukoencephalopathy in Greece. Mult Scler Relat Disord. 2014;3(2):203–10.
Hashimoto Y, Tashiro T, Ogawa R, Nakamichi K, Saijo M, Tateishi T. Therapeutic experience of progressive multifocal leukoencephalopathy development during ofatumumab therapy for chronic lymphocytic leukemia. Intern Med. 2021;60(24):3991–3.
Yoshida H, Ohshima K, Toda J, Kusakabe S, Masaie H, Yagi T, et al. Significant improvement following combination treatment with mefloquine and mirtazapine in a patient with progressive multifocal leukoencephalopathy after allogeneic peripheral blood stem cell transplantation. Int J Hematol. 2014;99(1):95–9.
Silverio KA, Patel SA. Progressive multifocal leukoencephalopathy with negative JC virus PCR following treatment of follicular lymphoma: implications for biologics in the era of targeted cancer therapy. Case Rep Oncol Med. 2015;2015: 534529.
Lindå H, von Heijne A. Presymptomatic diagnosis with MRI and adequate treatment ameliorate the outcome after natalizumab-associated progressive multifocal leukoencephalopathy. Front Neurol. 2013;4:11.
Nakayama K, Nakamura M, Konishi A, Kaneko S, Nakamichi K, Saijo M, et al. JC virus granule cell neuronopathy associated with Ruxolitinib: A case report and review of the literature. eNeurologicalSci. 100269, 2020.
Meister S, Benecke R, König FB, Großmann A, Zettl UK, Winkelmann A. Progressive multifocal leukoencephalopathy in a patient with pre-clinical primary biliary cirrhosis. Clin Neurol Neurosurg. 2014;123:45–9.
Kalisch A, Wilhelm M, Erbguth F, Birkmann J. Progressive multifocal leukoencephalopathy in patients with a hematological malignancy: review of therapeutic options. Chemotherapy. 2014;60(1):47–53.
Berntsson SG, Katsarogiannis E, Lourenço F, Moraes-Fontes MF. Progressive multifocal leukoencephalopathy and systemic lupus erythematosus: focus on etiology. Case Rep Neurol. 2016;8(1):59–65.
Takekoshi A, Yoshikura N, Ozawa K, Ikoma Y, Kitagawa J, Takeshima A, et al. A patient with progressive multifocal leukoencephalopathy who developed bálint syndrome improved by combination therapy using mefloquine and mirtazapine. Brain Nerve. 2019;71(3):281–6.
Ueno T, Sato N, Kon T, Haga R, Nunomura JI, Nakamichi K, et al. Progressive multifocal leukoencephalopathy associated with thymoma with immunodeficiency: a case report and literature review. BMC Neurol. 2018;18(1):37.
Schröder A, Lee DH, Hellwig K, Lukas C, Linker RA, Gold R. Successful management of natalizumab-associated progressive multifocal leukoencephalopathy and immune reconstitution syndrome in a patient with multiple sclerosis. Arch Neurol. 2010;67(11):1391–4.
Iwami K, Nakamichi K, Matsushima M, Nagai A, Shirai S, Nakakubo S, et al. Progressive multifocal leukoencephalopathy with mild clinical conditions and detection of archetype-like JC virus in cerebrospinal fluid. J Neurovirol. 2021;27(6):917–22.
Zucker BE, Stacpoole SRL. Progressive multifocal leukoencephalopathy in the absence of immunosuppression. J Neurovirol. 2018;24(1):119–22.
Hamaguchi M, Suzuki K, Fujita H, Uzuka T, Matsuda H, Shishido-Hara Y, et al. Successful treatment of non-HIV progressive multifocal leukoencephalopathy: case report and literature review. J Neurol. 2020;267(3):731–8.
Maréchal E, Beel K, Crols R, Hernalsteen D, Willekens B. Long-term survival after progressive multifocal leukoencephalopathy in a patient with primary immune deficiency and NFKB1 mutation. J Clin Immunol. 2020;40(8):1138–43.
Nishigori R, Warabi Y, Shishido-Hara Y, Nakamichi K, Nakata Y, Komori T, et al. Inflammatory cerebellar PML with a CD4/CD8 ratio of 29 showed a favorable prognosis in a patient with rheumatoid arthritis. Intern Med. 2019;58(22):3323–9.
Ikeda J, Matsushima A, Ishii W, Goto T, Takahashi K, Nakamichi K, et al. Brain biopsy is more reliable than the DNA test for JC virus in cerebrospinal fluid for the diagnosis of progressive multifocal leukoencephalopathy. Intern Med. 2017;56(10):1231–4.
Kano Y, Inoue H, Sakurai K, Yoshida M, Miura Y, Nakamichi K, et al. Idiopathic CD4-positive lymphocytopenia-associated progressive multifocal leukoencephalopathy confirmed by brain biopsy following negative results of repeated CSF-JC-virus tests: a case report. Rinsho Shinkeigaku. 2018;58(12):750–5.
Harel A, Horng S, Gustafson T, Ramineni A, Farber RS, Fabian M. Successful treatment of progressive multifocal leukoencephalopathy with recombinant interleukin-7 and maraviroc in a patient with idiopathic CD4 lymphocytopenia. J Neurovirol. 2018;24:652–5.
Lutz M, Schulze AB, Rebber E, Wiebe S, Zoubi T, Grauer OM, et al. Progressive multifocal leukoencephalopathy after Ibrutinib therapy for chronic lymphocytic Leukemia. Cancer Res Treat. 2017;49(2):548–52.
Kurmann R, Weisstanner C, Kardas P, Hirsch HH, Wiest R, Lämmleet B, et al. Progressive multifocal leukoencephalopathy in common variable immunodeficiency: mitigated course under mirtazapine and mefloquine. J Neuroviral. 2015;21:694–701.
Calic Z, Cappelen-Smith C, Hodgkinson SJ, McDougall A, Cuganesan R, Brew BJ. Treatment of progressive multifocal leukoencephalopathy–immune reconstitution inflammatory syndrome with intravenous immunoglobulin in a patient with multiple sclerosis treated with fingolimod after discontinuation of natalizumab. J Clin Neurosci. 2015;22(3):598–600.
van Oosten BW, Killestein J, Barkhof F, Polman CH, Wattjes MP. PML in a patient treated with dimethyl fumarate from a compounding pharmacy. N Engl J Med. 2013;368:1658–9.
Ermis U, Weis J, Schulz JB. PML in a patient treated with fumaric acid. N Engl J Med. 2013;368:1657–8.
Stoppe M, Thoma E, Liebert UG, Major EO, Hoffmann K, Claßen J, et al. Cerebellar manifestation of PML under fumarate and after efalizumab treatment of psoriasis. J Neurol. 2014;261:1021–4.
Dammeier N, Schubert V, Hauser TK, Bornemann A, Bischof F. Case report of a patient with progressive multifocal leukoencephalopathy under treatment with dimethyl fumarate. BMC Neurol. 2015;15:108.
Hoepner R, Faissner S, Klasing A, Schneider R, Metz I, Bellenberget B, et al. Progressive multifocal leukoencephalopathy during fumarate monotherapy of psoriasis. Neurol Neuroimmunol Neuroinflamm. 2015;2: e85.
Acknowledgements
Not applicable
Funding
This work was partially supported by the Research Committee of Prion Disease and Slow Virus Infection, Research on Policy Planning and Evaluation for Rare and Intractable Diseases, Health and Labour Sciences Research Grants, the Ministry of Health, Labour and Welfare, Japan (Grant Number 20FC1054), and JSPS KAKENHI (Grant Number 21K07450).
Author information
Authors and Affiliations
Contributions
NS, HN, MS, and KK were contributors in writing the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The research was conducted ethically in accordance with the World Medical Association Declaration of Helsinki. This single case report was ethically approved by the institutional research board at Showa University (IRB approval number CR2023005-B).
Consent for publication
Informed consent for publication was obtained from the patient and his family included in the study.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Table S1.
Summary of case reports treated with mefloquine and mirtazabine for non-HIV progressive multifocal leukoencephalopathy.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Sone, N., Nishiwaki, H., Shimokawa, M. et al. A case of progressive multifocal leukoencephalopathy in a post-kidney transplant patient with improvement after discontinuation of immunosuppressive drugs and combination therapy with mefloquine and mirtazapine. Ren Replace Ther 9, 62 (2023). https://doi.org/10.1186/s41100-023-00517-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41100-023-00517-9