Abstract
Background
Survival is equivalent between super high-flux hemodialysis (SHF-HD) and online hemodiafiltration (OHDF) with similar albumin leakage. According to the 2013 Japanese dialyzer performance classification, survival on HD is optimal when a type II dialyzer (β2-microglobulin clearance ≥ 70 mL/min) is used. Here, we investigated whether survival could be improved by SHF-HD using a type II-b dialyzer (sieving coefficient for albumin ≥ 0.03) with high albumin leakage compared with OHDF or SHF-HD using a type II-a dialyzer (sieving coefficient for albumin < 0.03) with low albumin leakage.
Methods
This 3-year retrospective observational propensity score-matched study included 738 patients receiving SHF-HD (n = 310) or OHDF (n = 428) with a type II dialyzer at our institution between April 1 and July 1, 2017. Three-year all-cause mortality was compared for SHF-HD with high estimated albumin leakage (EAL) versus OHDF and SHF-HD with low EAL. Kaplan–Meier survival curves were compared using the log-rank test and hazard ratios were calculated by Cox regression analysis.
Results
Mortality in SHF-HD with high EAL was significantly lower than OHDF with low EAL (each n = 52 after matching; P = 0.007, log-rank test). All the dialyzers used a polyethersulfone (PES) membrane, whereas none of the hemodiafilters had a PES membrane. In SHF-HD, mortality was significantly lower when EAL was ≥ 3.0 g/session than when EAL was < 3.0 g/session (each n = 115 after matching, P = 0.004, log-rank test) and when the dialyzer used was type II-b rather than type II-a (each n = 133 after matching, P = 0.001, log-rank test).
Conclusions
These findings suggest that survival is better on SHF-HD using a type II-b dialyzer with high albumin leakage than on OHDF with low albumin leakage or SHF-HD using a type II-a dialyzers. The PES used in the type II-b dialyzer may also have a beneficial effect on survival.
Similar content being viewed by others
Introduction
Hemodialysis (HD) using a high-flux membrane has limited ability to remove medium-middle and large-middle molecules. Therefore, online hemodiafiltration (OHDF), which has a high substitution volume, has been developed to remove these molecules by increasing the convection volume. In Europe, high-volume post-dilution OHDF (post-OHDF) using low-permeability membranes for albumin is the norm with albumin leakage not exceeding 3.4–5.0 g/session [1, 2]. In Japan, predilution OHDF (pre-OHDF) with a substitution volume of 24–84 L using membranes with low-to-high permeability for albumin is the norm, with albumin leakage set at no more than 5 g/session in many facilities; post-OHDF with a substitution volume of 6–16 L has also been performed although not often [3].
A prospective randomized controlled trial demonstrated that survival was significantly better in high-volume post-OHDF than in HD whether a high-flux membrane or a low-flux membrane was used [4]. However, three prospective randomized controlled trials using a high-flux [5, 6] or low-flux [7] membrane and a prospective observational study using both high-flux and low-flux membranes [8] have failed to demonstrate a survival advantage of high-volume post-OHDF over HD.
In 2004, the Ministry of Health, Labour and Welfare of Japan classified dialyzers into the following five functional types according to the β2-microglobulin (β2MG) clearance runder set conditions of membrane surface area 1.5 m2, blood flow rate (QB) 200 mL/min, dialysate flow rate (QD) 500 mL/min, and filtration flow rate 15 mL/min: type I, < 10 mL/min; type II, ≥ 10 and < 30 mL/min; type III, ≥ 30 and < 50 mL/min; type IV, ≥ 50 and < 70 mL/min; and type V, ≥ 70 mL/min) [9]. Subsequently, type I was defined as a low-flux membrane, types II and III as high-flux membranes, and types IV and V as super high-flux (SHF) membranes [10]. In 2013, the Japanese Society for Dialysis Therapy (JSDT) extended these five types of dialyzer to include a further four types determined by β2MG clearance and the sieving coefficient (SC) for albumin (type I-a, < 70 mL/min and < 0.03; type I-b, < 70 mL/min and ≥ 0.03; type II-a, ≥ 70 mL/min and < 0.03; type II-b, ≥ 70 mL/min and ≥ 0.03) and a type S (a dialyzer membrane with special features, such as those made from ethylene vinyl alcohol or polymethylmethacrylate) as shown in Additional file 1: Table S1 [11]. Although the JSDT allowed albumin leakage with SC ≥ 0.03, the basis for setting reference SC values for albumin and β2MG clearance was not clear.
In a 7-year observational study using dialyzers with β2MG clearance ≥ 10 mL/min (types II–V in the 2004 classification), Nagai et al. found that prognosis was better when the estimated albumin leakage (EAL) was ≥ 3.0 g/session than when it was < 3.0 g/session [12]. Abe et al. reported that mortality was significantly lower with SHF membranes that have β2MG clearance of ≥ 50 and < 70 mL/min than with those that have β2MG clearance of < 10 mL/min and was also significantly lower when β2MG clearance was ≥ 70 mL/min (type V in the 2004 classification and type II in the 2013 classification) than when it was ≥ 50 and < 70 mL/min (type IV in the 2004 classification and type I in the 2013 classification) [10, 11]. We have recently reported that survival is better with high albumin leakage than with low albumin leakage regardless of whether patients are receiving SHF-HD or OHDF, that survival is equivalent between these two dialysis modalities at a similar level of albumin leakage, and that survival in patients on OHDF is influenced by albumin leakage rather than substitution volume [13].
Based on these considerations, we hypothesized that survival would be better in SHF-HD using a dialyzer with β2MG clearance ≥ 70 mL/min and high albumin leakage than in either OHDF or SHF-HD with low albumin leakage. The aim of this study was to determine whether or not survival is better on SHF-HD with a type II-b dialyzer than on OHDF with low albumin leakage or SHF-HD with a type II-a dialyzer.
Methods
Patient selection
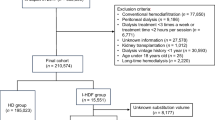
As shown in Fig. 1, 738 of 944 previously described patients undergoing maintenance dialysis with SHF-HD or OHDF at our institution and registered in our records database as of July 1, 2017 [14], were recruited to prepare a propensity score-matched (PSM) model. The patients were divided into those on SHF-HD with β2MG clearance ≥ 70 mL/min (n = 310) and those on OHDF (n = 428). The exclusion criteria were dialysis with β2MG clearance < 70 mL/min, a type S dialyzer, age younger than 20 years, a blood purification method other than HD or OHDF, dialysis frequency of fewer than 3 sessions/week, dialysis time less than 3 h, substitution volume for pre-OHDF < 60 L and post-OHDF < 8 L, missing covariate values, and pregnancy or lactation. Patients whose dialysis conditions (dialysis method, dilution method, substitution volume, and/or membrane material) at the start of the study on July 1, 2017, were different from those on April 1, 2017, were also excluded. Patients receiving SHF-HD or OHDF were defined as those confirmed annually to have received the same dialysis method for 3 years (July 1, 2017 to July 1, 2020). Switching between groups was censored in the Kaplan–Meier survival curve. The dialysis modality was chosen at the physician’s discretion. Blood test results were extracted from the medical records.
Preparation of propensity score-matched pairs
Patients with EAL corresponding to the third quartile or higher for SHF-HD vs. those corresponding to within the first quartile for OHDF and patients with EAL corresponding to the seventh octile or higher for SHF-HD vs. those corresponding to within the first octile for OHDF were used to investigate all-cause mortality between SHF-HD with high albumin leakage and OHDF with low albumin leakage. The propensity scores were matched for 117 pairs of patients receiving SHF-HD and 52 pairs of patients receiving OHDF. The following 14 items were used to calculate the propensity score for comparing patient survival outcomes: presence or absence of diabetes mellitus, age, dialysis vintage, body mass index, normalized protein catabolism rate, serum albumin, corrected calcium, phosphorus, hemoglobin, high-sensitivity C-reactive protein, Kt/V, systolic blood pressure, QB, and membrane surface area. The duration of each dialysis session was 4 h and both QD in HD and total QD (QD plus the substitution volume) in OHDF were fixed at 500 mL/min.
Next, patients with EAL ≥ 3.0 g/session vs. those with EAL < 3.0 g/session and patients treated using a type II-b dialyzer vs. a type II-a dialyzer were selected for investigation of all-cause mortality in SHF-HD according to whether albumin leakage was high or low; propensity scores were matched in 115 pairs and 133 pairs, respectively. The following 11 items were used to calculate the propensity score for comparing patient survival outcomes: presence or absence of diabetes mellitus, age, dialysis vintage, body mass index, normalized protein catabolism rate, serum albumin, corrected calcium, phosphorus, hemoglobin, high-sensitivity C-reactive protein, and Kt/V.
To calculate the propensity score for each patient, multivariable logistic regression analysis was performed using the treatment group as the dependent variable and 14 covariates as independent variables, followed by logit transformation. The propensity scores were calculated to 14 decimal places. Regardless of the number of cases, patients in the two groups were paired by nearest available matching at a ratio of 1:1 within a caliper (0.348014 for SHF-HD with high albumin leakage vs. OHDF with low albumin leakage; 0.173634 for EAL ≥ 3.0 g/session vs. EAL < 3.0 g/session in SHF-HD, and 0.144746 for type II-b dialyzers vs. type II-a dialyzers in SHF-HD) of 0.2 × SD of the logit values for all patients in both groups [15].
Estimation of amount of albumin leakage
The amount of albumin leakage was measured for each dialyzer or hemodiafilter by collecting whole dialysis waste liquid for 4 h; the average value was assigned according to the substitution volume. QB was 250 mL/min for HD and 280 mL/min for OHDF, and both QD in HD and total QD in OHDF were 500 mL/min. The substitution volumes were 60 L, 72 L, and 84 L for pre-OHDF and 8 L, 10 L, 12 L, and 16 L for post-OHDF. The dialyzers and hemodiafilters used in this study and the average EAL values are listed in Additional files 2 and 3: Tables S2 and S3. The albumin level was measured using a turbidimetric immunoassay for the dialysate and a photometric method using bromocresol green for serum.
Statistical analysis
Survival was determined from the medical records, which include information on deaths and transfers to other hospitals. A daily survival analysis was performed for the two groups, including censored cases, using the Kaplan–Meier method. Between-group differences were examined for statistical significance using the log-rank test. Cox regression analysis was used to calculate hazard ratios (HRs). All analyses were performed using SPSS Statistics for Windows, version 26 (IBM Corp., Armonk, NY, USA). A two-tailed P-value < 0.05 was considered statistically significant.
Results
Effect of SHF-HD with high EAL and OHDF with low EAL on survival
Variables for SHF-HD with EAL at or above the third quartile and OHDF with EAL within the first quartile and after PSM are compared in Table 1a. After PSM, there was a significant increase in the hemoglobin level and a significant decrease in the membrane surface area in SHF-HD compared with OHDF. EAL of 3.0 g/session corresponded to the third quartile for SHF-HD, and EAL of 3.4 g/session corresponded to the first quartile for OHDF. As shown in Fig. 2a, there was no significant difference in 3-year all-cause mortality (P = 0.477, log-rank test) despite a significant difference in EAL between SHF-HD and OHDF (3.9 ± 0.9 g/session vs. 2.7 ± 0.5 g/session, P < 0.001). All dialyzers with EAL ≥ 3.0 g/session were type II-b (Additional file 2: Table S2).
Comparison of patient survival outcomes between hemodialysis using SHF-HD) with high EAL and OHDF with low EAL. a SHF-HD with EAL at the third quartile or above versus OHDF with EAL within the first quartile. b SHF-HD with EAL at or above the seventh octile versus OHDF with EAL within the first octile. CI confidence interval; EAL estimated albumin leakage; HR hazard ratio; OHDF online hemodiafiltration; SHF-HD super high-flux hemodialysis
Variables for SHF-HD with EAL at or above the seventh octile and OHDF with EAL within the first octile before and after PSM are compared in Table 1b. All significant differences in covariates between SHF-HD and OHDF disappeared after PSM. EAL of 4.1 g/session corresponded to the seventh octile for SHF-HD and EAL of 2.4 g/session corresponded to the first octile for OHDF. Three-year all-cause mortality was significantly lower in SHF-HD than in OHDF (P = 0.007, log-rank test; Fig. 2b). However, the HR and 95% confidence interval (CI) could not be calculated because there were no deaths in SHF-HD. EAL was significantly higher in SHF-HD than in OHDF (4.2 ± 0.3 g/session vs. 2.3 ± 0.1 g/session, P < 0.001). All the dialyzers with EAL ≥ 4.2 g/session were type II-b and had a polyethersulfone (PES) membrane (Additional file 2: Table S2). None of the hemodiafilters with an EAL < 2.3 g/session had a PES membrane (Additional file 3: Table S3).
Comparison of SHF-HD according to whether EAL was high or low
Table 2a compares the variables recorded before and after PSM according to whether EAL was ≥ 3.0 g/session or < 3.0 g/session. After PSM, the corrected calcium level was significantly lower and the hemoglobin level was significantly higher when EAL was ≥ 3.0 g/session (mean 3.9 ± 0.8 g/session) than when it was < 3.0 g/session (mean 1.5 ± 0.2 g/session) (P < 0.001). Furthermore, 3-year all-cause mortality was significantly lower when the EAL was ≥ 3.0 g/session (P = 0.004, log-rank test; HR 0.37, 95% CI 0.18–0.75; Fig. 3a). Dialyzers with EAL ≥ 3.0 g/session were type II-b only while those with EAL < 3.0 g/session included type II-b and type II-a (Additional file 2: Table S2).
Variables measured before and after PSM are compared between type II-b and type II-a dialyzers in Table 2b. After PSM, the patient age and Kt/V level were significantly lower and dialysis vintage and serum albumin were significantly higher with a type II-b dialyzer (mean EAL 3.7 ± 1.0 g/session) than with a type II-a dialyzer (mean EAL 1.4 ± 0.2 g/session) (P < 0.001). The 3-year all-cause mortality rate was significantly lower for type II-b dialyzers than for type II-a dialyzers (P = 0.001, log-rank test; HR 0.34; 95% CI 0.17–0.68; Fig. 3b).
Discussion
This observational study is the first to suggest that survival on SHF-HD using a type II-b dialyzer (β2MG clearance ≥ 70 mL/min and SC for albumin ≥ 0.03) with high EAL is better than that on OHDF using a hemodiafilter with low EAL, that survival on SHF-HD using a type II dialyzer (β2MG clearance ≥ 70 mL/min) with EAL ≥ 3.0 g/session is better than that with EAL < 3.0 g/session, and that survival is better on SHF-HD using a type II-b dialyzer (SC for albumin ≥ 0.03) than on SHF-HD using a type II-a dialyzer (SC for albumin < 0.03). Moreover, it is possible that the PES membrane material itself, which allows high albumin leakage, contributes to the improvement in survival.
Albumin is a classic nutritional marker associated with mortality, and malnutrition, which can trigger hypoalbuminemia, may increase mortality in patients on HD [16]. Although high albumin leakage may induce hypoalbuminemia that worsens survival, it is possible that hypoalbuminemia resulting from excessive removal of albumin with degraded antioxidant activity by high albumin leakage leads to production of new albumin with normal antioxidant activity in the liver [17]. We have recently reported that high albumin leakage can improve survival to a similar extent on OHDF and SHF-HD even in the presence of mild-to-moderate hypoalbuminemia [13]. Of note is that in the European studies where no difference in mortality was found between patients on HD and those on OHDF, the mean serum albumin levels did not indicate hypoalbuminemia at baseline or during follow-up in any group [5,6,7], which suggests low albumin leakage. Endogenous uremic toxins have recently been classified as small, small-middle, medium-middle, large-middle, and large molecules [18]. The improved survival with high albumin leakage suggests the importance of removing not only large-middle molecules (25–58 kDa) but also large molecules (58–170 kDa), such as albumin-bound uremic toxins. In the present study, survival was better in patients on SHF-HD with a type II-b dialyzer and high albumin leakage than in those on OHDF with low albumin leakage. Therefore, aggressive albumin leakage may be important in patients on SHF-HD or OHDF, except for those with malnutrition or inflammation. Our present findings indicate that survival is improved by using a dialyzer with EAL ≥ 3.0 g/session or a type II-b dialyzer. SC for albumin of ≥ 0.03 corresponds to mean EAL of ≥ 3.0 g/session (Additional file 2: Table S2). However, there was a type II-b dialyzer that had mean EAL as low as 2.1 ± 1.1 g/session. For this reason, we should consider the effect of ultrafiltration volume and variation in performance when measuring albumin leakage.
In Europe, with QB set at 300–400 mL/min, low-flux membranes are defined as dialyzers having β2MG clearance of < 10 mL/min and SC for albumin of 0, high-flux membranes are defined as those having β2MG clearance of ≥ 20 and ≤ 40 mL/min and SC for albumin of < 0.01, medium cutoff membranes are defined as those having β2MG clearance > 80 mL/min and SC for albumin of < 0.01, and high cutoff membranes are defined as those having no β2MG clearance and SC for albumin of < 0.2 [19]. In 2013, the JSDT reclassified the dialyzers now used in clinical practice into five types as shown in Additional file 1: Table S1 [11]. Considering the findings of this study, we recommend use of a type II-b dialyzer in patients on HD who do not have malnutrition or an inflammatory condition. Considering that Japan and other countries use different dialysis conditions when measuring β2MG clearance and SC for albumin, a global dialyzer classification with measurements obtained under the same conditions is needed to compare the performance of dialyzers internationally. However, the JSDT has adopted a single classification for hemodiafilters in which β2MG clearance is ≥ 70 mL/min.
The only membrane material used in SHF dialyzers with EAL at or above the seventh octile is PES, which is not included in hemodiafilters that have EAL within the first octile. In 2017, Abe et al. reported a cohort PSM study in which they investigated the effects of dialysis membrane materials on the prognosis in 136,676 patients on dialysis in Japan [20]. They compared PES, cellulose triacetate, polyester polymer alloy, polymethyl-methacrylate, polyacrylonitrile, and ethylene vinyl alcohol membranes with polysulfone (PS) membranes for 2 years from 2009 and found that the survival was significantly better when a PES membrane was used rather than a PS membrane. This finding has prompted consideration of differences in biocompatibility arising from differences in membrane materials.
Although the removal of protein-bound and large-middle molecule uremic toxins by SHF-HD using a PES membrane was similar to that achieved by high-volume post-OHDF [21], it is not known whether this would improve survival. We have previously reported that SHF-HD improves survival to the same extent as OHDF with a similar level of albumin leakage [13]. However, in the present study, we found that survival was better for SHF-HD using a type II-b dialyzer with high albumin leakage than for OHDF with low albumin leakage.
The main limitation of this study was the accuracy of the EAL and its fluctuation, especially for hemodiafilters, as indicated previously [13]. The patient characteristics were different in three of the four groups, even after PSM, because of the small number of patients. Unlike in the nationwide database, there are no significant differences in unobserved background factors, such as quality of medical management or dialysis conditions, at our facilities [14]. Therefore, we consider it reliable to set a caliper value of 0.2 multiplied by the standard deviation of the logit transformed value of the propensity score for all cases [15]. We do not have data on residual kidney function, although the dialysis vintage for patients receiving SHF-HD or OHDF was more than 3 months. A randomized controlled trial is needed to confirm our findings.
Conclusions
This study is the first to suggest that better survival is achieved by SHF-HD using a type IIb dialyzer with high albumin leakage than by OHDF using a hemodiafilter with low albumin leakage, that survival is better for SHF-HD using a type II dialyzer with albumin leakage ≥ 3.0 g/session that with albumin leakage < 3.0 g/session, and that survival is improved on SHF-HD when a type II-b dialyzer is used. A PES membrane with high albumin leakage may also have a beneficial effect on patient survival.
Availability of data and materials
All data generated or analyzed during this study are included in this published article and its supplementary information files. Data supporting the results of the study are kept at Japan Institute of Statistical Technology (https://www.jiost.com/).
Abbreviations
- β2MG:
-
β2-Microglobulin
- BSA:
-
Bisphenol A
- CI:
-
Confidence interval
- EAL:
-
Estimated albumin leakage
- HD:
-
Hemodialysis
- HR:
-
Hazard ratio
- JSDT:
-
Japanese Society for Dialysis Therapy
- MCO:
-
Medium cutoff
- OHDF:
-
Online hemodiafiltration
- PES:
-
Polyethersulfone
- post-OHDF:
-
Post-dilution online hemodiafiltration
- pre-OHDF:
-
Predilution online hemodiafiltration
- PS:
-
Polysulfone
- PSM:
-
Propensity score matching
- QB:
-
Blood flow rate
- QD:
-
Dialysate flow rate
- RCT:
-
Randomized controlled trial
- SC:
-
Sieving coefficient
- SHF:
-
Super high-flux
References
van Gelder MK, Abrahams AC, Joles JA, Kaysen GA, Gerritsen KGF. Albumin handling in different hemodialysis modalities. Nephrol Dial Transplant. 2018;33:906–13. https://doi.org/10.1093/ndt/gfx191.
Potier J, Queffeulou G, Bouet J. Are all dialyzers compatible with the convective volumes suggested for postdilution online hemodiafiltration? Int J Artif Organs. 2016;39:460–70. https://doi.org/10.5301/ijao.5000525.
Kawanishi H. Development of effects of online hemodiafiltration in Japan. Ren Replace Ther. 2021;7:51. https://doi.org/10.1186/s41100-021-00370-8.
Maduell F, Moreso F, Pons M, Ramos R, Mora-Macià J, Carreras J, Soler J, Torres F, Campistol JM, Martinez-Castelao A, ESHOL Study Group. High-efficiency postdilution online hemodiafiltration reduces all-cause mortality in hemodialysis patients. J Am Soc Nephrol 2013;24:487–497. https://doi.org/10.1681/ASN.2012080875
Ok E, Asci G, Toz H, Ok ES, Kircelli F, Yilmaz M, Hur E, Demirci MS, Demirci C, Duman S, Basci A, Adam SM, Isuk IO, Zengin M, Suleymanlar G, Yilmaz ME, Ozkahya M, Turkish Online Haemodiafiltration Study. Mortality and cardiovascular events in online haemodiafiltration (OL-HDF) compared with high-flux dialysis: results from the Turkish OL-HDF Study. Nephrol Dial Transplant 2013;28:192–202. https://doi.org/10.1093/ndt/gfs407
Morena M, Jaussent A, Chalabi L, Leray-Moragues H, Chenine L, Debure A, Thibaudin D, Azzouz L, Patrier L, Maurice F, Nicoud N, Durand C, Seigneuric B, Dupuy A, Picot M, Cristol J, Canaud B. Treatment tolerance and patient-reported outcomes favor online hemodiafiltration compared to high-flux hemodialysis in the elderly. Kidney Int. 2017;91:1495–509. https://doi.org/10.1016/j.kint.2017.01.013.
Grooteman MPC, van den Dorpel MA, Bots ML, Penne L, van der Weerd NC, Mazairac AHA, den Hoedt CH, van der Tweel I, Lévesque R, Nubé MJ, ter Wee PM, Blankestijn PJ, CONTRAST Investigators. Effect of online hemodiafiltration on all-cause mortality and cardiovascular outcomes. J Am Soc Nephrol 23:1087–1096, 2012. https://doi.org/10.1681/ASN.2011121140
Locatelli F, Karaboyas A, Pisoni RL, Robinson BM, Fort J, Vanholder R, Rayner HC, Kleophas W, Jacobson SH, Combe C, Port FK, Tentori F. Mortality risk in patients on hemodiafiltration versus hemodialysis: a ‘real-world’ comparison from the DOPPS. Nephrol Dial Transplant 33:683–689. https://doi.org/10.1093/ndt/gfx277
Nakai S, Suzuki K, Masakane I, Wada A, Itami N, Ogata S, Kimata N, Shigematsu T, Shinoda T, Syouji T, Taniguchi M, Tsuchida K, Nakamoto H, Nishi S, Nishi H, Hashimoto S, Hasegawa T, Hanafusa N, Hamano T, Fujii N, Marubayashi S, Morita O, Yamagata K, Wakai K, Watanabe Y, Iseki K, Tsubakihara Y. Overview of Regular Dialysis Treatment in Japan (as of 31 December 2008). Ther Apher Dial. 2010;14:505–40. https://doi.org/10.1111/j.1744-9987.2010.00893.x.
Abe M, Masakane I, Wada A, Nakai S, Nitta K, Nakamoto H. Super high-flux membrane dialyzers improve mortality in patients on hemodialysis: a 3-year nationwide cohort study. Clin Kidney J. 2021;15:473–83. https://doi.org/10.1093/ckj/sfab177.
Kawanishi H, Mineshima M, Tomo M, Minakuchi J. Functional classification of (hollow-fiber) blood purification equipment 2013. J Jpn Soc Dial Ther. 2013;46:501–6 ((in Japanese)).
Nagai K, Tsuchida K, Ishihara N, Minagawa N, Ichien G, Yamada S, Hirose D, Michiwaki H, Kanayama H, Doi T, Minakuchi J. Implications of albumin leakage for survival in maintenance hemodialysis patients: a 7-year observational study. Ther Apher Dial. 2017;21:378–86. https://doi.org/10.1111/1744-9987.12526.
Okada K, Tashiro M, Michiwaki H, Inoue T, Shima H, Minakuchi J, Kawashima S. Effects of high albumin leakage on survival equivalently between online hemodiafiltration and super high-flux hemodialysis: the HISTORY study. Ren Replace Ther. 2022;8:52. https://doi.org/10.1186/s41100-022-00440-5.
Okada K, Michiwaki H, Tashiro M, Inoue T, Shima H, Minakuchi J, Kawashima S. Effects of Japanese-style online hemodiafiltration on survival and cardiovascular events. Ren Replace Ther. 2021;7:70. https://doi.org/10.1186/s41100-021-00385-1.
Austin PC. Optimal caliper widths for propensity-score matching when estimating differences in means and differences in proportions in observational studies. Pharm Stat. 2011;10:150–61. https://doi.org/10.1002/pst.433.
Lowrie EG, Lew NL. Death risk in hemodialysis patients: the predictive value of commonly measured variables and an evaluation of death rate differences between facilities. Am J Kidney Dis. 1990;15:458–82. https://doi.org/10.1016/s0272-6386(12)70364-5.
Nagai K, Tsuchida K, Hirose D, Michiwaki H, Hann M, Kanayama H, Doi T, Minakuchi J. The effect of albumin leakage in hemodialysis patients on redox status of serum albumin. J Artif Organs. 2016;19:310–4. https://doi.org/10.1007/s10047-016-0900-2.
Rosner MH, Reis T, Husain-Syed F, Vanholder R, Hutchison C, Stenvinkel P, Blankestijn PJ, Cozzolino M, Juillard L, Kashani K, Kaushik M, Kawanishi H, Massy Z, Sirich TL, Zuo L, Ronco C. Classification of uremic toxins and their role in kidney failure. CJASN. 2021;16:1918–28. https://doi.org/10.2215/CJN.02660221.
Ward RA, Beck W, Bernard AA, Alves FC, Stenvinkel P, Lindholm B. Hypoalbuminemia: a price worth paying for improved dialytic removal of middle-molecular-weight uremic toxins? Nephrol Dial Transplant. 2019;34:901–7. https://doi.org/10.1093/ndt/gfy236.
Abe M, Hamano T, Wada A, Nakai S, Masakane I. High-performance membrane dialyzers and mortality in hemodialysis patients: a 2-year cohort study from the annual survey of the Japanese Renal Data Registry. Am J Nephrol. 2017;46:82–92. https://doi.org/10.1159/000478032.
Thammathiwat T, Tiranathanagul K, Limjariyakul M, Chariyavilaskul P, Takkaratakarn K, Susantitaphong P, Meesangnin S, Wittayalertpanya S, Praditpornilpa K, Eiam-Ong S. Super high-flux hemodialysis provides comparable effectiveness with high-volume postdilution online hemodiafiltration in removing protein-bound and middle-molecule uremic toxins: a prospective cross-over randomized controlled trial. Ther Apher Dial. 2021;25:73–81. https://doi.org/10.1111/1744-9987.13508.
Acknowledgements
We are grateful to all of the staff in our medical corporation for providing a similar quality of healthcare management and dialysis conditions across facilities. We are also grateful to Dr. Shigeaki Ohtsuki of Japan Institute of Statistical Technology for performing the statistical analysis.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
KO interpreted the results of statistical analysis and was the major contributor to drafting the manuscript, and MT, HM, TI, HS, JM, and SK performed data collection. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the Research Ethics Committee of Kawashima Hospital and registered in the UMIN Clinical Trials Registry (UMIN000047948 registered on June 4, 2022—retrospectively registered, https://center6.umin.ac.jp/cgi-bin/ctr/ctr_view_reg.cgi?recptno=R000054664, and UMIN000050068 registered on January 18, 2023—retrospectively registered, https://center6.umin.ac.jp/cgi-bin/ctr/ctr_view_reg.cgi?recptno=R000056883). All clinical investigations were conducted according to the principles expressed in the Declaration of Helsinki. All patients gave informed consent for their data to be included in this study.
Consent for publication
Not applicable.
Competing interests
All of the authors report the funding of specific clinical research (Japan Registry of Clinical Trials with the registration number jRCTs062190020) from Asahi Kasei Medical Co., Ltd. and contract research from Nipro Co., Ltd.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Table S1
. Functional classification of dialyzers 2013 developed by the Japanese Society for Dialysis Therapy [11]
Additional file 2: Table S2
. Super high-flux dialyzers, mean estimated albumin leakage (EAL) and functional classification of dialyzers 2013 developed by the Japanese Society for Dialysis Therapy (JSDT).
Additional file 3: Table S3
. Hemodiafilters and mean estimated albumin leakage (EAL).
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Okada, K., Tashiro, M., Michiwaki, H. et al. Comparison of survival for super high-flux hemodialysis (SHF-HD) with high albumin leakage versus online hemodiafiltration or SHF-HD with low albumin leakage: the SUPERB study. Ren Replace Ther 9, 32 (2023). https://doi.org/10.1186/s41100-023-00490-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41100-023-00490-3