Abstract
Background
All-cause mortality is lower with a high substitution volume in predilution (pre) and postdilution (post) online hemodiafiltration (OHDF) than with hemodialysis (HD), and mortality does not significantly differ between pre- and post-OHDF groups. Despite the improved survival with a high substitution volume, there may be limitations. On the other hand, either normoalbuminemia or high albumin leakage in HD can reduce mortality, and super high-flux (SHF) membrane dialyzers can reduce mortality compared with low-flux and high-flux membrane dialyzers. Here, we investigated the associations of serum albumin concentration (s-Alb), albumin leakage, and substitution volume with all-cause mortality in OHDF and SHF-HD.
Methods
In a 3-year retrospective observational study of patients receiving dialysis from April 1 to July 1, 2017, we developed a propensity score-matched model using 783 stable patients (SHF-HD, 355; OHDF, 428). We used the log-rank test to compare Kaplan–Meier survival curves and Cox regression analysis to calculate hazard ratio (HR). Cox regression analysis was also used to compare the effect of estimated albumin leakage (EAL) and substitution volume on 3-year all-cause mortality.
Results
All-cause mortality was significantly lower with high EAL than with low EAL (SHF-HD: P = 0.012, log-rank test; HR, 0.44; 95% confidence interval [CI] 0.23–0.85; OHDF: P = 0.027, log-rank test; HR, 0.41; 95% CI 0.18–0.93). The mortality of high EAL was not significantly different between high and low s-Alb in SHF-HD (3.5 ± 0.1 and 3.2 ± 0.2 g/dL) and OHDF (3.6 ± 0.2 and 3.2 ± 0.1 g/dL), despite significant differences in s-Alb. Mortality did not significantly differ between SHF-HD and OHDF with higher EAL ranges or a lower difference in EAL. Mortality in pre-OHDF was significantly correlated with EAL (P = 0.007, beta − 0.32) rather than substitution volume, and mortality in post-OHDF was not analyzed because of fewer deaths.
Conclusions
The results suggest that survival is improved more with high EAL than with low EAL in both OHDF and SHF-HD patients, that high EAL with mild-to-moderate hypoalbuminemia does not necessarily worsen survival in OHDF and SHF-HD patients, and that survival is equivalent between OHDF and SHF-HD patients with a similar level of EAL.
Similar content being viewed by others
Introduction
Endogenous uremic toxins have recently been classified as follows: small molecules, < 0.5 kDa; small-middle molecules, 0.5–15 kDa; medium-middle molecules, 15–25 kDa; large-middle molecules, 25–58 kDa; and large molecules, 58–170 kDa [1]. Although high-flux membrane dialyzers generally perform well at removing small-middle molecules such as β2-microglobulin (β2MG), they perform poorly at removing medium-middle molecules. It is also considered important to remove large-middle molecules, which are dependent on convection and substitution volume. Therefore, online hemodiafiltration (OHDF) has been developed. In Europe, high-volume (16–26 L) postdilution OHDF (post-OHDF) using low-permeability membranes (defined as European-style post-OHDF) has been performed with albumin leakage not exceeding 3.4 g/session [2] or 5 g/session in a convection volume of 23 L/session/1.73 m2 [3], suggesting that the performance of hemodiafilters is of little concern. In contrast, in Japan, either post-OHDF with a substitution volume of 6–16 L or predilution OHDF (pre-OHDF) with a substitution volume of 24–84 L has been performed using moderate- to high-permeability membranes (defined as Japanese-style OHDF), and albumin leakage has been set at no more than 5 g/session in many facilities [4]. However, the acceptable serum albumin concentration (s-Alb) varies by institution, and low-permeability membranes have been used in patients with not only hypoalbuminemia (s-Alb ≤ 3.5 g/dL) but also normoalbuminemia (s-Alb ≥ 3.6 g/dL) in Japan.
Albumin is a classic nutritional marker associated with mortality. s-Alb < 4.0 g/dL is the parameter most strongly associated with probability of death, and malnutrition, which can trigger hypoalbuminemia, may increase mortality in HD patients [5]. The s-Alb, which comprises reduced human mercaptoalbumin (HMA) and oxidized human non-mercaptoalbumin, is the most important extracellular antioxidant in humans. Either a higher HMA level or higher estimated albumin leakage (EAL) per HD session has been related to lower mortality in HD patients [6, 7]. In addition, an increase in EAL was related to an elevated HMA ratio and inversely correlated with the s-Alb level [8], suggesting that hypoalbuminemia resulting from excessive removal of albumin with degraded antioxidant activity may lead to the production of new albumin with normal antioxidant activity in the liver. However, to our knowledge, there has been no report on EAL and survival in OHDF.
The Membrane Permeability Outcome study showed that patients with a s-Alb ≤ 4.0 g/dL had better survival on high-flux HD than on low-flux HD [9]. Mortality adjusted for confounding factors has been reported to not be significantly different between hypoalbuminemia without inflammation and normoalbuminemia without inflammation [10], suggesting that mild-to-moderate hypoalbuminemia due to albumin leakage without malnutrition or inflammation is not an independent predictor of mortality. In our facilities, hypoalbuminemia is tolerated up to approximately 3.0 g/dL, except in dialyzed patients with malnutrition or inflammation, because symptoms such as pruritus, restless legs syndrome, and fatigue can usually be improved by aggressive albumin leakage. Accordingly, we wondered whether European-style post-OHDF leaks enough albumin, considering that normoalbuminemia was observed in the CONTRAST study, in the Turkish study, and in the FRENCHIE study [11,12,13], which failed to demonstrate a survival advantage of post-OHDF over low-flux HD and high-flux HD.
In Japan, super high-flux (SHF) membrane dialyzers are defined as those with a β2MG clearance ≥ 50 mL/min, and they can reduce mortality compared with low-flux (< 10 mL/min clearance) and high-flux (≥ 10 and < 50 mL/min clearance) membrane dialyzers [14]. In Europe where the blood flow rate (QB) is considerably higher than in Japan, low-flux membranes are defined as those having a β2MG clearance < 10 mL/min with a sieving coefficient for albumin of 0, and high-flux membranes are defined as those having a β2MG clearance ≥ 20 mL/min and ≤ 40 mL/min clearance with a sieving coefficient for albumin of < 0.01 [15].
We therefore hypothesized that high albumin leakage in OHDF and SHF-HD improves survival compared with low albumin leakage, that high albumin leakage with hypoalbuminemia in OHDF and SHF-HD does not worsen survival, and that survival is more strongly affected by albumin leakage than by substitution volume in OHDF. The aim of this study was thus to elucidate the associations of s-Alb, albumin leakage, and substitution volume with all-cause mortality in OHDF and SHF-HD.
Methods
Patient selection
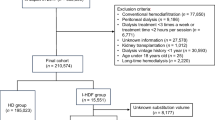
As shown in Fig. 1, of the 944 patients undergoing maintenance dialysis with SHF-HD or OHDF who were registered in the database from the medical records held by our corporation as of July 1, 2017, and were reported previously [16], 783 patients, comprising 355 receiving SHF-HD and 428 receiving OHDF (pre-OHDF, n = 333; post-OHDF, n = 95), were recruited to prepare a propensity score-matched (PSM) model. The exclusion criteria were age younger than 20 years, blood purification methods other than HD or OHDF, dialysis frequency of fewer than 3 sessions per week, dialysis time less than 3 h, substitution volumes for pre-OHDF < 60 L and post-OHDF < 8 L, missing values of covariates, and pregnancy or lactation. We also excluded patients whose dialysis conditions (dialysis method, dilution method, substitution volume, and membrane material) at the start of the study on July 1, 2017, differed from their dialysis conditions on April 1, 2017. Patients receiving HD or OHDF were defined as those who received the same dialysis method for 3 years (July 1, 2017, to July 1, 2020), with the dialysis method confirmed every year. Furthermore, when the two groups were compared, cases for which there was movement to another group across the median value were censored every year and the transfer was censored daily in the Kaplan–Meier survival curve. The period before the movement of another group was subjected to statistical analysis. The modality was chosen at the physician’s discretion. Blood test results were extracted from the medical records.
Flow diagram of participation in effects of high albumin leakage on survival between online hemodiafiltration and super high-flux hemodialysis: the HISTORY study. SHF-HD, super high-flux hemodialysis; OHDF, online hemodiafiltration; pre-OHDF, predilution online hemodiafiltration; post-OHDF, postdilution online hemodiafiltration; EAL, estimated albumin leakage; and s-Alb, serum albumin concentration
Preparation of propensity score-matched pairs
To analyze the effects of EAL at high and low doses on survival outcomes, propensity scores were matched in 69 and 147 pairs of patients receiving SHF-HD and OHDF, respectively. To compare the combined effects of EAL and s-Alb level on survival outcomes, propensity scores were matched in 79 and 81 pairs of patients receiving SHF-HD and OHDF for groups A (high EAL and high s-Alb) and B (high EAL and low s-Alb), 30 and 75 pairs of patients for groups A and C (low EAL and high s-Alb), and 19 and 39 pairs of patients for groups A and D (low EAL and low s-Alb), respectively. To directly compare the effects of the same ranges of the EAL in SHF-HD and OHDF patients on survival outcomes, propensity scores were matched in 75 pairs of patients with EAL < 2.5 g/session, 103 pairs of patients with 2.5 ≤ EAL < 5.0 g/session, and 20 pairs of patients with 5.0 ≤ EAL < 7.5 g/session.
Eleven items were used to calculate the propensity score for comparing patient survival outcomes in high vs. low EAL on SHF-HD and OHDF: age, dialysis vintage, presence or absence of diabetes mellitus, body mass index (BMI), normalized protein catabolism rate, s-Alb, corrected calcium, phosphorus, hemoglobin (Hb), high-sensitivity C-reactive protein (hs-CRP), and Kt/V. The other analyses were performed using 10 items, without s-Alb.
To calculate the propensity score for each patient, multivariable logistic regression analysis was performed using the treatment group as a dependent variable and the covariates as independent variables, followed by logit transformation. The propensity scores were calculated to a precision of 14 decimal places. Whether the number of cases was large or not large, patients in the two groups were paired by nearest available matching with a caliper (0.099884 for 11 items and 0.088768 for 10 items) of 0.2 × SD of the logit values of all patients in both groups [17].
Estimation of the amount of albumin leakage
The amount of albumin leakage was measured for each dialyzer or hemodiafilter by collecting whole dialysis waste liquid for 4 h; the average value was assigned according to the substitution volume. QB was 250 mL/min in HD and 280 mL/min in OHDF, and both the dialysate flow rate (QD) in HD and the total QD (QD plus the substitution volume) in OHDF were 500 mL/min. The substitution volumes were 60, 72, and 84 L for pre-OHDF and 8, 10, 12, and 16 L for post-OHDF. The dialyzers and hemodiafilters used in this study and the average EAL are listed in Additional file 1: Table S1 and Additional file 2: Table S2. The albumin level was measured by a turbidimetric immunoassay for dialysate and by a photometric method using bromocresol green for serum.
Survival analysis and statistics
Survival was analyzed using patients’ medical records, which include information on deaths, hospitalizations, discharges, transfers to other hospitals, and operations and interventions. A daily survival analysis was performed for the two groups with censored cases using the Kaplan–Meier method. Statistical significance between the groups was determined with the log-rank test. Cox regression analysis was used to calculate the hazard ratio (HR) and was performed using all-cause mortality as an independent variable and the EAL and substitution volume as dependent variables.
All analyses were performed using SPSS Statistics for Windows, version 25 (IBM Corporation, Armonk, NY), and P < 0.05 (two-tailed) was considered statistically significant.
Results
Comparison of SHF-HD and OHDF patient survival by high versus low EAL
A comparison of SHF-HD and OHDF variables between high and low EAL and before and after PSM is shown in Table 1. After PSM, BMI and Hb levels in SHF-HD patients and age and Hb levels in OHDF patients were significantly different between the high EAL and low EAL groups. The median EAL values were 1.4 g/session in SHF-HD patients and 5.0 g/session in OHDF patients. As shown in Fig. 2, 3-year all-cause mortality in SHF-HD and OHDF was significantly lower in the high EAL groups than in the low EAL groups (P = 0.012, log-rank test; HR, 0.44; 95% confidence interval [CI] 0.23–0.85; and P = 0.027, log-rank test; HR, 0.41; 95% CI 0.18–0.93, respectively).
Comparison of patient survival outcomes by estimated albumin leakage (EAL) and serum albumin concentration (s-Alb) in super high-flux hemodialysis patients. a High EAL and high s-Alb group vs. high EAL and low s-Alb group. b High EAL and high s-Alb group vs. low EAL and high s-Alb group. c High EAL and high s-Alb group versus low EAL and low s-Alb group. HR, hazard ratio; CI, confidence interval
Comparison of SHF-HD and OHDF patient survival by EAL and s-Alb
We compared the 3-year all-cause mortality in group A with that in groups B, C, and D. After PSM, the variables of group A in SHF-HD and OHDF patients were not significantly different from those of the other groups, except for Hb and hs-CRP between groups A and D in OHDF patients (Tables 2 and 3). The median s-Alb was the same (3.4 g/dL) for both SHF-HD and OHDF.
Table 4a shows the mean EAL and s-Alb in each group. Three-year all-cause mortality was not significantly different between groups A and B (SHF-HD: HR, 0.35; 95% CI 0.11–1.11; P = 0.062, log-rank test; OHDF: HR, 1.01; 95% CI 0.14–7.19; P = 0.990, log-rank test) (Figs. 3 and 4), despite a significant difference in mean s-Alb levels (SHF-HD: 3.5 ± 0.1 vs. 3.2 ± 0.2 g/dL [P < 0.001]; OHDF: 3.6 ± 0.2 vs. 3.2 ± 0.1 [P < 0.001]). In SHF-HD patients, 3-year all-cause mortality was only significantly lower in group A than in group D (EAL: 2.2 ± 1.0 vs. 1.1 ± 0.4 g/session [P < 0.001]; s-Alb: 3.5 ± 0.2 vs. 3.0 ± 0.3 g/dL [P < 0.001]) based on a log-rank test (P = 0.034), and the HR and 95% CI could not be calculated because there were no deaths in group A. Although the EAL and s-Alb levels in group A were significantly increased compared with those in group D (EAL: 7.1 ± 2.6 vs. 3.5 ± 0.7 g/session [P < 0.001]; s-Alb: 3.6 ± 0.2 vs. 3.2 ± 0.1 g/dL [P < 0.001]) in OHDF patients, there was no significant difference in all-cause mortality.
Comparison of patient survival outcomes by estimated albumin leakage (EAL) and serum albumin concentration (s-Alb) in online hemodiafiltration patients. a High EAL and high s-Alb group versus high EAL and low s-Alb group. b High EAL and high s-Alb group vs. low EAL and high s-Alb group. c High EAL and high s-Alb group versus low EAL and low s-Alb group. HR, hazard ratio; CI, confidence interval
Comparison of SHF-HD and OHDF patient survival by EAL range
The EAL in SHF-HD and OHDF patients was divided into three groups: < 2.5 g/session, ≥ 2.5 to < 5.0 g/session, and ≥ 5.0 to < 7.5 g/session. The variables in each SHF-HD and OHDF group before and after PSM are shown in Table 5. Although there were significant differences in the dialysis vintage and hs-CRP after PSM in the EAL < 2.5 g/session group, the other groups showed no significant differences in variables. There was a significant difference between the two groups in the mean EAL level at < 2.5 g/session and ≥ 2.5 to < 5.0 g/session. On the other hand, there was no significant difference in s-Alb between the two groups for the three ranges (Table 4 (b)). Although 3-year all-cause mortality was not significantly different between the ≥ 2.5 to < 5 g/session group (mean difference in EAL, 0.2 g/session) and ≥ 5 to < 7.5 g/session group (mean difference in EAL, 0.5 g/session), it was significantly lower in OHDF patients with EAL < 2.5 g/session group (mean difference in EAL, 1.0 g/session) than in SHF-HD patients based on the log-rank test (P < 0.001) and a HR of 0.31 (95% CI 0.16–0.61) (Fig. 5).
Comparison of patient survival outcomes by estimated albumin leakage (EAL) between super high-flux hemodialysis (SHF-HD) and online hemodiafiltration (OHDF) patients. a EAL < 2.5 g SHF-HD group versus EAL < 2.5 g OHDF group. b 2.5 ≤ EAL < 5.0 g SHF-HD group versus 2.5 ≤ EAL < 5.0 g OHDF group. c 5.0 ≤ EAL < 7.5 g SHF-HD group versus 5.0 ≤ EAL < 7.5 g OHDF group. HR, hazard ratio; CI, confidence interval
Direct comparison of pre-OHDF and post-OHDF patient survival by EAL and substitution volume
Because there were only 3 deaths among the 95 post-OHDF patients, this analysis was not performed in these patients. In pre-OHDF patients, 3-year all-cause mortality was significantly correlated with EAL (P = 0.007, beta − 0.32) rather than the substitution volume (P = 0.212, beta 0.02).
Discussion
This study is the first to suggest (i) that survival is improved more with high EAL than with low EAL in both OHDF and SHF-HD patients, (ii) that high EAL with mild-to-moderate hypoalbuminemia does not necessarily worsen survival in OHDF and SHF-HD, (iii) that survival is equivalent between OHDF and SHF-HD patients with a similar level of EAL, and (iv) that survival in pre-OHDF patients is influenced by albumin leakage rather than substitution volume. In our previous study using a similar database [16], QB—which was excluded from the PSM items assessed in the present study—was significantly higher for OHDF than for SHF-HD (279.5 ± 20.3 vs. 268.0 ± 19.8 mL/min, P < 0.001). Despite the fact that high QB improves survival, survival was found to be comparable between SHF-HD and OHDF patients under certain conditions.
There are multiple studies of European-style post-OHDF with different mortality outcomes [11,12,13, 18,19,20,21,22]. The CONVINCE and H4RT definitive trials are therefore being conducted to determine whether high-volume post-OHDF is preferable to high-flux HD [23, 24]. In Japan, pre-OHDF has commonly been selected for fear of filter clogging and excessive albumin leakage due to post-OHDF with a lower QB. In a previous study, we found that Japanese-style post-OHDF improves all-cause mortality to a level similar to that of pre-OHDF and that it improves both all-cause mortality and cardiovascular events [16], similar to European-style post-OHDF [18]. In addition, there was a limited effect of increased substitution volume in Japanese-style pre-OHDF on survival, considering that there was no significant difference in 3-year all-cause mortality between the high-volume (80.4 ± 5.5 L) and low-volume (58.7 ± 5.1 L) pre-OHDF groups [16]. It is therefore likely that there is a limit to the substitution volume in post-OHDF as well. In studies where no difference in mortality was observed, the mean levels of s-Alb were from 3.8 to 4.1 g/dL at baseline and during follow-up in all groups [11,12,13]. On the other hand, the time effect of s-Alb was significantly decreased from 4.1 g/dL at baseline to 3.9 g/dL during follow-up in the ESHOL study, which observed a significant difference in mortality [19]. Based on this, we consider that HD and post-OHDF in Europe does not leak enough albumin.
The mean s-Alb level in both HD and OHDF was 3.7 g/dL in the Japanese Society for Dialysis Therapy Renal Data Registry 2012 database [25]. One of the reasons why the annual crude mortality rate in Japan (9.7%) [26] is lower than that in other countries is suggested to be a higher albumin leakage with a lower s-Alb level. This possibility is supported by the fact that the crude mortality rate in 2020 at our institutions was 7.1% (Additional file 3: Figure S1), even though the mean s-Alb level was 3.3 g/dL in SHF-HD and 3.4 g/dL in OHDF. All-cause mortality was significantly correlated with the EAL rather than the substitution volume. It is therefore presumed that the reason why the survival improvement depended on the substitution volume in European-style post-OHDF was not only the increase in the substitution volume itself, but also the higher albumin leakage associated with the increase in the substitution volume.
The results of the present study suggest that high EAL contributes to survival and that high EAL with mild-to-moderate hypoalbuminemia does not necessarily worsen survival in SHF-HD and OHDF. Although all-cause mortality in SHF-HD was significantly lower with high EAL and high s-Alb compared with low EAL and low s-Alb, there were no significant differences in OHDF patients. The reason why only OHDF did not show a significant difference may be that a low EAL in OHDF patients (3.5 ± 0.7 g/session) was still high compared with that in SHF-HD patients (1.1 ± 0.4 g/session).
In a comparison of the survival of SHF-HD and OHDF patients in the same EAL ranges, there was a significant difference in all-cause mortality in the low range but not in the middle and high ranges, despite the significantly higher QB in OHDF compared with SHF-HD [16]. EAL was significantly different between SHF-HD and OHDF patients in the low and middle ranges but not in the high range. The mean differences between SHF-HD and OHDF patients were 1.0 g/session in the low range, 0.2 g/session in the middle range, and 0.5 g/session in the high range. The levels of hypoalbuminemia were not significantly different in all ranges. It is therefore suggested that survival in both groups of patients with mild-to-moderate hypoalbuminemia was equivalent when EAL was higher or the difference between the two groups in EAL was smaller.
Our results suggest that the aggressive albumin leakage in both OHDF and SHF-HD may be important because high albumin leakage with mild-to-moderate hypoalbuminemia can improve survival. Although the removal of uremic toxins up to large-middle molecules with low albumin leakage can improve survival, the removal of uremic toxins with large molecules would be more important for avoiding severe hypoalbuminemia. Because the removal of protein-bound and large-middle molecule uremic toxins by HD using SHF membrane dialyzers with high albumin leakage was similar to that by high-volume post-OHDF [27], it seems important to use SHF membrane dialyzers with high albumin leakage in HD patients. Also, because the average serum β2MG level is 27.0 mg/L in both HD and OHDF in Japan [28], it is suggested that there is no difference in the removal of small-middle molecules between the two methods. Because high-flux HD does not adequately remove medium-middle molecules, high-volume post-OHDF is considered necessary for removing larger molecules. However, we believe that SHF-HD with sufficient albumin leakage has a similar capacity for removing large-middle molecules compared with high-volume post-OHDF. Therefore, the results of present study suggest that the prognosis for survival is the same regardless of whether OHDF or SHF-HD is used, so long as the amount of albumin leakage is similar. In cases with malnutrition, high-volume pre-OHDF using a hemodiafilter with less albumin leakage may be a better option in order to reduce loss of amino acids. European-style high-volume post-OHDF with a low-permeability hemodiafilter reduces the increase in transmembrane pressure (TMP) and filtration fraction (FF), both of which are indicators for stabilizing the hemodiafilter environment by increasing the QB. However, if albumin leakage is the same, a lower-volume post-OHDF with a higher-permeability hemodiafilter is a more physiologic method because it reduces TMP and FF to a greater extent.
The main limitation of this study was the accuracy of the EAL and its fluctuation, especially for hemodiafilters. Because it is not possible to collect all of the whole dialysis waste liquid for 4 h from all patients at the same time, we evaluated the average albumin leakage before the start of the study in 6 patients. In addition, the average values of EAL were assigned to each patient based on the dialyzers, hemodiafilters, and substitution volume (Additional file 1: Table S1 and Additional file 2: Table S2). The EAL of the dialyzers varies little by lot number, but that of the hemodiafilters is affected not only by the lot number but also by fluctuations in TMP and FF, especially in higher-volume post-OHDF. It is thus necessary to determine an index for the removal rate of large-middle molecules or large molecules that can be measured simultaneously in all patients instead of the EAL. The Stokes radii of free α1-microglobulin (α1MG) and albumin are similar, at 28.6 Å [29] and 35.5 Å [30], respectively, despite having different molecular weights (33–66 kDa, respectively). Although α1MG forms complexes with IgA, prothrombin, and albumin [31], the Stokes radii of these complexes are not known. In our preliminary study, the relationship between the removal amount of α1MG and the reduction rate (RR) of α1MG corrected by the hematocrit level to exclude concentration effects with respect to the amount of albumin leakage was depicted by a logarithmic regression curve (Additional file 4: Figure S2a, b) after obtaining the equation for the logarithmic regression model using the logarithmic value of the amount of albumin leakage. Both equations showed significant regression coefficients, and their respective coefficients of determination R2 were 0.547 and 0.435, indicating a strong relationship between the removal amount of α1MG and RR of α1MG with respect to albumin leakage. Next, the relationship between the removal amount of α1MG and RR of α1MG was depicted by a regression line (Additional file 4: Figure S2c) after obtaining the equation for the linear regression model. The obtained regression coefficient was significant and the coefficient of determination R2 was 0.507, indicating a strong relationship between them. Thus, RR of α1MG can be measured simultaneously in all cases and may therefore be a potential replacement for the measurement of albumin leakage. It should be noted, however, that this strong correlation disappeared when albumin leakage exceeded 8.0 g/session or when the removal amount of α1MG exceeded 250 mg/session (Additional file 5: Figure S3 and Additional file 6: Figure S4). Regarding the dilution method, albumin leakage and the removal amount of α1MG were significantly correlated in both pre-OHDF and post-OHDF (Additional file 7: Figure S5) but were both significantly higher in post-OHDF than in pre-OHDF (8.7 ± 5.1 vs. 6.0 ± 3.5 g/session, P < 0.001 and 199.9 ± 65.7 vs. 173.0 ± 62.2 mg/session, P < 0.001). When albumin leakage was aligned at 3.0 to < 5.0 g/session, there were no significant differences in albumin leakage and the removal amount of α1MG between pre-OHDF and post-OHDF (3.9 ± 0.5 vs. 4.0 ± 0.5 g/session, P = 0.579, 141.7 ± 31.4 vs. 143.2 ± 37.9 mg/session, P = 0.897), meaning that albumin leakage and the removal amount of α1MG significantly correlated only in pre-OHDF (Additional file 8: Figure S6). The patient characteristics were different in 4 of the 11 groups, even after PSM, because the number of patients was small. Our corporation, which includes seven facilities, has created a unified basic healthcare management policy that covers dialysis conditions, dry weight, chronic kidney disease-related mineral and bone disorders, chronic kidney disease-related anemia, and vascular access, with the aim of eliminating any differences in the healthcare quality provided at our facilities. This policy is regularly reviewed by the Blood Purification Management Center. In principle, QB should be 250–300 mL/min, with a QD and total QD constant of 500 mL/min. There does not appear to be any significant differences in unobserved background factors such as quality of healthcare management and dialysis conditions. Therefore, even if there are significant differences in observed background factors, the results when using PSM with caliper values [17] are considered reliable. We do not have data on residual kidney function, although the dialysis vintage for patients receiving SHF-HD or OHDF was more than 3 months. A randomized controlled trial is needed to confirm our findings.
Conclusions
This study is the first to suggest that high albumin leakage improves survival equivalently between OHDF and SHF-HD, even under mild-to-moderate hypoalbuminemia, and that aggressive removal of solutes, including large molecules, avoids severe hypoalbuminemia, thereby improving survival in patients without malnutrition or inflammation.
Availability of data and materials
All data generated or analyzed during this study are included in this published article and its supplementary information files.
Abbreviations
- α1MG:
-
α1-Microglobulin
- β2MG:
-
β2-Microglobulin
- BMI:
-
Body mass index
- CI:
-
Confidence interval
- EAL:
-
Estimated albumin leakage
- FF:
-
Filtration fraction
- Hb:
-
Hemoglobin
- HD:
-
Hemodialysis
- HMA:
-
Human mercaptoalbumin
- HR:
-
Hazard ratio
- hs-CRP:
-
High-sensitivity C-reactive protein
- OHDF:
-
Online hemodiafiltration
- post-OHDF:
-
Postdilution online hemodiafiltration
- pre-OHDF:
-
Predilution online hemodiafiltration
- PSM:
-
Propensity score matching
- QB:
-
Blood flow rate
- QD:
-
Dialysate flow rate
- HR:
-
Hazard ratio
- s-Alb:
-
Serum albumin concentration
- SHF:
-
Super high-flux
- TMP:
-
Transmembrane pressure
References
Rosner MH, Reis T, Husain-Syed F, Vanholder R, Hutchison C, Stenvinkel P, Blankestijn PJ, Cozzolino M, Juillard L, Kashani K, Kaushik M, Kawanishi H, Massy Z, Sirich TL, Zuo L, Ronco C. Classification of uremic toxins and their role in kidney failure. CJASN. 2021;16:1918–28. https://doi.org/10.2215/CJN.02660221.
van Gelder MK, Abrahams AC, Joles JA, Kaysen GA, Gerritsen KGF. Albumin handling in different hemodialysis modalities. Nephrol Dial Transplant. 2018;33:906–13. https://doi.org/10.1093/ndt/gfx191.
Potier J, Queffeulou G, Bouet J. Are all dialyzers compatible with the convective volumes suggested for postdilution online hemodiafiltration? Int J Artif Organs. 2016;39:460–70. https://doi.org/10.5301/ijao.5000525.
Kawanishi H. Development of effects of online hemodiafiltration in Japan. Ren Replace Ther. 2021;7:51. https://doi.org/10.1186/s41100-021-00370-8.
Lowrie EG, Lew NL. Death risk in hemodialysis patients: the predictive value of commonly measured variables and an evaluation of death rate differences between facilities. Am J Kidney Dis. 1990;15:458–82. https://doi.org/10.1016/s0272-6386(12)70364-5.
Terawaki H, Takada Y, Era S, Funakoshi Y, Nakayama K, Nakayama M, Ogura M, Ito S, Hosoya T. The redox state of albumin and serious cardiovascular incidence in hemodialysis patients. Ther Apher Dial. 2010;14:465–71. https://doi.org/10.1111/j.1744-9987.2010.00841.x.
Nagai K, Tsuchida K, Ishihara N, Minagawa N, Ichien G, Yamada S, Hirose D, Michiwaki H, Kanayama H, Doi T, Minakuchi J. Implications of albumin leakage for survival in maintenance hemodialysis patients: a 7-year observational study. Ther Apher Dial. 2017;21:378–86. https://doi.org/10.1111/1744-9987.12526.
Nagai K, Tsuchida K, Hirose D, Michiwaki H, Hann M, Kanayama H, Doi T, Minakuchi J. The effect of albumin leakage in hemodialysis patients on redox status of serum albumin. J Artif Organs. 2016;19:310–4. https://doi.org/10.1007/s10047-016-0900-2.
Locatelli F, Martin-Malo A, Hannedouche T, Loureiro A, Papadimitriou M, Wizemann V, Jacobson SH, Czekalski S, Ronco C, Vanholder R. Membrane permeability outcome (MPO) study group: effect of membrane permeability on survival of hemodialysis patients. J Am Soc Nephrol. 2009;20:645–54. https://doi.org/10.1681/ASN.2008060590.
Alves FC, Sun J, Qureshi AR, Dai L, Snaedal S, Barany P, Heimburger O, Lindholm B, Stenvinkel P. The higher mortality associated with low serum albumin is dependent on systemic inflammation in end-stage kidney disease. PLoS One. 2018;13: e0190410. https://doi.org/10.1371/journal.pone.0190410.
Grooteman MPC, van den Dorpel MA, Bots ML, Penne L, van der Weerd NC, Mazairac AHA, den Hoedt CH, van der Tweel I, Lévesque R, Nubé MJ, ter Wee PM, Blankestijn PJ, CONTRAST Investigators. Investigators: effect of online hemodiafiltration on all-cause mortality and cardiovascular outcomes. J Am Soc Nephrol. 2012;23:1087–96. https://doi.org/10.1681/ASN.2011121140.
Ok E, Asci G, Toz H, Ok ES, Kircelli F, Yilmaz M, Hur E, Demirci MS, Demirci C, Duman S, Basci A, Adam SM, Isuk IO, Zengin M, Suleymanlar G, Yilmaz ME, Ozkahya M, Turkish Online Haemodiafiltration Study. Mortality and cardiovascular events in online haemodiafiltration (OL-HDF) compared with high-flux dialysis: results from the Turkish OL-HDF Study. Nephrol Dial Transplant. 2013;28:192–202. https://doi.org/10.1093/ndt/gfs407.
Morena M, Jaussent A, Chalabi L, Leray-Moragues H, Chenine L, Debure A, Thibaudin D, Azzouz L, Patrier L, Maurice F, Nicoud N, Durand C, Seigneuric B, Dupuy A, Picot M, Cristol J, Canaud B. Treatment tolerance and patient-reported outcomes favor online hemodiafiltration compared to high-flux hemodialysis in the elderly. Kidney Int. 2017;91:1495–509. https://doi.org/10.1016/j.kint.2017.01.013.
Abe M, Masakane I, Wada A, Nakai S, Nitta K, Nakamoto H. Super high-flux membrane dialyzers improve mortality in patients on hemodialysis: a 3-year nationwide cohort study. Clin Kidney J. 2021;15:473–83. https://doi.org/10.1093/ckj/sfab177.
Ward RA, Beck W, Bernard AA, Alves FC, Stenvinkel P, Lindholm B. Hypoalbuminemia: a price worth paying for improved dialytic removal of middle-molecular-weight uremic toxins? Nephrol Dial Transplant. 2019;34:901–7. https://doi.org/10.1093/ndt/gfy236.
Okada K, Michiwaki H, Tashiro M, Inoue T, Shima H, Minakuchi J, Kawashima S. Effects of Japanese-style online hemodiafiltration on survival and cardiovascular events. Ren Replace Ther. 2021;7:70. https://doi.org/10.1186/s41100-021-00385-1.
Austin PC. Optimal caliper widths for propensity-score matching when estimating differences in means and differences in proportions in observational studies. Pharm Stat. 2011;10:150–61. https://doi.org/10.1002/pst.433.
Peters SAE, Bots ML, Canaud B, Davenport A, Grooteman MPC, Kircelli FK, Locatelli F, Maduell F, Morena M, Nube MJ, Ok E, Torres F, Woodward M, Blankesrijn PJ, HDF Pooling Project Investigators. Haemodaiafiltration and mortality in end-stage kidney disease patients: a pooled individual participant data analysis from four randomized controlled trials. Nephrol Dial Transplant. 2016;31:978–84. https://doi.org/10.1093/ndt/gfv349.
Maduell F, Moreso F, Pons M, Ramos R, Mora-Macià J, Carreras J, Soler J, Torres F, Campistol JM, Martinez-Castelao A, ESHOL Study Group. High-efficiency postdilution online hemodiafiltration reduces all-cause mortality in hemodialysis patients. J Am Soc Nephrol. 2013;24:487–97. https://doi.org/10.1681/ASN.2012080875.
Mostovaya IM, Blankestijn PJ, Bots ML, Covic A, Davenport A, Grooteman MPC, Hegbrant J, Locatelli F, Vanholder R, Nubé MJ, EUDIAL1–an official ERA-EDTA Working Group. Clinical evidence on hemodiafiltration: a systematic review and a meta-analysis. Semin Dial. 2014;27:119–27. https://doi.org/10.1111/sdi.12200.
Davenport A, Peters SAE, Bots ML, Canaud B, Grooteman MPC, Asci G, Locatelli F, Maduell F, Morena M, Nubé MJ, Ok E, Torres F, Woodward M, Blankestijn PJ, HDF Pooling Project Investigators. Higher convection volume exchange with online hemodiafiltration is associated with survival advantage for dialysis patients: the effect of adjustment for body size. Kidney Int. 2016;89:193–9. https://doi.org/10.1038/ki.2015.264.
Locatelli F, Karaboyas A, Pisoni RL, Robinson BM, Fort J, Vanholder R, Rayner HC, Kleophas W, Jacobson SH, Combe C, Port FK, Tentori F. Mortality risk in patients on hemodiafiltration versus hemodialysis: a ‘real-world’ comparison from the DOPPS. Nephrol Dial Transplant. 2018;33:683–9. https://doi.org/10.1093/ndt/gfx277.
Blankestin PJ, Fischer K, Barth C, Cromm K, Canaud B, Davenport A, Grobbee DE, Hegbrant J, Roes KC, Rose M, Strippoli G, Vernooij RWM, Woodward M, de Wit GA, Bots M. Benefits and harms of high-dose haemodiafiltration versus high-flux haemodialysis: the comparison of high-dose haemodiafiltration with high-flux haemodialysis (CONVINCE) trial protocol. BMJ Open. 2020;10: e033228. https://doi.org/10.1136/bmjopen-2019-033228.
University of Bristol: the high-volume haemodiafiltration versus high-flux haemodialysis registry trial (H4RT). Available at: https://www.bristol.ac.uk/population-health-sciences/projects/h4rt-trial/patient-and-public-information/. Accessed 8 Jun 2022.
Kikuchi K, Hamano T, Wada A, Nakai S, Masakane I. Predilution online hemodiafiltration is associated with improved survival compared with hemodialysis. Kidney Int. 2019;95:929–38. https://doi.org/10.1016/j.kint.2018.10.036.
Masakane I, Taniguchi M, Nakai S, Tsuchida K, Wada A, Ogata S, Hasegawa T, Hamano T, Hanafusa N, Hoshino J, Goto S, Yamamoto K, Minakuchi J, Nakamoto H, Japanese Society for Dialysis Therapy Renal Data Registry Committee. Annual dialysis data report 2016, JSDT renal data registry. Ren Replace Ther. 2018;4:45. https://doi.org/10.1186/s41100-018-0183-6.
Thammathiwat T, Tiranathanagul K, Limjariyakul M, Chariyavilaskul P, Takkaratakarn K, Susantitaphong P, Meesangnin S, Wittayalertpanya S, Praditpornilpa K, Eiam-Ong S. Super high-flux hemodialysis provides comparable effectiveness with high-volume postdilution online hemodiafiltration in removing protein-bound and middle-molecule uremic toxins: a prospective cross-over randomized controlled trial. Ther Apher Dial. 2021;25:73–81. https://doi.org/10.1111/1744-9987.13508.
Nitta K, Masakane I, Hanafusa N, Taniguchi M, Hasegawa T, Nakai S, Goto S, Wada A, Hamano T, Hoshino J, Joki N, Abe M, Yamamoto K, Nakamoto H, Japanese Society for Dialysis Therapy Renal Data Registry Committee. Annual dialysis data report 2017, JSDT renal data registry. Ren Replace Ther. 2019;5:53. https://doi.org/10.1186/s41100-019-0248-1.
Wester L, Johansson MU, Akerström B. Physicochemical and biochemical characterization of human alpha 1-microglobulin expressed in baculovirus-infected insect cells. Protein Expr Purif. 1997;11:95–103. https://doi.org/10.1006/prep.1997.0760.
Erickson HP. Size and shape of protein molecules at the nanometer level determined by sedimentation, gel filtration, and electron microscopy. Biol Proced Online. 2009;11:32–51. https://doi.org/10.1007/s12575-009-9008-x.
Berggård T, Thelin N, Falkenberg C, Enghild JJ, Åkerström B. Prothrombin, albumin and immunoglobulin a form covalent complexes with α1-microglobulin in human plasma. Eur J Biochem. 1997;245:676–83. https://doi.org/10.1111/j.1432-1033.1997.00676.x.
Acknowledgements
We are grateful to all of the staff in our medical corporation for providing a similar quality of healthcare management and dialysis conditions. We are also grateful to Dr. Shigeaki Ohtsuki of Japan Institute of Statistical Technology for the statistical analysis.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
KO interpreted the results of statistical analysis and was the major contributor to drafting the manuscript, and MT, HM, TI, HS, JM, and SK performed data collection. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the Research Ethics Committee of Kawashima Hospital and registered in UMIN Clinical Trials Registry (UMIN000045112 registered on August 10, 2021). All clinical investigations were conducted according to the principles expressed in the Declaration of Helsinki. All patients gave informed consent for their data to be included in this study.
Consent for publication
Not applicable.
Competing interests
All of the authors report the funding of specific clinical research (Japan Registry of Clinical Trials with the registration number jRCTs062190020) from Asahi Kasei Medical Co., Ltd. and contract research from Nipro Co., Ltd.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file1
. Table S1. Dialyzers and mean estimated albumin leakage (EAL)
Additional file2
. Table S2. Hemodiafilters and mean estimated albumin leakage (EAL)
41100_2022_440_MOESM3_ESM.tif
Additional file3. Fig. S1 Comparison of annual crude mortality rate in the Japanese Society for Dialysis Therapy Renal Data Registry (JRDR) versus our institutions
Additional file4
. Fig. S2. Relationship among albumin leakage, removal amount of α1-microglobulin (α1MG), and reduction rate of α1MG. a Albumin leakage and removal amount of α1MG . b Albumin leakage and reduction rate of α1MG. c Removal amount of α1MG and reduction rate of α1MG
Additional file5
. Fig. S3. Relationships of albumin leakage over 8.0 g/session with removal amount of α1-microglobulin (α1MG) and reduction rate of α1MG. a Albumin leakage and removal amount of α1MG. b Albumin leakage and reduction rate of α1MG
Additional file6
. Fig. S4. Relationships of removal amount of α1-microglobulin (α1MG) over 250 mg/session with albumin leakage and reduction rate of α1MG. a Removal amount of α1MG and albumin leakage. b Removal amount of α1MG and reduction rate of α1MG
Additional file7
. Fig. S5. Relationship between albumin leakage and the removal amount of α1-microglobulin (α1MG). a Predilution online hemodiafiltration. b Postdilution online hemodiafiltration
Additional file8
. Fig. S6. Relationship between albumin leakage in the range of 3.0 to <5.0 g/session and the removal amount of α1-microglobulin (α1MG). a Predilution online hemodiafiltration. b Postdilution online hemodiafiltration
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Okada, K., Tashiro, M., Michiwaki, H. et al. Effects of high albumin leakage on survival between online hemodiafiltration and super high-flux hemodialysis: the HISTORY study. Ren Replace Ther 8, 52 (2022). https://doi.org/10.1186/s41100-022-00440-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41100-022-00440-5