Abstract
Hemodiafiltration (HDF) therapy has become standard treatment in Japan and Europe, but evidence from Europe is not directly applicable to HDF in Japan because HDF therapy differs greatly in the two regions. Japanese dialysis membranes vary widely, including use of protein-leaking and non-leaking membranes, and the molecular weight of solutes that can be removed is generally larger in Japan than in Europe. Given the characteristics of pre-dilution, the volume of replacement fluid itself cannot be used as a marker for solute removal, and the relationship of this volume to life prognosis is still unknown. Under these circumstances, the JAMREDS, a multicenter study led by the Japanese Society for Hemodiafiltration, was started in April 2020. The goal of the study is to determine whether α1-microglobulin reduction rate can be used as a marker for the prognosis of hemodialysis patients, including life prognosis and cardiovascular event onset. The JAMREDS is being performed from a new perspective of solute removal by HDF. This research design is reasonable and highly original for HDF in Japan, in view of the wide variety of membrane types and treatment modes, and the results of the study will be of particular interest.
Similar content being viewed by others
Introduction
The molecular weight range of solutes removed by blood purification therapies has changed over time, and a dialyzer with clearance of β2-microglobulin (β2-MG; molecular weight 11,800) exceeding 70 ml/min has now been developed. The principle of diffusion is now sufficient for managing β2-MG removal. After β2-MG, removal of substances with a molecular weight close to albumin (67,000) and large-middle molecular weight uremic toxins has been targeted. In Japan, removal of α1-microglobulin (α1-MG; molecular weight 33,000) has been achieved by hemodiafiltration (HDF) and it has become clear that α1-MG removal improves several clinical symptoms [1,2,3,4]. However, the effects of α1-MG reduction rate on survival and prognosis are still unknown. To examine the effects of this rate on prognosis of hemodialysis (HD) patients, three academic societies, the Japanese Society for Hemodiafiltration, the Japanese Society of High Performance Membranes for Blood Purification, and the Japanese Society of Intermittent Infusion Hemodiafiltration started the JAMREDS (https://center6.umin.ac.jp/cgi-open-bin/ctr/ctr_view.cgi?recptno=R000043823), as a multicenter prospective observational study, in April 2020.
HDF as standard treatment
An advantage of HDF is that it can efficiently remove middle molecular weight solutes that are difficult to remove using conventional HD. At a time when performance of membranes was not as good as it is today, HDF was developed mainly for removal of small-middle-sized molecules (i.e., β2-MG) by filtration.
To date, various clinical effects have been reported, such as 1) improvement of β2-MG removal, dialysis amyloidosis, pruritus, irritability, restless leg syndrome, bone/joint pain, loss of appetite, and other general malaise, 2) improvement of anemia, 3) improvement of inflammation, and 4) improvement of dialysis related hypotension [1,2,3,4,5,6,7,8,9,10]. Recent reports showing improvement of life prognosis have established the effectiveness of HDF in dialysis treatment [11,12,13,14,15,16,17].
HDF has always been widely recognized as a useful treatment modality in Japan, but its widespread use was initially limited because it was not covered by national health insurance. In 2010, online HDF equipment was approved and coverage of online HDF was included in national health insurance. However, the low coverage rate still limited use of HDF. In 2012, insurance reimbursement was set at a higher rate, leading to a rapid increase in clinical use and making HDF a standard treatment as a form of dialysis therapy. A national survey by the Japanese Society for Dialysis Therapy (https://docs.jsdt.or.jp/overview/index.html) showed that the percentage of HDF patients among all dialysis patients has increased rapidly, reaching 47.1% at the end of 2020.
α1-MG as a marker for solute removal in HDF
Once it was shown that β2-MG can be removed with conventional HD therapy, the next removal target for HDF in Japan was in the region of α1-MG, which is a large-middle molecular weight of 33,000, and the α1-MG reduction rate has become a target value for improving various clinical symptoms. A recent HDF treatment standard has been proposed with reduction rates of β2-MG of ≥ 80% and α1-MG of ≥ 35%, with 3–5 g of albumin leakage [2, 4]. Albumin leakage is unavoidable under HDF conditions that give high α1-MG removal, since the Stokes radius of α1-MG is about 80% that of albumin, but this is relatively well tolerated in Japan based on our experience of the clinical effects. For example, the symptoms of restless leg syndrome, which is a particularly clinically intractable complaint, can be improved with an α1-MG reduction rate of ≥ 40% and albumin leakage of ≥ 5 g [3].
α1-MG as a bioactive substance
α1-MG has been regarded as a marker for removal of uremic toxins in the α1-MG size range, as a so-called surrogate marker. However, recently, the physiological functions of α1-MG itself have been recognized and its role in disease has gained attention [18,19,20,21]. α1-MG is a low molecular weight glycoprotein that is mainly produced in the liver [22]. A free form of α1-MG and a form bound to IgA are present in the blood at approximately the same level in healthy subjects [23]. The physiological activity of α1-MG as a strong radical scavenger has become of particular interest in recent years [18,19,20,21]. The reduced form of α1-MG is relatively low in dialysis patients with high oxidative stress, while the degraded oxidized form of α1-MG is higher, indicating that the original function of α1-MG as a radical scavenger may be reduced, despite its higher blood concentration [4]. α1-MG has a much faster metabolic turnover than albumin, taking only a few hours, and a large reserve capacity for liver production that exceeds the amount removed [4]. Therefore, even if α1-MG is removed by HDF or other methods, its concentration will not decrease to normal after treatment [4]. Regardless, one of the reasons why the high α1-MG reduction rate contributes to the improvement of clinical symptoms may be to promote the turnover of α1-MG and increases the amount of the reduced form, which restores the original function of α1-MG as a radical scavenger and brings about various clinical benefits.
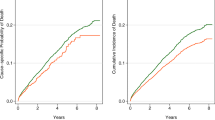
Research on life prognosis of HDF and the JAMREDS (Fig. 1)
In the first part of the 2010s, several overseas randomized controlled trials (RCTs) [13,14,15,16] demonstrated positive effects of post-dilution online HDF on life prognosis and suggested a relationship with convection volume, but the mechanism has yet to be determined. From the perspective of solute removal, HDF in European countries uses non-protein leaking membranes, and thus, the convection volume is thought to reflect removal of solutes with a molecular weight of < 20 kDa, which is a smaller target than that used in Japan. It can be inferred that removal of uremic toxins of this size improves life prognosis. In 2019, a Japanese observational study of the effects of pre-diluted online HDF on life prognosis using propensity score matching (a quasi-RCT) suggested the significance of a high replacement fluid volume on life prognosis [17]. However, protein-leakage and non-leakage membranes are used in HDF in Japan, and the removable molecular weight is likely to be larger. Given the characteristics of pre-dilution, the volume of replacement fluid is not likely to be a direct marker of solute removal, and a clear answer on how this volume is related to life prognosis has not been obtained.
The results of the recent 2018 Euro-DOPPS 4–5 real-world data study did not show superiority of HDF [24], and the weakness of the study design in previous RCTs has been pointed out [25, 26]. In these circumstances, in the 2020s, new RCTs such as CONVINCE [26] and H4RT [25] are being performed for further verification in Europe. In Japan, the JAMREDS (https://center6.umin.ac.jp/cgi-open-bin/ctr/ctr_view.cgi?recptno=R000043823), a multicenter study led by the Japanese Society for Hemodiafiltration, was started in April 2020, with the goal of determining whether α1-MG reduction rate is a marker of prognosis, including life prognosis and cardiovascular events. The abbreviation “JAMREDS” was coined by Dr. Kenji Sakurai from the “Japanese study of the effects of AMG (α1-microglobulin) reduction rates on survival, complications and prognosis in dialysis patients.” The observation period of the JAMREDS will continue until the end of 2023, after which the data will be cleaned and confirmed before moving to analysis.
Conclusion
HDF is a standard treatment in Europe and Japan, but differs between these regions, and evidence from Europe cannot be used directly for HDF in Japan. The JAMREDS is a clinical study designed from the new perspective of examining solute removal by HDF. This approach is reasonable and innovative for HDF in Japan, due to the use of a wide variety of membrane types and treatment modes, and the results of this study are highly anticipated.
Availability of data and materials
The data and materials were all included in the manuscript.
Abbreviations
- HDF:
-
Hemodiafiltration
- β2-MG:
-
β2-Microglobulin
- α1-MG:
-
α1-Microglobulin
- RCTs:
-
Randomized controlled trials
References
Sakurai K. Biomarkers for evaluation of clinical outcomes of hemodiafiltration. Blood Purif. 2013;35(Suppl 1):64–8.
Yamashita AC, Sakurai K. Clinical effect of pre-dilution hemodiafiltration based on the permeation of the hemodiafilter. Contrib Nephrol. 2015;185:1–7.
Sakurai K, Saito T, Hosoya H, Kurihara Y, Yamauchi F. Therapeutic effect of high-efficiency online hemodiafiltration for recurrent restless legs syndrome in dialysis patients. J Artif Organs. 2020;23(3):296–301.
Sakurai K, Hosoya H, Kurihara Y, Saito T. Suitability of α1-microglobulin reduction rate as a biomarker of removal efficiency of online hemodiafiltration: a retrospective cohort study. Renal Replacement Therapy. 2021;7(1).
Locatelli F, Marcelli D, Conte F, Limido A, Malberti F, Spotti D. Comparison of mortality in ESRD patients on convective and diffusive extracorporeal treatments. The Registro Lombardo Dialisi E Trapianto. Kidney Int. 1999;55(1):286–93.
Nakai S, Iseki K, Tabei K, Kubo K, Masakane I, Fushimi K, et al. Outcomes of hemodiafiltration based on Japanese dialysis patient registry. Am J Kidney Dis. 2001;38(4 Suppl 1):S212–6.
McKane W, Chandna SM, Tattersall JE, Greenwood RN, Farrington K. Identical decline of residual renal function in high-flux biocompatible hemodialysis and CAPD. Kidney Int. 2002;61(1):256–65.
Locatelli F, Altieri P, Andrulli S, Bolasco P, Sau G, Pedrini LA, et al. Hemofiltration and hemodiafiltration reduce intradialytic hypotension in ESRD. J Am Soc Nephrol. 2010;21(10):1798–807.
Locatelli F, Altieri P, Andrulli S, Sau G, Bolasco P, Pedrini LA, et al. Predictors of haemoglobin levels and resistance to erythropoiesis-stimulating agents in patients treated with low-flux haemodialysis, haemofiltration and haemodiafiltration: results of a multicentre randomized and controlled trial. Nephrol Dial Transplant. 2012;27(9):3594–600.
Susantitaphong P, Siribamrungwong M, Jaber BL. Convective therapies versus low-flux hemodialysis for chronic kidney failure: a meta-analysis of randomized controlled trials. Nephrol Dial Transplant. 2013;28(11):2859–74.
Canaud B, Morena M, Leray-Moragues H, Chalabi L, Cristol JP. Overview of clinical studies in hemodiafiltration: What do we need now? Hemodial Int. 2006;10(Suppl 1):S5–12.
Panichi V, Rizza GM, Paoletti S, Bigazzi R, Aloisi M, Barsotti G, et al. Chronic inflammation and mortality in haemodialysis: effect of different renal replacement therapies. Results from the RISCAVID study. Nephrol Dial Transplant. 2008;23(7):2337–43.
Grooteman MP, van den Dorpel MA, Bots ML, Penne EL, van der Weerd NC, Mazairac AH, et al. Effect of online hemodiafiltration on all-cause mortality and cardiovascular outcomes. J Am Soc Nephrol. 2012;23(6):1087–96.
Maduell F, Moreso F, Pons M, Ramos R, Mora-Macia J, Carreras J, et al. High-efficiency postdilution online hemodiafiltration reduces all-cause mortality in hemodialysis patients. J Am Soc Nephrol. 2013;24(3):487–97.
Ok E, Asci G, Toz H, Ok ES, Kircelli F, Yilmaz M, et al. Mortality and cardiovascular events in online haemodiafiltration (OL-HDF) compared with high-flux dialysis: results from the Turkish OL-HDF Study. Nephrol Dial Transplant. 2013;28(1):192–202.
Morena M, Jaussent A, Chalabi L, Leray-Moragues H, Chenine L, Debure A, et al. Treatment tolerance and patient-reported outcomes favor online hemodiafiltration compared to high-flux hemodialysis in the elderly. Kidney Int. 2017;91(6):1495–509.
Kikuchi K, Hamano T, Wada A, Nakai S, Masakane I. Predilution online hemodiafiltration is associated with improved survival compared with hemodialysis. Kidney Int. 2019;95(4):929–38.
Olsson MG, Olofsson T, Tapper H, Akerstrom B. The lipocalin alpha1-microglobulin protects erythroid K562 cells against oxidative damage induced by heme and reactive oxygen species. Free Radic Res. 2008;42(8):725–36.
Olsson MG, Rosenlof LW, Kotarsky H, Olofsson T, Leanderson T, Morgelin M, et al. The radical-binding lipocalin A1M binds to a Complex I subunit and protects mitochondrial structure and function. Antioxid Redox Signal. 2013;18(16):2017–28.
Wester-Rosenlof L, Casslen V, Axelsson J, Edstrom-Hagerwall A, Gram M, Holmqvist M, et al. A1M/alpha1-microglobulin protects from heme-induced placental and renal damage in a pregnant sheep model of preeclampsia. PLoS ONE. 2014;9(1): e86353.
Kristiansson A, Ahlstedt J, Holmqvist B, Brinte A, Tran TA, Forssell-Aronsson E, et al. Protection of kidney function with human antioxidation protein alpha1-microglobulin in a mouse (177)Lu-DOTATATE radiation therapy model. Antioxid Redox Signal. 2019;30(14):1746–59.
Akerstrom B, Gram M. A1M, an extravascular tissue cleaning and housekeeping protein. Free Radic Biol Med. 2014;74:274–82.
Berggard T, Thelin N, Falkenberg C, Enghild JJ, Akerstrom B. Prothrombin, albumin and immunoglobulin A form covalent complexes with alpha1-microglobulin in human plasma. Eur J Biochem. 1997;245(3):676–83.
Locatelli F, Karaboyas A, Pisoni RL, Robinson BM, Fort J, Vanholder R, et al. Mortality risk in patients on hemodiafiltration versus hemodialysis: a “real-world” comparison from the DOPPS. Nephrol Dial Transplant. 2018;33(4):683–9.
Caskey FJ, Procter S, MacNeill SJ, Wade J, Taylor J, Rooshenas L, et al. The high-volume haemodiafiltration vs high-flux haemodialysis registry trial (H4RT): a multi-centre, unblinded, randomised, parallel-group, superiority study to compare the effectiveness and cost-effectiveness of high-volume haemodiafiltration and high-flux haemodialysis in people with kidney failure on maintenance dialysis using linkage to routine healthcare databases for outcomes. Trials. 2022;23(1):532.
Vernooij RWM, Bots ML, Strippoli GFM, Canaud B, Cromm K, Woodward M, et al. CONVINCE in the context of existing evidence on haemodiafiltration. Nephrol Dial Transplant. 2022;37(6):1006–13.
Acknowledgements
Not applicable.
Funding
The authors declare that there is no funding related to this manuscript.
Author information
Authors and Affiliations
Contributions
T.N. (corresponding author) contributed to the concept and writing. Y.T., N.K. and H.K. reviewed and revised the manuscript. All authors read and approved the final manuscript.
Ethics declarations
Ethics approval and consent to participate
Not applicable since this paper is a review article.
Consent for publication
Not applicable since this paper is a review article.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Naganuma, T., Takemoto, Y., Kamada, N. et al. Hemodiafiltration in Japan: current status and future directions. Ren Replace Ther 9, 23 (2023). https://doi.org/10.1186/s41100-023-00471-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41100-023-00471-6