Abstract
Background
Globally, there is increased clinical interest and uptake of hemodiafiltration (HDF) for increased removal of uremic toxins. To date, there has been no epidemiological analysis of HDF in China. We present HDF practice patterns and associated mortality risk in Shanghai.
Methods
This is an observational, prospectively collected, retrospective analysis of 9351 Chinese patients initiating hemodialysis in Shanghai from 2007 to 2014. The primary exposure was hemodialysis sub-modality at inception, classified into hemodiafiltration (HDF) and hemodialysis (HD), with adjustment for concommitant hemoperfusion. The primary outcome was patient mortality. We used Cox proportional hazards regression and Fine and Gray’s proportional subhazards regression, with multiple imputation of missing co-variates by the chained equation method, adjusting for demographic and clinical variables.
Results
Overall, patients in the cohort were younger, with a more males, and with a lower body mass index when compared to corresponding non-Asian cohorts. Mortality rate was low although it doubled over the period of observation. HDF utilization increased from 7% of patients in 2007 to 42% of patients in 2014. The majority of patients received HDF once a week. The adjusted hazard ratio of death (95% confidence intervals) for HDF versus HD was 0.85 (0.71–1.03), and corresponding sub-hazard ratio 0.86 (0.71–1.03). There was strong effect modification by age. In those aged 40–60 years, the hazard ratio (95% confidence intervals) was 0.65 (0.45–0.94), and sub-hazard ratio also 0.65 (0.45–0.95).
Conclusions
Our study has certain limitations resulting from the limited number of co-variates available for modelling, missing data for some co-variates, and the lack of verification of data against source documentation. Notwithstanding, there is evidence of clinical benefit from HDF in China, and potential to improve patient outcomes through the greater removal of middle and larger uremic solutes.
Similar content being viewed by others
Background
China has one of the largest – if not the largest - chronic kidney disease (CKD) populations on the globe [1]. According to the latest report from the Chinese Renal Data System [2], there were 447,435 prevalent patients on hemodialysis (HD) and 74,138 on peritoneal dialysis (PD) at the end of 2016 (https://www.cnrds.net). There has been rapid growth in dialysis for several reasons. Firstly, there is an increasing prevalence of risk factors for progressive CKD, most notably diabetes mellitus and increased body size [3,4,5]. Secondly, coverage for dialysis via social insurance has expanded markedly over the last 5 years, and dialysis is increasingly offered to patients. Thirdly, there is increasing health literacy amongst healthcare consumers in developed areas of China, who have grown accustomed to advanced standards in healthcare, especially in larger cities. Finally, the largest rural to urban migration in human history has led to generally better access to health services and higher incomes for most people [6].
Globally, there is increased clinical interest and uptake of therapies that have increased removal of uremic toxins relative to high flux HD. The currently available methods for doing so include more intensive HD [7, 8], leakier dialyzer membranes [9, 10], or greater convective clearance [11,12,13,14,15,16,17,18,19]. At the present time, greater convective clearance using hemodiafiltration (HDF) is the most commonly applied means for extending uremic solute clearance in routine clinical practice. To date, there has been no epidemiological analysis of HDF in China. In this article, we describe and analyses the evolving practice patterns and outcomes of HDF in China using the Shanghai Renal Registry (SRR), which was begun in 1996 by the Shanghai Society of Nephrology and Shanghai Center for Hemodialysis Quality Control (http://sh.cnrds.org). The registry prospectively collects data on all patients treated with maintenance renal replacement therapy in that city, and at the time of this study covered all 66 dialysis providers. We report on HDF experience in a large cohort of Chinese patients initiating HD in Shanghai from 2007 to 2014.
Methods
Study design
We performed an retrospective observational cohort study using an intention-to-treat framework [20]. None of the authors of the manuscript had access to any information that could be used to identify individual participants or their treating center during or after data collection. The Ethics Committee of the Shanghai Clinical Research Center (http://www.scrcnet.org/IEC_en.asp) reviewed and approved the study design and execution, and waived the need for individual consent to participate on the basis of the research being a non-interventional study with unidentified data.
Participants and data source
The SRR is a government-mandated registry that collects information on all treated end-stage kidney disease (ESKD) patients from all dialysis centers in Shanghai, China. The SRR began as the Shanghai Dialysis Registry in 1996 [21], and includes those treated with all forms of renal replacement therapy (RRT) including transplantation, followed from renal replacement inception until death or loss to follow-up. The SRR excludes those with acute kidney injury, and defines treated ESKD patients as those for whom dialysis is intended to be indefinite, and offers general guidance that patients with a treatment period of less than 90 days should not be included. Minimum data collected for the registry includes the centre, patient demographics, exact dates for dialysis inception, each change of renal replacement modality, and loss to follow-up or death. Discretionary data include details of patient co-morbidity, biochemical and laboratory tests, details of renal replacement regimens, and medications. Data are updated quarterly via an online user interface, and facility specific reports provided on an annual basis. Structure and methods of the registry have been reported elsewhere [22, 23].
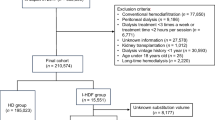
From the larger dataset, we created a cohort of incident adult patients (aged > = 18 years) who initiated RRT as either inpatients or outpatients between January 1, 2007 and December 31, 2014 in Shanghai, China. This inception cohort was restricted to only those initiating RRT with HD, HDF, or hemoperfusion (HP). Patients were followed up until death, dropout, return of renal function, transfer out of the SRR network, permanent switch to PD (defined as more than 30 days of continuous treatment), kidney transplantation or December 31, 2014, whichever occurred first.
Exposure variables
The primary exposure was treatment with HDF. We adjusted for patient-related factors using the first recorded observations for each patient. These data are intended to reflect status at RRT inception or after a short period of stabilization, although the SRR network does not specify collection period or procedures. The following data were included: age, gender, primary kidney disease as recorded in the SRR (primary nephropathies [glomerulonephritis], secondary nephropathies [diabetes, hypertension, systemic diseases with renal manifestations], and other causes [urinary tract infection (UTI) / stones / urological / malignancy]), pre-dialysis weight, body mass index (BMI), serum albumin, serum (unadjusted) calcium, serum phosphate, total cholesterol, estimated glomerular filtration rate (eGFR) by the abbreviated Modification of Diet in Renal Disease (MDRD) formula for Chinese [24], creatinine index from Canaud et al. [25], and year of dialysis inception.
We also adjusted for treatment-related factors also using the first recorded observations for each patient. These included: interdialytic weight gain (as a % of pre-dialysis weight), RRT frequency (per week), dialysis dose (single pool Kt/V per treatment), vascular access (arteriovenous fistula/graft, central venous catheter [CVC] /other), and the application of HP (e.g such as using a neutral macroporous resin apparatus [26]).
Continuous or ordinal co-variates other than age were modeled as clinically relevant quantiles in order to avoid the assumption of linear relationships.
Outcome variable
The primary outcome was death on HD. The recorded outcome of “withdrawal from dialysis” was modelled as death. We assessed switch to PD, kidney transplantation, transfer out of the SRR network and loss to follow-up for unrecorded reasons as competing risks, since patients reaching these endpoints were no longer at risk of dying while being on HD.
Statistical methods
For the primary analysis, we firstly constructed models for survival using Cox proportional hazards regression, censoring for all competing risks such as switch to PD, kidney transplantation, transfer out of the SRR network and loss to follow-up for unrecorded reasons. In such statistical models, probabilities of death in censored patients are still modelled - their deaths are calculated as happening at a time after the competing event, with the same probability (conditional on covariates) as those who had remained on HD and already died.
To more carefully account for competing risks, we also constructed models using Fine and Gray’s proportional subhazards model, where switch to PD and kidney transplantation were not censored but modelled as competing risks [27]. In this model and the Cox proportional hazards model, we censored patients for transfer out of the SRR network and loss to follow-up for unrecorded reasons, under the assumption of independent and non-informative censoring.
The SRR is intended to record only those patients with end-stage kidney disease, and not those with acute kidney injury. We could not be sure about adherence to this guideline by contributing HD units, however, and we therefore performed supplementary analyses in a restricted dataset excluding those with less than 90 days on dialysis.
We removed covariates from the model using a backward stepwise process starting with the covariate with the highest P value from two-tailed Wald tests of the individual coefficients, using the partial likelihood ratio test to compare the new model with the older one. We selected confounders for the final model according to both biological plausibility and comprehensibility, aiming for the most parsimonious model based upon the significance of the covariate within the model as assessed by the two-tailed partial likelihood ratio test P value at a level of 0.2 when jointly adjusted for other covariates.
We examine effect modification by era, serum albumin, and patient age using two-way interaction terms in the main-effects models. These interactions were chosen as being clinically plausible, as indicated by both published literature as well as the cumulative clinical experience of the research team. The significance of interaction terms were assessed by the two-tailed partial likelihood ratio test P value at a level of < 0.05 when jointly adjusted for other covariates.
We tested the proportional hazards assumption quantitatively using scaled Schoenfeld residuals, and qualitatively by using -ln [−ln(survival)] versus ln(analysis time) plots and comparing the goodness of fit between plots of Kaplan-Meier observed survival curves to the corresponding curves predicted by Cox models.
The data used for this study had important degrees of missingness. We examined missingness of other covariates using logistic regression. Missingness was found to depend on observed data on regression analysis, implying a “missing at random” (MAR) rather than “missing completely at random” (MCAR) data structure. We therefore imputed missing values to avoid distorted inference from complete case analysis in a non-representative sample of the study population. For imputation, we used the chained equation method based on iterative multiple regression models with all other variables included (with categorical covariates expanded as indicator variables) [28, 29]. We included all patient-related covariates, treatment-related covariates, and the dependent variable (primary and competing outcomes, loss to follow-up/transfer) as co-variates in imputation models [29, 30], and constrained imputation of missing variables to the observable data range using truncated regression. We ran imputation models separately for each variable before full imputation, to test for convergence and misspecification, and to assess for interactions [29].
For all imputation, we included potential interactions between the imputed variables as covariate terms, thus avoiding imputed values reflecting only linear relationships. We imputed within different subsamples for era of inception and renal replacement treatment frequency, to preserve higher-order dependencies for these variables. We imputed 20 data sets to reduce sampling variability from the imputation process [31, 32], and combined results of analyses using Rubin’s rules [33]. This number of imputations was guided by inspection of Monte Carlo errors, and the achievement of what has been suggested to be an acceptable amount of error 1) the error for a coefficient is less than or equal to 10% the coefficient’s standard error 2) the error of a coefficient’s T-statistic is less than or equal to 0.1, and 3) the error of a coefficient’s P-value is less than or equal to 0.01 if the true P-value is 0.05, or less than or equal to 0.02 if the true P-value is 0.1 [29].
Where necessary, we made comparisons between non-imputed groups using the Fisher’s exact and Wilcoxon rank sum test, and comparisons involving imputed groups using linear or quantile regression [34]. We computed effect size statistics for non-imputed groups only using Cohen’s d for continuous variables and Cohen’s ω for categorical ones [35]. Statistical significance was attributed to associations if the two-tailed P value was < 0.05.
Analyses were performed using Stata Intercooled MP/14.2 (StataCorp, www.stata.com).
Results
Participants and outcome data
The inception cohort contained 14,941 patients with complete primary exposure and outcomes data. Of these, there were 5590 patients without complete data for outcomes, age, gender, primary kidney disease, and renal replacement modality / dialysis characteristics. These patients were excluded from further analysis, leaving 9351 with complete data for the above variables. The excluded and included datasets are shown in Additional file 1: Table S1 (available as online supplementary material), along with their respective degrees of missingness. There were some minor differences between the excluded and included datasets, although all differences were small or very small in terms of effect size.
Of the 9351 patients in the included dataset, 4201 had no missing covariate data at all. The other 5150 required imputation for some degree of missingness for covariates other than the ones mentioned above (complete case and imputed variables shown in Additional file 2, available as online supplementary material). The final dataset for analysis consisted of 9351 patients over 29,250 patient-years, in which 982 patients died, 259 underwent kidney transplants, 240 changed to PD, and 7870 patients were censored (5484 because of end of follow-up, 64 for return of renal function, 2294 for transfer out to the SRR network, and 28 for loss to follow-up).
The annual mortality rate (95% confidence interval) was 3.4 per 100 pt./years (3.2–3.6) overall, although this increased rapidly over the period of observation. Figure 1 shows the probability (from Kaplan Meier estimates) and cumulative incidence (from competing risks regression) of death over the follow up.
Descriptive data
Table 1 summarizes the clinical characteristics of the final dataset, and also compares these characteristics by HDF versus HD. The following key observations can be made.
Overall, the patients comprise a relatively young dialysis population, with a preponderance of males. Body size (weight, height and mass index) tended to be low compared to North American, European, or Australasian cohorts. Of note there are a relatively large number of patients on twice a week HD, a common feature of HD in China generally.
Patient characteristics differed significantly in those receiving HDF compared to those receiving no HDF. Those treated with HDF tended to have greater body size, were more likely to be males, more likely to be have a higher serum albumin, more likely to be on three-times a week therapy, and more likely to have an AV access.
The practice patterns around HDF also warrant comment. Overall 51 patients (0.5%) were treated with HDF once a month, 578 (6%) once every two weeks, 1585 (16%) once a week, and 34 (0.3%) twice or three times a week. Figure 2 illustrates the increase in HDF utilization by year, from 7% of patients in 2007 to 52% of patients in 2014. Overall, however, the application of HDF once a week remains the most common practice pattern over this period of observation. The distribution of the frequency of HDF varies by gender, age, and body size, indicating that HDF is applied slightly more frequently in younger, larger, males (Fig. 3).
Main results
The two primary main-effects models are summarized in Fig. 4, and shown in full in Additional file 3: Figures S1 and Additional file 4: Figure S2 (available as online supplementary material). There was reasonable convergent validity between two co-primary models, with only minor differences.
In the full models, increased patient mortality risk was associated with increasing age, male gender, hypoalbuminemia, anemia, CVC access, and era of dialysis inception, starting dialysis in the later part of the period of observation. Decreased mortality risk was associated with primary nephropathy as a cause of end stage kidney failure, and a trend to decreased mortality risk associated with lager body size, higher creatinine index, lower interdialytic weight gain, and less than thrice weekly renal replacement therapy. The covariates of Kt/V (p = 0.926, p = 0.651 in categories compared to reference category), blood purification using the neutral macroporous resin apparatus (p = 0.305 compared to no hemoperfusion), serum calcium (p = 0.993, p = 0.630 in categories compared to reference category), serum phosphate (p = 0.242, p = 0.630 in categories compared to reference category), and total cholesterol (P = 0.494 compared to reference category) were not associated with changes in mortality risk and were dropped from the model.
In terms of exposure of interest, HDF was associated with a trend to reduced mortality multivariable analyses, which fell short of being statistically significant at the 5% level. This trend was adjusted for other confounding, and independent of other risk factors for mortality included in Table 1 and also independent of era.
Other results
There was no statistically significant interaction (P < 0.05) in the two primary models related to era, serum albumin, or frequency of renal replacement therapy, but a strong interaction by age (P = 0.0014). The interaction models are also summarized in Fig. 4. The effect modification by age is such that there is a trend towards improved mortality associated with HDF to improved mortality in those < 40 years of age, a statistically significant associated improvement in mortality risk in those 40–60 years, and no associated change in mortality risk in those greater than 60 years.
Supplementary analysis excluding those without 90 days of follow-up yielded similar estimates to the primary analyses - the adjusted hazard ratio (95% confidence interval) for mortality was 0.84 (0.68–1.03) using Cox proportional hazards regression, and the corresponding adjusted sub-hazard ratio (95% confidence interval) using competing risks regression was 0.84 (0.68–1.04).
Discussion
There are four key findings in this study. The first is that HDF in China is most often applied once a week. There are two likely reasons for this practice pattern. The first is resource limitation – in the current China market, HDF machines are more expensive than HD machines, and HDF is not available in all facilities or to all patients within a given facility. The second concerns restrictions in reimbursement from social insurance, which is not universal in every part of China, with limited ability of many patients to self-pay for HDF.
The second key finding is that, even at a reduced frequency, HDF is independently associated with improved mortality risk in younger patients, and with a trend to improved outcomes in the population overall. This is directionally consistent with definitive evidence [11,12,13,14,15,16,17,18,19], and has not been reported before in the Chinese setting. Existing reports on HDF in China are limited to surrogate outcomes, most commonly clearance [36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58]. What reports there are, however, suggest that HDF in China is often applied with less than an optimal convective dose: blood flow rates range between 200 and 350 mL/min, dialysate flow between 500 and 800 mL/min, and convective dose in post-dilution mode of 15–25 L per treatment. In settings other than China, this has led to a paradoxical finding that HDF does not always lead to observable benefit in a “real world” setting [59]. In China, based on this study, this may not be the case.
The third finding is that mortality on HD in Shanghai is low. The annual mortality rate in our study for those initiating dialysis since 2010 was 5.4 per 100 pt./years, compared with 8.2 most recently in Europe [60], 9.8 in Japan [61], and 16.9 in the United States [62]. Data at hand do not allow detailed comparison with these other cohorts, although the mortality rate in our patients appears similar to those from the ongoing ChinaQ trial in China (NCT02378350, personal communication Xueqing Yu, Principal Investigator, November 2018), as well as another recent Chinese cohort study in which the 2010 annual mortality rate was reported as 7.7 per 100 pt./years [63, 64].
This low mortality rate is important in terms of external validity. In every population, surviving CKD to the point of dialysis selects hardy people [65], and this situation is exaggerated in our study. A decade or more ago, lack of reimbursement for dialysis in China resulted in markedly positive selection bias in dialysis patients. Anecdotally, these were affluent people who could afford dialysis, with a predicted longevity on dialysis that would justify dialysis in a resource-challenged healthcare setting. Over time, the increased access to care in China has led to a greater number of patients on HD; for example, in Shanghai the point prevalence of HD rose from 176 pmp in 2005 [66] to 380 pmp in 2014 [23]. This broadening of acceptance onto dialysis in Shanghai as well as elsewhere in China has led to patients with progressively less favourable prognoses, and is reflected by the decreasing survival on HD in Shanghai both in our study (Fig. S1), as well as in other Chinese cohorts [63, 64]. The relatively healthy nature of our cohort should be considered carefully, and the extent to which their response to therapy is more broadly applicable.
The final key finding in our study concerns the satisfactory outcomes with carefully applied utilization of < 3 x week RRT, which is consistent with other recent studies [67,68,69,70]. In China, resource constraints lead most nephrologists to delay initiation of RRT until there are clear clinical indications and/or symptoms of uraemia, and use < 3 x week RRT as much as possible. In our study, both higher eGFR and thrice weekly regimens at dialysis inception were both associated with increased mortality risk. This reflects customary practice in Shanghai - earlier and more intensive dialysis is reserved for sicker patients in an effort to preserve or improve health status, while the many healthier patients start only after the onset of clinical uraemia, in a carefully-monitored and incremental fashion.
Our study is an important one as it provides census-based and longitudinal approach. We can contrast the difference between our results and those of the recent Dialysis Outcome and Practice Patterns Study (DOPPS) data published as a cross-sectional study of prevalent patients across China [68]. Important differences include: Gender - 46% female in the DOPPS, 39% in this study; hemoglobin - 106 g/l in the DOPPS, 95.9 g/l in this study; frequency of RRT <3x/week - 26% in DOPPS, 21% in this study; AVF/AVG prevalence - 89.8% in the DOPPS, 46% in this study. These differences reflect differences between prevalent and cross-sectional (DOPPS) versus incident and longitudinal (SRR) cohorts.
Our study has three major weaknesses. The most important is residual confounding. Despite our efforts to account for differences between groups treated with HDF and HD, there is likely to be residual confounding that no amount of modelling can abrogate due to the non-availability of potentially important covariates. A large but unquantifiable part of the demonstrated association between HDF and better outcomes may therefore be from unmeasured differences between the cohorts. For instance, we do not have comprehensive information on medical co-morbidity such as the presence of diabetes mellitus, and no way for us to ensure balance between groups for characteristics such as these. As importantly, we do not have data on patient income status to identify financial advantage in one group or the other, and this may be favouring the HDF group and positively affecting mortality risk. As a result, our analyses cannot be considered conclusive, and the effect of HDF per se on the patient survival must still be a matter of speculation. In our study, there remains an unquantifiable but potentially high likelihood of important selection bias between groups as a result of unavailable covariates.
The second limitation is the significant amounts of missing data. In our study, 5590 out of 14,941 patients were excluded since they did have complete data for outcomes, age, gender, primary kidney disease, and renal replacement modality / dialysis characteristics. This ratio is not dissimilar to some other registry analyses [71], such as a recent United Kingdom renal registry analysis where ~ 5500 of 11,000 patients were excluded for missing data. The included patients in our study had complete data for these core variables (i.e. outcomes, age, gender, primary kidney disease, and renal replacement modality / dialysis characteristics), and acceptable amounts of missing data for imputation of other covariates. As acknowledged by others [71,72,73,74,75,76], the imputation of missing values of other variables is critical to avoid bias from complete case analysis with missingness that is not completely at random.
The third limitation is that the SRR (like all registries) is likely to be affected by significant ascertainment bias. Endpoints were recorded by electronic returns, but not validated against source documentation or other records.
Conclusions
In conclusion, there is evidence of changing HD practice patterns in Shanghai, and increased HDF utilization. Our study suggests that the enhanced removal of middle sized and larger molecules improves outcomes in end-stage kidney failure populations from larger metropolitan centers, even when used within the resource constraints of the world’s most rapidly expanding and largest CKD population. The benefit of HDF is reasonably accepted to depend on convective dose [12], although it possible that the current standards for adequacy proposed from data in Europeans may be inappropriate for Chinese patients who are significantly smaller. Nonetheless, it is possible or even likely that even greater improvements might be seen in China with therapy that is applied in more standard fashion at three time a week with greater attention to convective dose.
Abbreviations
- AVF:
-
Arteriovenous fistula
- AVG:
-
Arteriovenous (prosthetic bridge) graft
- BMI:
-
Body mass index
- CI:
-
Confidence interval
- CKD:
-
Chronic kidney disease
- CVC:
-
Central venous catheter
- DOPPS:
-
Dialysis Outcome and Practice Patterns Study
- eGFR:
-
Estimated glomerular filtration rate
- ESKD:
-
End-stage kidney disease
- HD:
-
Hemodialysis
- HDF:
-
Hemodiafiltration
- HP:
-
Hemoperfusion
- IDWG:
-
Inter-dialytic weight gain
- MAR:
-
Missing at random
- MCAR:
-
Missing completely at random
- MDRD:
-
Modification of Diet in Renal Disease
- PD:
-
Peritoneal dialysis
- PH:
-
Proportional hazards
- RRT:
-
Renal replacement therapy
- SRR:
-
Shanghai Renal Registry
- UTI:
-
Urinary tract infection
References
Zhang L, Wang F, Wang L, Wang W, Liu B, Liu J, et al. Prevalence of chronic kidney disease in China: a cross-sectional survey. Lancet. 2012;379(9818):815–22. https://doi.org/10.1016/S0140-6736(12)60033-6.
Xie F, Zhang D, Wu J, Zhang Y, Yang Q, Sun X, et al. Design and implementation of the first nationwide, web-based Chinese renal data system (CNRDS). BMC Med Inform Decis Mak. 2012;12:11. https://doi.org/10.1186/1472-6947-12-11.
Cao Y, Li W, Yang G, Liu Y, Li X. Diabetes and hypertension have become leading causes of CKD in Chinese elderly patients: a comparison between 1990-1991 and 2009-2010. Int Urol Nephrol. 2012;44(4):1269–76. https://doi.org/10.1007/s11255-012-0194-0.
Cao X, Zhou J, Yuan H, Wu L, Chen Z. Chronic kidney disease among overweight and obesity with and without metabolic syndrome in an urban Chinese cohort. BMC Nephrol. 2015;18(16):85. doi:D - NLM: PMC4471928 EDAT- 2015/06/19 06:00 MHDA- 2015/06/19 06:00 CRDT- 2015/06/19 06:00 PHST- 2014/11/04 [received] PHST- 2015/06/04 [accepted] PHST- 2015/06/18 [aheadofprint] AID - https://doi.org/10.1186/s12882-015-0083-8 [doi] AID - https://doi.org/10.1186/s12882-015-0083-8 [pii] PST - epublish.
Qian Y, Ren H, Wang ZH, Li X, Chen XN, Chen N. Staging and risk factors of chronic kidney disease of outpatients in Shanghai. Ren Fail. 2014;36(7):1018–22. https://doi.org/10.3109/0886022X.2014.926923.
Gong P, Liang S, Carlton EJ, Jiang Q, Wu J, Wang L, et al. Urbanisation and health in China. Lancet. 2012;379(9818):843–52. https://doi.org/10.1016/S0140-6736(11)61878-3.
Collins AJ, Chan CT. Intensive Hemodialysis: Time to Give the Therapy Greater Consideration. Am J Kidney Dis. 2016;68(5S1):S1–4. https://doi.org/10.1053/j.ajkd.2016.05.027.
Marshall MR, Polkinghorne KR, Kerr PG, Hawley CM, Agar JW, McDonald SP. Intensive hemodialysis and mortality risk in Australian and New Zealand populations. Am J Kidney Dis. 2016;67(4):617–28. https://doi.org/10.1053/j.ajkd.2015.09.025.
Kirsch AH, Rosenkranz AR, Lyko R, Krieter DH. Effects of hemodialysis therapy using dialyzers with medium cut-off membranes on middle molecules. Contrib Nephrol. 2017;191:158–67. https://doi.org/10.1159/000479264.
Kirsch AH, Lyko R, Nilsson LG, Beck W, Amdahl M, Lechner P, et al. Performance of hemodialysis with novel medium cut-off dialyzers. Nephrol Dial Transplant. 2017;32(1):165–72. https://doi.org/10.1093/ndt/gfw310.
Maduell F, Moreso F, Pons M, Ramos R, Mora-Macia J, Carreras J, et al. High-efficiency postdilution online hemodiafiltration reduces all-cause mortality in hemodialysis patients. J Am Soc Nephrol. 2013;24(3):487–97. https://doi.org/10.1681/ASN.2012080875.
Mostovaya IM, Blankestijn PJ, Bots ML, Covic A, Davenport A, Grooteman MP, et al. Clinical evidence on hemodiafiltration: a systematic review and a meta-analysis. Semin Dial. 2014;27(2):119–27.
Nistor I, Palmer SC, Craig JC, Saglimbene V, Vecchio M, Covic A, et al. Haemodiafiltration, haemofiltration and haemodialysis for end-stage kidney disease. Cochrane Database Syst Rev. 2015;5:CD006258. https://doi.org/10.1002/14651858.CD006258.pub2.
Susantitaphong P, Siribamrungwong M, Jaber BL. Convective therapies versus low-flux hemodialysis for chronic kidney failure: a meta-analysis of randomized controlled trials. Nephrol Dial Transplant. 2013;28(11):2859–74. https://doi.org/10.1093/ndt/gft396.
Wang AY, Ninomiya T, Al-Kahwa A, Perkovic V, Gallagher MP, Hawley C, et al. Effect of hemodiafiltration or hemofiltration compared with hemodialysis on mortality and cardiovascular disease in chronic kidney failure: a systematic review and meta-analysis of randomized trials. Am J Kidney Dis. 2014;63(6):968–78. https://doi.org/10.1053/j.ajkd.2014.01.435.
Webster A, Nistor I. Haemodiafiltration, haemofiltration and haemodialysis for end-stage kidney disease. Nephrology (Carlton). 2016;21(6):526–7. https://doi.org/10.1111/nep.12756.
Marshall MR. Measuring the patient response to dialysis therapy: hemodiafiltration and clinical trials. Kidney Int. 2017;91(6):1279–82. https://doi.org/10.1016/j.kint.2017.02.024.
Morena M, Jaussent A, Chalabi L, Leray-Moragues H, Chenine L, Debure A, et al. Treatment tolerance and patient-reported outcomes of online hemodiafiltration versus high-flux hemodialysis in elderly: results from the FRENCHIE (FRENch Convective vs Hemodialysis In Elderly) randomized trial. Kidney Int. 2017;91(6):1495–509 In press.
Peters SA, Bots ML, Canaud B, Davenport A, Grooteman MP, Kircelli F, et al. Haemodiafiltration and mortality in end-stage kidney disease patients: a pooled individual participant data analysis from four randomized controlled trials. Nephrol Dial Transplant. 2016;31(6):978–84. https://doi.org/10.1093/ndt/gfv349.
Vonesh E, Schaubel D, Hao W, Collins A. Statistical methods for comparing mortality among ESRD pateints: examples of regional/international variations. Kidney Int. 2000;57(Suppl 74):S19–27.
Liu FX, Rutherford P, Smoyer-Tomic K, Prichard S, Laplante S. A global overview of renal registries: a systematic review. BMC Nephrol. 2015;16:31. https://doi.org/10.1186/s12882-015-0028-2.
Zhang W, Qian J. Current status of dialysis therapy in Shanghai (results from Shanghai renal Registry, 2011). Chin J Blood Purif. 2012;11(5):233–6.
Zhang W, Mei C, Chen N, Chen X, Zou J, Xue J, et al. Improving adequacy of hemodialysis in Shanghai: perspectives from the quality control Group of the Shanghai Renal Registry Network (SRRN). Med Sci Tech. 2015;56:78–83.
Ma YC, Zuo L, Chen JH, Luo Q, Yu XQ, Li Y, et al. Modified glomerular filtration rate estimating equation for Chinese patients with chronic kidney disease. J Am Soc Nephrol. 2006;17(10):2937–44. https://doi.org/10.1681/ASN.2006040368.
Canaud B, Granger Vallee A, Molinari N, Chenine L, Leray-Moragues H, Rodriguez A, et al. Creatinine index as a surrogate of lean body mass derived from urea Kt/V, pre-dialysis serum levels and anthropometric characteristics of haemodialysis patients. PLoS One. 2014;9(3):e93286. https://doi.org/10.1371/journal.pone.0093286.
Chen SJ, Jiang GR, Shan JP, Lu W, Huang HD, Ji G, et al. Combination of maintenance hemodialysis with hemoperfusion: a safe and effective model of artificial kidney. Int J Artif Organs. 2011;34(4):339–47. https://doi.org/10.5301/IJAO.2011.7748.
Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. Journal of the American Statistical Association. 1999;94:496–509.
Sterne JA, White IR, Carlin JB, Spratt M, Royston P, Kenward MG, et al. Multiple imputation for missing data in epidemiological and clinical research: potential and pitfalls. BMJ. 2009;338:b2393. https://doi.org/10.1136/bmj.b2393.
White IR, Royston P, Wood AM. Multiple imputation using chained equations: issues and guidance for practice. Stat Med. 2011;30(4):377–99. https://doi.org/10.1002/sim.4067.
van Buuren S, Boshuizen HC, Knook DL. Multiple imputation of missing blood pressure covariates in survival analysis. Stat Med. 1999;18(6):681–94.
Graham JW, Olchowski AE, Gilreath TD. How many imputations are really needed? Some practical clarifications of multiple imputation theory. Prev Sci. 2007;8(3):206–13. https://doi.org/10.1007/s11121-007-0070-9.
Kenward MG, Carpenter J. Multiple imputation: current perspectives. Stat Methods Med Res. 2007;16(3):199–218. https://doi.org/10.1177/0962280206075304.
Rubin DB. Multiple imputation for nonresponse in surveys. John Wiley and Sons: New York; 2004.
Lachenbruch PA. Stata tip 89: estimating means and percentiles following multiple imputation. Stata J. 2010;3:496–9.
Cohen J. Statistical power analysis for the behavioral sciences. 2nd ed. Hillsdale, NJ: Lawrence Erlbaum Associates; 1988.
Cai J-W. Effects of different hemodialysis methods on serum pathogenic factors in patients with renal failure. Zhejiang Journal of Clinical Medicine. 2015;17(5):759–60.
Jia P, Jin W, Teng J, Zhang H, Zou J, Liu Z, et al. Acute effects of Hemodiafiltration versus conventional hemodialysis on endothelial function and inflammation: a randomized crossover study. Medicine. 2016;95(16):e3440. https://doi.org/10.1097/md.0000000000003440.
Jiang X, Ji F, Chen ZW, Huang QL. Comparison of high-flux hemodialysis with hemodialysis filtration in treatment of uraemic pruritus: a randomized controlled trial. Int Urol Nephrol. 2016;48(9):1533–41. https://doi.org/10.1007/s11255-016-1364-2.
Kuo HL, Chou CY, Liu YL, Yang YF, Huang CC, Lin HH. Reduction of pro-inflammatory cytokines through hemodiafiltration. Ren Fail. 2008;30(8):796–800. https://doi.org/10.1080/08860220802272589.
Li X, Li M, Liu T, Li L, Duan L, Li Y, et al. Hemofiltration or hemodiafiltration with on-line production of substitution fluid: clinical observation of safety and effectiveness. Chin Med J. 1997;110(7):520–5.
Lin CL, Huang CC, Chang CT, Wu MS, Hung CC, Chien CC, et al. Clinical improvement by increased frequency of on-line hemodialfiltration. Ren Fail. 2001;23(2):193–206.
Lin CL, Huang CC, Yu CC, Yang HY, Chuang FR, Yang CW. Reduction of advanced glycation end product levels by on-line hemodiafiltration in long-term hemodialysis patients. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2003;42(3):524–31.
Liu H, Chen X, Zhao Y, Song J, Yi N. The curative effects of different dialysis therapy for end-stage of renal failure. Chinese Journal of Metallurgical Industry. 2008;25:5.
Su SF, Ng HY, Huang TL, Chi PJ, Lee YT, Lai CR, et al. Survey of depression by Beck depression inventory in uremic patients undergoing hemodialysis and hemodiafiltration. Therapeutic apheresis and dialysis : official peer-reviewed journal of the International Society for Apheresis, the Japanese Society for Apheresis, the Japanese Society for Dialysis Therapy. 2012;16(6):573–9. https://doi.org/10.1111/j.1744-9987.2012.01094.x.
Wang C, Lou TQ, Tang H, Chen ZJ, Yin PD, Yu XQ. [clearance effect of different blood purification techniques on parathyroid hormone in renal function failure patients on maintenance hemodialysis]. Zhongguo wei zhong bing ji jiu yi xue = Chinese critical care medicine =. Zhongguo weizhongbing jijiuyixue. 2004;16(12):753–5.
Wang L. Nutrition and prognosis of maintenance hemodialysis patients with different dialysis regimens: Shandong University; 2012.
Wang L, Liu Z, Zhou Z, Liu X, Wang P, Ren W, et al. Maintenance hemodialysis patients with different dialysis regimen: prognosis and comparative analysis. Chinese Journal of Blood Purification. 2002;1(4):198–226.
Wang X, Zeng L, Xie J. Comparison and clinical observation of three kinds of blood purification methods for medium molecular clearance. Contemporary Medicine. 2011;17(6):67.
Weng CJ, Chang MY, Chen YC, Tian YC, Fang JT, Yang CW. Long-term online hemodiafiltration does not reduce the frequency and severity of acquired cystic kidney disease in hemodialysis patients. Ren Fail. 2009;31(7):555–61.
Xu B, Chen L, CY HDL, Ling Y, Guan T. Effects of different blood purification methods on the removal of advanced oxidized protein products and the impact on cardiovascular prognosis. J Clin Nephrol. 2013;13(2):60–3.
Yang X, Chen X, Shi M, Ding G. Different blood purification methods for maintenance hemodialysis: a systematic review of prognostic effects. Internatonal Journal of Transplantation and Hemopurification. 2014;12(2):23–30.
Zhang DL, Liu J, Liu S, Zhang Y, Liu WH. The differences of asymmetric dimethylarginine removal by different dialysis treatments. Ren Fail. 2010;32(8):935–40. https://doi.org/10.3109/0886022x.2010.502281.
Zhang L, Yang J, Eastwood GM, Zhu G, Tanaka A, Bellomo R. Extended daily Dialysis versus continuous renal replacement therapy for acute kidney injury: a meta-analysis. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2015;66(2):322–30. https://doi.org/10.1053/j.ajkd.2015.02.328.
Zhang Y. Effect of hemodiafiltration on survival rate of patients with maintenance dialysis. International Journal of Nursing. 2015;34(4):565–7.
Zhu H, Qi Z, Chen J-H. On-line hemodiafiItration to correct calcium and phosphate metabolic disorder in uremia hemodialysis patients. Clinical Focus. 2012;27(20):1765–7.
Bai H. Hemodialysis in the treatment of end - stage renal disease with chronic congestive heart failure - clinical analysis. Aerospace Medicine. 2001;21(12):2197–8.
Peng B, Xu T, Wang N, Sheng X. Impact of hemodialysis combined with hemodiafiltraton on cardiac structure and function in maintenance hemodialysis pateints with diabetic nephrology. Chinese Journal of Integrated Traditional and Western Nephrology. 2014;15(2):129–33.
Rong S, Ye C, Ma Y, Chen J, Zhang B, Ji Y, et al. Comparison of efficacy and safety of high-flux hemodialysis and conventional hemodialysis combining hemodiafiltration. J Clinical Nephrology. 2013;13(2):56–9.
Locatelli F, Karaboyas A, Pisoni RL, Robinson BM, Fort J, Vanholder R, et al. Mortality risk in patients on hemodiafiltration versus hemodialysis: a 'real-world' comparison from the DOPPS. Nephrol Dial Transplant. 2017. https://doi.org/10.1093/ndt/gfx277.
Registry ERA-EDTA. ERA-EDTA Registry annual report 2013: Academic Medical Center. Amsterdam, The Netherlands: Department of Medical Infromatics; 2015.
Masakane I, Nakai S, Ogata S, Kimata N, Hanafusa N, Hamano T, et al. An overview of regular Dialysis treatment in Japan (as of 31 December 2013). Ther Apher Dial. 2015;19(6):540–74. https://doi.org/10.1111/1744-9987.12378.
United States Renal Data System. 2014 USRDS annual data report: Epidemiology of kidney disease in the United States: National Institutes of Health. Bethesda, MD: National Institute of Diabetes and Digestive and Kidney Diseases; 2014.
Cheng X, Nayyar S, Wang M, Li X, Sun Y, Huang W, et al. Mortality rates among prevalent hemodialysis patients in Beijing: a comparison with USRDS data. Nephrol Dial Transplant. 2013;28(3):724–32. https://doi.org/10.1093/ndt/gfs326.
Zuo L, Wang M, for the Beijing Blood Purification Quality Control Improvement Center. Current status of maintenance hemodialysis in Beijing, China. Kidney Int Suppl (2011). 2013;3(2):167–9. https://doi.org/10.1038/kisup.2013.6.
Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351(13):1296–305. https://doi.org/10.1056/NEJMoa041031.
Yao Q, Zhang W, Qian J. Dialysis status in China: a report from the Shanghai Dialysis Registry (2000-2005). Ethn Dis. 2009;19(1 Suppl 1):S1 -23-6.
Lin X, Yan Y, Ni Z, Gu L, Zhu M, Dai H, et al. Clinical outcome of twice-weekly hemodialysis patients in shanghai. Blood Purif. 2012;33(1–3):66–72. https://doi.org/10.1159/000334634.
Bieber B, Qian J, Anand S, Yan Y, Chen N, Wang M, et al. Two-times weekly hemodialysis in China: frequency, associated patient and treatment characteristics and quality of life in the China Dialysis outcomes and practice patterns study. Nephrol Dial Transplant. 2014;29(9):1770–7. https://doi.org/10.1093/ndt/gft472.
Mathew A, Obi Y, Rhee CM, Chen JL, Shah G, Lau WL, et al. Treatment frequency and mortality among incident hemodialysis patients in the United States comparing incremental with standard and more frequent dialysis. Kidney Int. 2016;90(5):1071–9. https://doi.org/10.1016/j.kint.2016.05.028.
Marshall MR. Observations of twice a week hemodialysis. Kidney Int. 2016;90(5):936–8. https://doi.org/10.1016/j.kint.2016.06.040.
Wagner M, Ansell D, Kent DM, Griffith JL, Naimark D, Wanner C, et al. Predicting mortality in incident dialysis patients: an analysis of the United Kingdom renal Registry. Am J Kidney Dis. 2011;57(6):894–902. https://doi.org/10.1053/j.ajkd.2010.12.023.
Couchoud C, Labeeuw M, Moranne O, Allot V, Esnault V, Frimat L, et al. A clinical score to predict 6-month prognosis in elderly patients starting dialysis for end-stage renal disease. Nephrol Dial Transplant. 2009;24(5):1553–61. https://doi.org/10.1093/ndt/gfn698.
Jassal SV, Karaboyas A, Comment LA, Bieber BA, Morgenstern H, Sen A, et al. Functional dependence and mortality in the international Dialysis outcomes and practice patterns study (DOPPS). Am J Kidney Dis. 2016;67(2):283–92. https://doi.org/10.1053/j.ajkd.2015.09.024.
van Walraven C, Austin PC, Knoll G. Predicting potential survival benefit of renal transplantation in patients with chronic kidney disease. CMAJ. 2010;182(7):666–72. https://doi.org/10.1503/cmaj.091661.
Floege J, Gillespie IA, Kronenberg F, Anker SD, Gioni I, Richards S, et al. Development and validation of a predictive mortality risk score from a European hemodialysis cohort. Kidney Int. 2015;87(5):996–1008. https://doi.org/10.1038/ki.2014.419.
Allison PD. Multiple imputation for missing data: a cautionary tale. Sociol Methods Res. 2000;28(3):301–9. https://doi.org/10.1177/0049124100028003003.
Acknowledgements
None.
Funding
No specific funding was required or utilized for this study.
Availability of data and materials
Data and materials are available on request from the Shanghai Society of Nephrology and Shanghai Center for Hemodialysis Quality Control (http://sh.cnrds.org).
Declarations
The results presented in this paper have not been published previously in whole or part, except in abstract format.
Author information
Authors and Affiliations
Contributions
Authors WZ, HL, MRM, MJW contributed equally to the work, and undertook overall study design, analysis and drafting and completion of the manuscript. Authors CM, NC, XD, ZN, CH, JHZ, JYZ, NW, GJ, ZG, CY, YD, QY, JQ contributed equally to the work, and undertook data collection, oversight of analysis, and revision of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The Ethics Committee (IRB00009803, https://ohrp.cit.nih.gov/search) of the Shanghai Clinical Research Center (IORG0008177, https://ohrp.cit.nih.gov/search) reviewed and approved the design and execution of our study (Submission Number2018-08-01) (http://www.scrcnet.org/IEC_en.asp). The committee waived the need for individual consent to participate on the basis of the research being a retrospective observational study with unidentified data.
Consent for publication
The requirement for individual consent to publish was waived by the reviewing ethics committee, under the framework of retrospective audit of clinical records. All data in the study are de-identified, and none of the authors of the manuscript had access to any information that could be used to identify individual participants or their treating center during or after data collection.
Competing interests
Haiming Li and Qiang Yao are fulltime employees of Baxter Healthcare (China) Ltd. Mark R Marshall is a fulltime employee of Baxter Healthcare (Asia-Pacific) Ltd., a part-time employee of University of Auckland (New Zealand) as an adjunct Associate Professor, and a part-time employee of Counties Manukau Health (New Zealand) as a clinical nephrologist. No other researcher has any potential conflicts of interest to declare.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Additional files
Additional file 1:
Table S1. Table of patient characteristics at renal replacement therapy (RRT) inception, comparing the included and excluded cohorts. (PDF 38 kb)
Additional file 2:
Complete case and imputed variables (twenty imputations) used in the modelling. (PDF 149 kb)
Additional file 3:
Figure S1. Full Cox proportional hazards (PH) main-effects model, fully adjusted for the main effects confounders listed in Table 1 (the marker represents point estimates, the whiskers, 95% confidence intervals). Abbreviations: HDF, hemodiafiltration; HB, hemoglobin; BMI, body mass index; eGFR, estimated glomerular filtration rate; RRT, renal replacement therapy; AV, arteriovenous; IDWG, inter-dialytic weight gain. (PDF 270 kb)
Additional file 4:
Figure S2. Full competing risks main-effects model, fully adjusted for the main effects confounders listed in Table 1 (the marker represents point estimates, the whiskers, 95% confidence intervals). Abbreviations: HDF, hemodiafiltration; HB, hemoglobin; BMI, body mass index; eGFR, estimated glomerular filtration rate; RRT, renal replacement therapy; AV, arteriovenous; IDWG, inter-dialytic weight gain. (PDF 283 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Zhang, W., Mei, C., Chen, N. et al. Outcomes and practice patterns with hemodiafiltration in Shanghai: a longitudinal cohort study. BMC Nephrol 20, 34 (2019). https://doi.org/10.1186/s12882-019-1219-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12882-019-1219-z