Abstract
Background
Fungal peritonitis is a common and serious complication associated with peritoneal dialysis (PD), and it is often refractory to treatment.
Case presentation
A 70-year-old patient undergoing peritoneal dialysis was admitted to our hospital with fever and cloudy PD fluid. A diagnosis of yeast-like fungal peritonitis was made by examining the PD fluid. After starting intravenous caspofungin acetate, the PD catheter was removed. A fungal pathogen was isolated from the peritoneal fluid and identified as Cryptococcus sp. Based on the results of antifungal susceptibility testing, the treatment was changed to oral voriconazole and continued for 6 weeks. However, because of the discrepancy between the morphological findings and culture results, we performed genetic analysis, which uncovered Moesziomyces antarcticus. This patient was diagnosed with PD-related peritonitis caused by M. antarcticus and was successfully treated with voriconazole and removal of the PD catheter.
Conclusions
Reports of human infection by M. antarcticus are rare, and only two cases have been recognized. This may be the first case of infection detected in PD fluid.
Similar content being viewed by others
Background
Peritonitis is one of the most common complications associated with peritoneal dialysis (PD). Although the incidence of peritonitis is only 5%, it can be fatal and a direct cause of death in patients undergoing PD [1]. Of the different types, fungal peritonitis is a rare but serious complication associated with high mortality. Even when it does not lead to death, it is often refractory to treatment and subsequent withdrawal from PD [2].
Moesziomyces antarcticus (formerly known as Pseudozyma antarctica), a basidiomycete yeast, was originally isolated from sediment samples obtained from Lake Banda in Antarctica, and later reports indicated that it is mainly present on plant surfaces [3]. The species has been classified and described as M. antarcticus since 2015 [4]. Current reports of human infection by M. antarcticus are scarce, and to the extent that they have been confirmed, they include a case reported in 2003 in Thailand that was identified from blood [5]. In 2019, a 93-year-old Chinese patient with chronic kidney disease, chronic obstructive pulmonary disease, and dementia was diagnosed with M. antarcticus infection in blood [6]. A summary of each case is presented in Table 1. In the present study, we experienced a case of peritonitis caused by M. antarcticus in an adult patient on PD. To the best of our knowledge, this is the first report of the successful treatment of PD-related peritonitis associated with M. antarcticus using voriconazole and catheter removal.
Case presentation
A 70-year-old man with hypertensive nephropathy had been receiving continuous ambulatory peritoneal dialysis (CAPD) since the age of 69. The patient’s life history included gardening as a hobby. The patient did not use a sterile connection ultraviolet flush device when changing the PD bag, and the procedure was performed manually. At 70 years of age, CAPD was changed to the combination of PD and hemodialysis (HD). The PD bag exchange protocol was as follows: 1.5 L of 1.5% glucose-based solution three times a day during the daytime and 2 L of icodextrin solution overnight. He was not treated with any immunosuppressive therapies, and a serological test for human immunodeficiency virus was negative. The patient had experienced PD-related peritonitis for 1 month prior to this episode. At that time, Streptococcus salivarius was detected and eradicated using cefazolin.
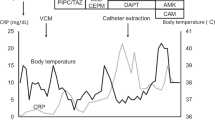
He presented to our hospital with complaints of fever and cloudy PD fluid. His vital signs were as follows: blood pressure, 142/86 mmHg; pulse, 87 beats/min; respiratory rate, 17 breaths/min; and temperature, 37.1 °C. Physical examination revealed mild abdominal tenderness but no muscular defenses. The clinical laboratory data at admission were as follows: peripheral white blood cell (WBC) count, 6600 cells/mm3 (neutrophils, 66.2%; lymphocytes, 24.1%; eosinophils, 3.8%); hemoglobin level, 10.1 g/dL; and C-reactive protein (CRP) level, 0.32 mg/dL. A Tenckhoff catheter had been placed in the left lower quadrant, and it did not appear to be infected. The WBC count in the first cloudy PD fluid sample was 295 cells/μL. Blood cultures collected at admission were negative. We initially treated the patient with intraperitoneal tobramycin and cefazolin in combination. Gram staining of the centrifuged PD fluid sediment was performed, and yeast-like fungi were identified by microscopy. This result was also confirmed in PD fluid on day 5. These findings suggested fungal peritonitis, and the administration of intravenous caspofungin acetate was initiated. However, the number of WBCs in the PD fluid remained above 100/μL. The peritoneal catheter was removed on day 11 after admission according to the recommendation of the International Society for Peritoneal Dialysis (ISPD) guidelines [7]. PD catheter removal was successful without any adverse events. There was no sign of infection around the cuffs, and cultures of the tip of the catheter were negative. Subsequently, HD was performed as renal replacement therapy. A fungal pathogen grew from the initial PD fluid, and the isolate was identified as a Cryptococcus species (C. laurentii) by a commercial laboratory (method: matrix-assisted laser desorption ionization–time-of-flight mass spectrometry) 15 days after admission. On the basis of the antifungal susceptibility test results, the treatment was switched to oral voriconazole. The patient’s CRP levels improved, and he was discharged on day 22 after admission. However, because of the discrepancy between the morphological findings (Fig. 1) and culture results, we asked the Medical Mycology Research Center, Chiba University (Chiba, Japan) to perform an additional detailed examination. We ultimately identified the strain as M. antarcticus based on sequencing of the internal transcribed spacer and D1/D2 regions of the 28S ribosomal DNA by performing a Basic Local Alignment Search Tool search in GenBank. We continued voriconazole administration for 6 weeks and then terminated the treatment. The clinical course is summarized in Fig. 2. The patient has been in good condition for more than 1 year after completing antibiotic therapy.
Morphological and physiological characteristics of the isolated Moesziomyces antarcticus specimen. a Smooth colonies of M. antarcticus after 3 days growth on Sabouraud medium at 30 °C. b Colony morphology of M. antarcticus after 3 days growth on Sabouraud medium at 35 °C. c Direct microscopic examination of fungi in blood culture flasks. d Gram staining of fungi in blood culture flasks
Discussion and conclusions
The causes of peritonitis in patients undergoing PD are touch contamination, exit site or tunnel infection, and gastrointestinal infection [8]. Eisenberg et al. [9] identified the following risk factors for the development of fungal peritonitis: bacterial peritonitis within the past month, the use of antibiotics, hospitalization within the past 10 days, extraperitoneal infection, bowel perforation, peritoneal-vaginal traffic, and the use of immunosuppressive drugs. In this case, there were no signs of tunnel or gastrointestinal infections, but there was prior bacterial peritonitis 1 month before presentation. Staphylococcus epidermidis and S. aureus are the most common causative organisms of peritonitis secondary to PD, and fungal peritonitis is a common subsequent microbial infection. Fungal peritonitis is a serious complication in patients undergoing PD, leading to death in approximately 25% of episodes [10, 11]. According to recommendations from the ISPD, catheters should be removed immediately after the diagnosis of fungal peritonitis in addition to initiating antifungal therapy [7].
In the Wright Valley, South Victoria Land, Antarctica in 1965, a research team dispatched from Japan isolated a basidiomycetous yeast strain from the sediment of Lake Vanda and named it S. antarcticus [12]. After several taxonomic changes, the species was combined with the genus Pseudozyma and described as P. antarctica in 1995 [13]. In 2015, P. antarctica was transferred to the genus Moesziomyces, and the strain was named M. antarcticus [4]. Moesziomyces species are mainly isolated from plant surfaces, and they provide natural sources of protection against powdery mildews. Several Moesziomyces species have been reported to exhibit biological activity against biodegradable plastics, which are typically used in a number of industrial processes [14]. In recent years, some cases of patients infected by plant fungus have been reported [5, 15]. As previously mentioned, reports of human infection by M. antarcticus are rare, with only two cases recognized. In a previous report of human infection by M. antarcticus, both cases were detected in blood samples. This may be the first case of infection detected in PD fluid.
Since its discovery in Antarctica, M. antarcticus has also been isolated from rice paddies, water bodies, and oceans in regions in East Asia, including Japan. The microbe was also detected on an ovary of barnyardgrass (Echinochloa crus-galli) in Japan [16]. It is presumed that this patient had extensive contact with plants based on his hobby of gardening and thus opportunities to be exposed to M. antarcticus. Microbial substitution from the most recent episode of bacterial peritonitis may have also contributed to the onset of this episode. An M. antarcticus strain isolated by Sugita and colleagues in Thailand in 2003 was sensitive to fluconazole and itraconazole. Conversely, an isolate identified by Liu et al. in China in 2009 was resistant or weakly susceptible to flucytosine, fluconazole, voriconazole, and itraconazole and was only sensitive to amphotericin B (Table 2). In this case, the fungus was resistant to fluconazole and flucytosine, and it had relatively low susceptibility to caspofungin. We chose voriconazole because it has good sensitivity results, it is available in an oral form, and it is relatively simple to adjust its dose [17], even in patients with renal failure. We believe the patient's CRP level clearly improved after catheter removal, suggesting that this was the most effective treatment in this case. We believe that voriconazole effectively prevented postoperative wound infection and recurrent or refractory peritonitis.
Experience regarding the optimal duration of treatment for M. antarcticus infection is limited because of the small number of reported cases and the lack of controlled trial data. In the present case, treatment was successful after 6 weeks of voriconazole administration and removal of the peritoneal catheter. In the future, the accumulation of data on cases of successful treatment should facilitate selection of the optimal antifungal agent and optimization of the duration of treatment for PD-related peritonitis associated with M. antarcticus.
Availability of data and materials
All data and materials were included in the manuscript.
Abbreviations
- CAPD:
-
Continuous ambulatory peritoneal dialysis
- CRP:
-
C-reactive protein
- HD:
-
Hemodialysis
- ISPD:
-
International Society for Peritoneal Dialysis
- PD:
-
Peritoneal dialysis
- WBC:
-
White blood cell
References
Miles R, Hawley CM, McDonald SP, Brown FG, Rosman JB, Wiggins KJ, et al. Predictors and outcomes of fungal peritonitis in peritoneal dialysis patients. Kidney Int. 2009;76(6):622–8.
Matuszkiewicz-Rowinska J. Update on fungal peritonitis and its treatment. Perit Dial Int. 2009;29(Suppl 2):S161–5.
Sugiyama J, Sugiyama Y, Iizuka H, Torii T. Report of the Japanese summer parties in dry valleys, Victoria Land, 1963–1965. IV. Mycological studies of the Antarctic fungi 2. Mycoflora of Lake Vanda, an ice-free lake. Antarctic Rec. 1967;28:23–32.
Wang QM, Begerow D, Groenewald M, Liu XZ, Theelen B, Bai FY, et al. Multigene phylogeny and taxonomic revision of yeasts and related fungi in the Ustilaginomycotina. Stud Mycol. 2015;81:55–83. https://doi.org/10.1016/j.simyco.2015.10.004.
Sugita T, Takashima M, Poonwan N, Mekha N, Malaithao K, Thungmuthasawat B, et al. The first isolation of ustilaginomycetous anamorphic yeasts, Pseudozyma species, from patients’ blood and a description of two new species: P. parantarctica and P. thailandica. Microbiol Immunol. 2003;47:183–90. https://doi.org/10.1111/j.1348-0421.2003.tb03385.x.
Liu Y, Zou Z, Hu Z, Wang W, Xiong J. Morphology and molecular analysis of Moesziomyces antarcticus isolated from the blood samples of a Chinese patient. Front Microbiol. 2019;10:254.
Li PK, Szeto CC, Piraino B, de Arteaga J, Fan S, Figueiredo AE, et al. ISPD peritonitis recommendations: 2016 update on prevention and treatment. Perit Dial Int. 2016;36(5):481–508.
Keane WF, Bailie GR, Boeschoten E, Gokal R, Golper TA, Holmes CJ, et al. Adult peritoneal dialysis-related peritonitis treatment recommendations: 2000 update. Perit Dial Int. 2000;20(4):396–411.
Eisenberg ES, Leviton I, Soeiro R. Fungal peritonitis in patients receiving peritoneal dialysis: experience with 11 patients and review of the literature. Rev Infect Dis. 1986;8(3):309–21.
Wang AY, Yu AW, Li PK, Lam PK, Leung CB, Lai KN, et al. Factors predicting outcome of fungal peritonitis in peritoneal dialysis: analysis of a 9-year experience of fungal peritonitis in a single center. Am J Kidney Dis. 2000;36(6):1183–92.
Prasad KN, Prasad N, Gupta A, Sharma RK, Verma AK, Ayyagari A. Fungal peritonitis in patients on continuous ambulatory peritoneal dialysis: a single centre Indian experience. J Infect. 2004;48(1):96–101.
Goto S, Sugiyama J, Iizuka H. A taxonomic study of Antarctic yeasts. Mycologia. 1969;61(4):748–74.
Boekhout T. Pseudozyma Bandoni emend. Boekhout, a genus for yeast-like anamorphs of Ustilaginales. J Gen Appl Microbiol. 1995;41:359–66.
Kitamoto H, Yoshida S, Koitabashi M, Yamamoto-Tamura K, Ueda H, Yarimizu T, et al. Enzymatic degradation of poly-butylene succinate-co-adipate film in rice husks by yeast Pseudozyma antarctica in indoor conditions. J Biosci Bioeng. 2018;125(2):199–204.
Lin SS, Pranikoff T, Smith SF, Brandt ME, Gilbert K, Palavecino EL, et al. Central venous catheter infection associated with Pseudozyma aphidis in a child with short gut syndrome. J Med Microbiol. 2008;57(Pt 4):516–8.
Tanaka E, Koitabashi M, Kitamoto H. A teleomorph of the ustilaginalean yeast Moesziomyces antarcticus on barnyardgrass in Japan provides bioresources that degrade biodegradable plastics. Antonie Van Leeuwenhoek. 2019;112(4):599–614.
Peng LW, Lien YH. Pharmacokinetics of single, oral-dose voriconazole in peritoneal dialysis patients. Am J Kidney Dis. 2005;45:162–6.
Acknowledgements
We would like to thank Rio Seki (Medical Mycology Research Center, Chiba University) and Shuhei Yamamoto (Takamatu Red Cross Hospital) for their valuable technical assistance. We thank Joe Barber Jr., Ph.D., from Edanz (https://jp.edanz.com/ac) for editing a draft of this manuscript.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
SN, MY, YK, YA, MH, TY, TO, and TS participated in discussions of this case. SN drafted the manuscript, and this author is responsible for the final version of the manuscript. MO and KK discussed the fungus in detail. SN, KK, and TS reviewed and revised the manuscript. All authors read and approved the manuscript and agreed with its submission to this journal.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This report has been prepared in compliance with the Declaration of Helsinki. Ethical approval was not required for this type of case report.
Consent for publication
Written informed consent was obtained from the patient for the publication of this case report.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Nishioka, S., Yamanaka, M., Kunisho, Y. et al. A case of Moesziomyces antarcticus peritonitis in a patient undergoing peritoneal dialysis. Ren Replace Ther 8, 29 (2022). https://doi.org/10.1186/s41100-022-00419-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41100-022-00419-2