Abstract
Background
Insulin antibody appears in approximately 40% of insulin users using human insulin and insulin analog formulations. Insulin antibody, characterized by high affinity and low insulin antibody binding capacity (% bound), rarely causes clinical problems including poor glycemic control. Recently, the insulin antibody against human insulin causing deterioration of glycemic control, characterized by low affinity and high insulin antibody binding capacity similar to those observed in insulin autoimmune syndrome, has been reported.
Case presentation
A 62-year-old male developing fulminant type 1 diabetes showing rapid hyperglycemia (602 mg/dL) and normal range of glycoalbumin (GA) (18.4%) in the introductory stage of hemodialysis (HD) was transferred to our hospital 3 weeks after the onset of diabetes. He had been administered insulin intensification therapy in other medical clinic; however, his glycemic control remained poor, showing ketoacidosis. After hospitalization, he was initially administered intravenous insulin for 2 days and then switched to subcutaneous intensification therapy (24 U/day); the dose was gradually increased maximum of 72 U/day within 2 months. His glycemic control deteriorated despite high-dose insulin administration and change in the insulin type. Insulin antibody was detected, insulin antibody binding capacity (% bound) was of a high value (89.5%), GA was 32.9%, and casual blood glucose widely changed in continuous glucose monitor, suggesting that unstable blood glucose fluctuations caused by insulin antibody; therefore, treatment with double filtration plasmapheresis (DFPP) and prednisolone (PSL) was performed. The insulin dose reduced, and glycemic control improved from 1 month after the commencement of treatment. Subsequently, insulin antibody level remained high; however, after 6 months, % bound declined (20.9%), and glycemic control improved (GA 23.7%) by PSL (gradually reduced to 5 mg/day). The Scatchard plots also indicated the effectiveness with DFPP and PSL therapy.
Conclusions
The authors present a unique case of fulminant type 1 diabetes developing during the HD introduction period. Although this is a rare complication, physicians specializing in dialysis therapy should also keep in mind that this is a life-threatening complication if the treatment is delayed and that concomitant therapy with apheresis such as DFPP and long-term steroids is effective in some cases when glycemic control deteriorates attributable to insulin antibody.
Similar content being viewed by others
Background
Insulin antibody appear in approximately 40% of insulin users administered human insulin and insulin analog formulations. Insulin antibody, characterized by high affinity and low insulin antibody binding capacity (% bound), rarely causes clinical problems including glycemic control [1]. In contrast, insulin antibody against human insulin, characterized by low affinity and high insulin antibody binding capacity similar to those observed in insulin autoimmune syndrome, has been reported recently [2], with reports mentioning that glycemic control was difficult [3]. The authors herein report a case of fulminant type 1 diabetes developing during the hemodialysis (HD) introduction period complicated by renal cell carcinoma (RCC). Firstly, insulin therapy was introduced. Secondly, nephrectomy was performed. Glycemic control deteriorated thereafter. The cause of poor glycemic control could be associated with insulin antibody. Treatment with double filtration plasmapheresis (DFPP) and prednisolone (PSL) was performed for the case. The insulin antibody binding capacity decreased, as was also confirmed in Scatchard analysis. In the present case, DFPP and PSL successfully improved a long-term glycemic control.
Case presentation
A 62-year-old Japanese male under HD due to IgA nephropathy for approximately 1 week (at second HD session) with no previous history of diabetes mellitus was diagnosed and treated with insulin at other medical clinic. He had not been treated with corticosteroid for IgA nephropathy. His glycemic profile during the 2 years just before the introduction of HD was within normal range (fasting blood glucose level 78–98 mg/dL and hemoglobin A1c (HbA1c; NGSP) level 5.5–5.8%). The final monitoring for blood glucose and HbA1c levels were performed 3 months before onset of diabetes (fasting blood glucose 89 mg/dL, HbA1c 5.5%). Any precipitating event such as pancreatitis, sepsis, medication, or any traumatic event that might have caused impaired glucose tolerance did not appear. He complained of thirst, nausea, and vomiting; however, he did not show bronchitis. His body weight decreased 2.8 kg (height 163 cm, body weight 49.1 kg, body temperature 37.3 °C) compared with after the first HD session, and his blood glucose level was 602 mg/dL (HbA1c 5.9%, glycoalbumin (GA) 18.4%). Because his glycemic control was poor using insulin lispro and deemir, he was transferred to our hospital approximately 3 weeks after the onset of diabetes. On admission to our hospital, his blood glucose level was 642 mg/dL with ketoacidosis (HbA1c 8.3%, GA 32.9%, pH 7.04, HCO3 − 12 mEq/L, plasma ketone 284 μmol/L, anion gap 19 mEq/L, plasma osmolality 331 mOsm/L). His diabetic ketoacidosis was managed using standard treatment protocols. The authors managed to keep his blood glucose concentrations within 150–200 mg/dL with continuous intravenous infusion (intravenous insulin therapy approximately 40 U/day for 2 days). Following recovery, he was introduced to an intensive insulin therapy (insulin glulisine and glargine) and maintained his blood glucose between 100 and 200 mg/dL (insulin glulisine as bolus 6-6-6 U, glargine as basal 0-0-6 U, a total of 24 U/day).
His blood count was as follows: white blood cells, 6900/μL; red blood cells, 328 × 104/μL; hemoglobin, 11.8 g/dL; hematocrit, 32.9%; platelet, 21.6 × 104/μL; using epoetin beta pegol 25 μg/2 weeks.
His serum albumin was 4.0 g/dL, alkaline phosphatase (ALP) 174 U/L, asparate aminotransferase (AST) 15 U/L, alanine aminotransferase (ALT) 14 U/L, gamma-glutamyl transpeptidase (γ-GTP) 17 U/L, blood urea nitrogen (BUN) 89, creatinine 7.54, and uric acid 5.9 mg/dL.
The glucagon, IgG, IgA, IgM, IgG4, carcinoembryonic antigen (CEA), and carbohydrate antigen (CA) 19-9 values were within normal limits. Serum interleukin-2 receptor level was slightly elevated at 1040 U/mL. Serum anti-glutamic acid decarboxylase (GAD) antibody, islet antigen 2 (IA-2) antibody, islet cell antibody (ICA), parathyroid hormone-related protein, anti-thyroid peroxidase (TPO) antibody, anti-thyroglobulin antibody, calcitonin, myeloperoxidase anti-neutrophil cytoplasmic antibody (MPO-ANCA), and protease 3 (PR3)-ANCA were not elevated. The serum C-peptide immunoreactivity (CPR) level was 0.04 ng/mL, and glucagon tolerance test revealed no elevation of CPR (Δ CPR 0 ng/mL).
Human leukocyte antigen (HLA) typing showed A2/24, B35/52, DR4(DRB1*04:01), DR15(DRB1*15:01), and DQB1*04:02:01/DQB1*06:02:01.
The pancreatic exocrine enzymes were slightly elevated as follows: pancreatic amylase, 155 U/l; elastase 1176 ng/dL; and lipase 65 U/L (slightly elevated compared with normal limits).
Abdominal ultrasonography revealed no remarkable findings, such as pancreatic enlargement, poorly defined edges, or ascites retention; however, it revealed a mass in the left kidney. Contrast computed tomography (CT) of the abdomen was subsequently performed and revealed a 43-mm mass in the left kidney (Fig. 1). Two months after admission, the mass was removed using a laparoscopic approach, and no gross metastasis was evident at surgery. Pathology of the mass was consistent with clear cell adenocarcinoma with no invasion of the capsule and renal pelvis. The margins of the resection were clear with no evidence of tumor in the renal veins.
Ultrasonography of the thyroid gland revealed no morphological defects, and the fundus findings of the patient were normal.
In the evaluation of glycemic daily profile using a continuous glucose monitor (CGM), his blood glucose level widely fluctuated, with a tendency for hyperglycemia during the day and nocturnal hypoglycemia. Two weeks after admission, insulin antibody was detected, and the insulin antibody binding capacity was 89.1%.
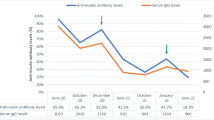
First, insulin intensification therapy was introduced (at first, insulin lispro as bolus 6-6-6 U, detemir as basal 0-0-6 U, total of 24 U/day; and then changed insulin glulisine as bolus 6-6-6 U, glargine as basal 0-0-6 U, total of 24 U/day); however, maintaining his glycemic control in good condition was difficult. The dosage was therefore increased to a maximum of 72 U/day within 2 months (Fig. 2). His glycemic control deteriorated after nephrectomy despite treatment with high dose of insulin; therefore, he was introduced to insulin pump therapy (glulisine as bolus 22-18-20 U, glargine as basal; 12–7 am 0.1, 7–12 pm 0.8, 12–7 pm 0.4, 7–12 am 0.1 U/h, total of 8 U/day, grand total of 68 U/day). Because his casual blood glucose fluctuated widely at 246 ± 99 mg/dL in CGM (nocturnal hypoglycemia was frequently observed), insulin pump therapy was switched back to intensive insulin therapy (glulisine as bolus 18-17-18 U, glargine as basal; 8 U, a total of 61 U/day), resulting in an improvement in his casual blood glucose to 154 ± 55 mg/dL in CGM. Second, the insulin antibody and antibody binding capacity was high value; therefore, DFPP was performed twice a week for 3 h before HD for 5 weeks, totaling 10 times (concentration of replacement albumin fluid was 8%, plasma fractionator Evaflux 2A-20, Kawasumi Laboratories, Tokyo, Japan, and plasma separator Plasmacure PE-05, Kawasumi Laboratories, Tokyo, Japan), to eliminate the antibody. Third, PSL (Predonisolone, Takeda, Osaka, Japan) was commenced starting at 30 mg/day, after which the dosage was gradually decreased to 5 mg/day over 6 months. On comparing the insulin antibody binding capacity before treatment with that after treatment with DFPP and PSL, the value gradually decreased from 89.1 to 20.9% (6 months), while the casual blood glucose improved to 154 ± 55 mg/dL CGM, and the GA level declined from 36.5 to 23.7% (6 months). Furthermore, the frequency of hypoglycemia decreased, and the amount of insulin administered decreased from 61 to 38 U/day (6 months). These results suggested the long-term effectiveness of DFPP and PSL therapy (Fig. 2).
The insulin antibody binding capacity declined from 89.5% before treatment with DFPP and PSL to 23.7% at 6 months after introduction of the treatment. Further, using a Scatchard analysis to compare human insulin before with after treatment for qualitative evaluation of the antibody, low affinity and high binding capacity was indicated before treatment, at k1 = 0.0034 × 108 M−1, R1 = 112 × 10−8M; however, the affinity elevated, and the number of binding capacity significantly declined after treatment, at k1 = 0.04 × 108 M−1, R1 = 9.1 × 10−8M (Fig. 3).
Scatchard plots of insulin antibody in this patient. Before treatment of double filtration plasmapheresis and prednisolone (circle, k1 = 0.0032 × 108 M−1, R1 = 112 × 10−8 M) and after treatment (triangle, k1 = 0.0400 × 108 M−1, R1 = 9.1 × 10−8 M). Bound/free insulin at low concentration of bound insulin is thought to reflect the degree of affinity of the antibody. To assess the quantity of high-affinity antibody in relation to the level of affinity, the authors calculated the value of X intercept (R1) multiplied by the Y intercept (k1R1) of the Scatchard line for high-affinity binding sites, where k1 and R1 were affinity constant and binding capacity in high-affinity binding sites, respectively. These plots of after treatment indicate the degree of affinity of the antibody increased, whereas the degree of binding capacity dramatically decreased compared with before treatment
The patient has continued self-monitoring of blood glucose and self-administration of insulin and undergoing chronic maintenance HD 3 times a week in our hospital as an outpatient. He is clinically well, showing no signs of exacerbation of poor glycemic control, with a GA level of approximately 21%, even 12 months after the initiation of the treatment (total insulin of 32 U/day and PSL of 2.5 mg/day).
Discussion
The authors found out three important clinical issues in the present case: First, poor glycemic control following administration of insulin was attributable to insulin antibody during insulin treatment for rare fulminant type 1 diabetes developing shortly after the introduction of HD. Second, after the treatment with DFPP and PSL to reduce insulin antibody, the insulin antibody value was still high despite the results as follows: (1) a significant decrease in the insulin antibody binding capacity, (2) glycemic control improved, and (3) the amount of insulin administered was reduced. Third, a Scatchard analysis was useful for evaluating the qualitative changes in the insulin antibody level; high affinity and a decline in the number of binding sites were observed after treatment. These results indicated the effectiveness of the treatment with DFPP and PSL.
In general, while the pathogenesis of fulminant type 1 diabetes remains unknown, this is a subtype of non-autoimmune, fulminant disorder characterized by a remarkably abrupt onset (exhibiting hyperglycemia and ketoacidosis), elevated serum pancreatic enzyme concentrations, depletion of endogenous insulin secretion, and the absence of insulitis and diabetes-related antibody within several days after onset [4]. HbA1c level is often normal or slightly increased. The association between the disease and viral infections has been discussed as the cause [5], the pathogenesis is still unknown. Approximately 10% of new-onset type 1 diabetes cases in Japan has been reported to be fulminant type. Moreover, antecedent infection is observed in approximately 70%, along with complications of the upper respiratory symptoms and digestive symptoms [6]. Among HLA genes, several haplotypes constructing the DRB1 and DQB1 genes have been reported to relating to the susceptibility and resistance of the onset of type 1 diabetes [7]. However, it still remains unknown in detail. Fulminant type 1 diabetes is a fatal disease that requires rapid diagnosis and treatment. Regardless of the blood glucose level, patients under HD generally complain of dry mouth attributable to water restrictions. Moreover, they tend to cause as follows: (1) pseudo-hypo HbA1c values attributable to renal anemia and (2) shortened hemoglobin cycle. Moreover, to distinguish the present disease from dialysis disequilibrium syndrome might be difficult if the present disease developed shortly after introduction of HD. In patients under HD with no medical history of diabetes complicated with acute hyperglycemia and metabolic acidosis, HbA1c and diabetes-related auto-antibodies should be examined.
Next, regarding insulin antibody, it could be produced by two pathways as follows: (1) exogenous insulin formulations and (2) produced against the patient’s own insulin, such as insulin autoimmune syndrome. In the present case, the clinical course suggested that insulin antibody were induced by exogenous insulin from the results as follows: (1) no characteristic HLA DR4 (DRB1*04:06) in the insulin autoimmune syndrome was observed, (2) the patient had no history of taking drugs that might induce the syndrome, and (3) the patient was undergoing exogenous insulin treatment.
Although there are no established treatments for insulin antibody, reports are available concerning the effectiveness of apheresis such as DFPP, steroids, and other immunosuppressive drugs [8]. As the molecular weight of insulin antibody has not been well known, DFPP was selected as an apheresis therapy to remove wide range of molecular weight in the present case. DFPP might be effective in HD patients complicated by fulminant type 1 diabetes with a high insulin antibody value and poor glycemic control, as well as HD patients complicated by other forms of insulin-dependent diabetes. Apheresis could be one of the most efficient therapies to eliminate antibodies; however, the therapeutic effect is temporary and disappears within a short-term of approximately 6 months; therefore, several reports are available mentioning the effectiveness of the long-term treatment with apheresis repeatedly, as well as the concomitant use of insulin-like growth factor (IGF) I for maintaining favorable glycemic control [8]. Discontinuation of insulin for the purpose of reducing insulin antibody is also one of the options [9].
Several reports in the literature describe the effectiveness of steroid therapy; however, the dosage of steroids at the time of introduction, methods for gradually reducing the dosage, and the presence of steroid pulse therapy (not performed in the present case) differ from reports. A certain consensus concerning the long-term prognosis of steroid treatment has not been obtained [10].
In the present case, since achieving good glycemic control was difficult due to the presence of insulin antibodies, the possibility remained that monotherapy (DFPP or steroid) might be insufficient; therefore, the authors performed two kinds of treatments (both DFPP and steroid) at the same time.
According to the laboratory results of the present case on admission, an abdominal CT scan revealed no findings such as pancreatic enlargement, poorly defined edges, or ascites retention. In contrast, a mild increase in pancreatic exocrine enzymes was observed. A thyroid gland ultrasound revealed no morphological defects. Despite severe hyperglycemia (602 mg/dL) at the onset of diabetes, HbA1c (5.9%) and GA (18.4%) poorly elevated. Three weeks later (when transferred to our hospital), his glycemic profile changed as follows: blood glucose 642 mg/dL, HbA1c 8.3, and GA 32.9%. Three weeks interval of acute onset of hyperglycemia and poor glycemic control might affect the deterioration of HbA1c and GA levels. Moreover, serum CPR level (0.04 ng/mL) and CPR value (0 ng/mL) by glucagon tolerance testing, indicating depletion of insulin secretion. Pancreatic islet-related auto-antibodies were negative.
In the present case, serological typing was DR15, and DNA typing was DR15 (DRB1*15:01), DQB1*04:02:01/DQB1*06:02:01 by HLA testing. These results showed the resistance to HLA associated with type 1 diabetes [7]. Moreover, HLA DRB1*04:05-DQB1*04:01 [11, 12] was not observed, which provided the potential association with fulminant type 1 diabetes. According to “the diagnostic criteria of fulminant type 1 diabetes [13],” association with HLA DRB1*04:05-DQB1*04:01 was not observed in the reference findings; however, the present case was diagnosed as fulminant type 1 diabetes that appeared almost simultaneously with the introduction of dialysis. No reports were available concerning fulminant type 1 diabetes complicated with dialysis therapy or renal disease. The relationship between fulminant type 1 diabetes and dialysis therapy was not clarified in this case. It seems accordingly that fulminant type 1 diabetes is merely an incidental complication in a HD patient.
In the present case, the clinical course suggested that poor glycemic control was attributable to insulin antibody after insulin administration; however, few reports were available showing the relationship between insulin antibody and poor glycemic control such as in the present case.
The patient did not require much insulin initially, but the insulin dose gradually increased from 24 U to a maximum of 72 U/day within 2 months. Insulin intensification therapy was introduced (at first, insulin lispro as bolus and detemir as basal and then changed insulin glulisine as bolus, glargine as basal); however, his glycemic control did not improve. This showed that changeness of the type of insulin did not effect in this case.
The number of daily exogenous insulin injections has been reported to be a factor influencing insulin antibody production [1]. Insulin intensification therapy could be related to the production of high amounts of insulin antibodies within a short period of time in this patient accordingly.
Hattori et al. reported as follows: (1) insulin antibody developed even in patients who have never used insulin (2.7%), (2) insulin glargine and aspart were more antigenic than other insulin analogs, (3) the time to produce insulin antibody after the start of insulin administration varied from several days to several months [14].
In the present case, the possibility remains as follows: (1) insulin antibody might be produced early after the start of the insulin treatment (he had been administered glargine at the beginning of the treatment), (2) the patient might have insulin antibody before insulin treatment; thereby, a large amount of insulin was required from the early stage of the treatment.
The insulin antibody value was remarkably elevated in the present case. Previous studies reported that insulin antibody observed among insulin users rarely effects on glycemic control [1]. In contrast, as in the present case, several reports in the literature describing poor glycemic control caused by insulin antibody have recently increased [3]. In the present case, glycemic control deteriorated despite strict self-administration of insulin. The main cause could be due to the involvement of insulin antibody as follows: (1) changes in the glycemic control and (2) the qualitative changes of the insulin antibody, as shown in the Scatchard analysis comparing the values before and after treatment with DFPP and PSL. A Scatchard analysis revealed that the patient had low affinity and high binding capacity before treatment, at k1 (affinity); 0.0034 × 108 M−1 and R1 (binding capacity), 112 × 10−8 M, characteristics that seemed similar to the insulin antibody findings in patients with insulin autoimmune syndrome [2]. In cases with poor glycemic control attributable to the appearance of insulin antibody after the administration of insulin, as in the present case, the Scatchard plot results showed insulin antibody characteristics similar to those in patients with insulin autoimmune syndrome [2]. Furthermore, previous reports also found that the insulin activity could be suppressed by increasing the insulin antibody binding capacity, thereby causing hyperglycemia in the daytime the large amount of insulin binding to the antibody in the daytime due to low affinity gradually dissociated in the nighttime, thereby causing nocturnal hypoglycemia [2]. The similar fluctuations of blood glucose concentration were also observed in the CGM in the present case.
A Scatchard analysis revealed “qualitative changes” in the insulin antibodies after DFPP and long-term PSL treatment in the present case, and significant improvement in the reduction of insulin dosage, blood glucose fluctuations (CGM), glycemic control (GA value), and the reduction of insulin antibody binding capacity was observed. The combination therapy of DFPP and long-term PSL treatment was effective. In contrast, the insulin antibody remained high value. These results show the effectiveness of treatment with DFPP, and PSL should be evaluated not only using the insulin antibody value but also using the CGM, GA value, and insulin antibody binding capacity in similar cases.
Although the present case has still been administered PSL with gradual reduction (5 mg/day), the possibility remains that glycemic control might deteriorate attributable to a reduction or discontinuation of PSL in the future. Insulin treatment is essential to the present case; therefore, monitoring long-term glycemic daily profile should be continued. Moreover, fulminant type 1 diabetes has been reported to be a high-risk group prone to the advancement of microvascular diseases attributable to poor glycemic control [15]. Long-term evaluation of daily glycemic profile is necessary for reducing the risk of cardiovascular disease.
Conclusions
In conclusion, the authors present a unique case of fulminant type 1 diabetes developing during the HD introduction period. The present case is rare; however, physicians specializing in endocrinology, nephrology, and dialysis therapy also should keep in mind that this is a life-threatening complication if the treatment is delayed and that concomitant therapy with apheresis such as DFPP and long-term PSL is effective in some cases when glycemic control deteriorates attributable to the appearance of insulin antibody. No established protocol concerning the dosage of steroids, gradual reduction method, or criteria for discontinuation has been available. Further accumulation and investigation of similar cases should be required.
Abbreviations
- ALP:
-
Alkaline phosphatase
- ALT:
-
Alanine aminotransferase
- ANCA:
-
Anti-neutrophil cytoplasmic antibody
- AST:
-
Asparate aminotransferase
- BUN:
-
Blood urea nitrogen
- CA19-9:
-
Carbohydrate antigen 19-9
- CEA:
-
Carcinoembryonic antigen
- CGM:
-
Continuous glucose monitor
- CPR:
-
C-peptide immunoreactivity
- CT:
-
Computed tomography
- DFPP:
-
Double filtration plasmapheresis
- GA:
-
Glycoalbumin
- GAD:
-
Glutamic acid decarboxylase
- HbA1c:
-
Hemoglobin A1c
- HD:
-
Hemodialysis
- HLA:
-
Human leukocyte antigen
- IA-2:
-
Islet antigen-2
- ICA:
-
Islet cell antibody
- IGF:
-
Insulin-like growth factor
- MPO:
-
Myeloperoxidase
- PR3:
-
Protease 3
- PSL:
-
Prednisolone
- RCC:
-
Renal cell carcinoma
- TPO:
-
Thyroid peroxidase
- γ-GTP:
-
Gamma-glutamyl transpeptidase.
References
Van Haeften TW. Clinical significance of insulin antibodies in insulin-treated diabetic patients. Diabetes Care. 1989;12:641–8.
Uchigata Y, Eguchi Y, Takayama-Hasumi S, Omori Y. Insulin autoimmune syndrome (Hirata disease): clinical features and epidemiology in Japan. Diabetes Res Clin Pract. 1994;22:89–94.
Hoshina S, Kondo T, Fujioka M, Gocho N, Togawa A, Tanaka H, et al. A case of type 2 diabetes mellitus with polymyalgia rheumatica successfully treated with long-term corticosteroid administration for nocturnal hypoglycemia possibly caused by insulin antibody. J Japan Diab Soc. 2012;55:470–6 [in Japanese].
Imagawa A, Hanafusa T, Miyagawa J, Matsuzawa Y. A novel subtype of type 1 diabetes mellitus characterized by a rapid onset and an absence of diabetes-related antibodies. N Eng J Med. 2000;342:301–7.
Imagawa A, Hanafusa T. Fulminant type 1 diabetes—an important subtype in East Asia. Diabetes Metab Res Rev. 2011;27:959–64.
Imagawa A, Hanafusa T, Uchigata Y, Kanatsuka A, Kawasaki E, Kobayashi T, et al. Fulminant type 1 diabetes: a nationwide survey in Japan. Diabetes Care. 2003;26:2345–52.
Kawabata Y, Ikegami H, Kawaguchi Y, Fujisawa T, Shintani M, Ono M, et al. Asian-specific HLA haplotypes reveal heterogeneity of the contribution of HLA-DR and -DQ haplotypes to susceptibility to type 1 diabetes. Diabetes. 2002;51:545–51.
Koyama R, Nakanishi K, Kato M, Yamashita S, Kuwahara H, Katori H. Hypoglycemia and hyperglycemia due to insulin antibodies against therapeutic human insulin: treatment with double filtration plasmapheresis and prednisolone. Am J Med Sci. 2005;329:259–64.
Hara K, Tobe K, Uchigata Y, Nakazono M, Yasuda K, Terauchi Y, et al. Antibody-mediated insulin resistance treated by cessation of insulin administration. Intern Med. 2000;39:143–5.
Matsuyoshi A, Shimoda S, Tsuruzoe K, Taketa K, Chirioka T, Sakamoto F, et al. A case of slowly progressive type 1 diabetes with unstable glycemic control caused by unusual insulin antibody and successfully treated with steroid therapy. Diabetes Res Clin Pract. 2006;72:238–43.
Kawabata Y, Ikegami H, Awata H, Imagawa A, Maruyama T, Kawasaki E, et al. Differential association of HLA with three subtypes of type 1 diabetes: fulminant, slowly progressive and acute-onset. Diabetologia. 2009;52:2513–21.
Tsutsumi C, Imagawa A, Ikegami H, Makino H, Kobayashi T, Hanafusa T, on behalf of the Japan Diabetes Society Committee on Type 1 Diabetes Mellitus Research. Class II HLA genotype in fulminant type 1 diabetes: a nationwide survey with reference to glutamic acid decarboxylase antibodies. J Diabetes Investig. 2012;3:62–9.
Imagawa A, Hanafusa T, Awata T, Ikegami H, Uchigata Y, Osawa H, et al. Report of the Committee of the Japan Diabetes Society on the Research of Fulminant and Acute-onset Type 1 Diabetes Mellitus: new diagnositic criteria of fulminant type 1 diabetes mellitus. J Diabetes Investig. 2012;3:536–9.
Hattori N, Duhita MR, Mukai A, Matsueda M, Shimatsu A. Development of insulin antibodies and changes in titers over a long-term period in patients with type 2 diabetes. Clin Chim Acta. 2014;433:135–8.
Murase Y, Imagawa A, Hanafusa T, Iwahashi H, Uchigata Y, Kanatsuka A, et al. Fulminant type 1 diabetes as a high risk group for diabetic microangiopathy—a nationwide 5-year-study in Japan. Diabetologia. 2007;50:531–7.
Acknowledgements
The authors would like to express our sincere thanks to Dr Tomomi Hakoda for giving us valuable advice and the medical staffs of Nippon Kokan Fukuyama Hospital, Hiroshima, Japan for collecting samples of the patient and analyzing the samples. The authors also thank to Mr. Brain Quinn for his skillful English editing.
Funding
Not applicable. Our manuscript does not contain funding.
Availability of data and materials
The data and materials were all included in the manuscript.
Authors’ contributions
KW is responsible for the manuscript. KW and YW performed acquisition of data and interpretation of data, drafted, and revised manuscript. HAU helped to draft the manuscript. All authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Written informed consent was obtained from the patient for publication of this case report and any accompanying images.
Ethics approval and consent to participate
Written informed consent was obtained from the patient. This case report was approved by the Ethical Committee of the Nippon Kokan Fukuyama Hospital (IRB approval number 2016-01) and was conducted in compliance with the Declaration of Helsinki.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Wada, K., Uchida, H.A. & Wada, Y. Poor glycemic control attributable to insulin antibody in a fulminant type 1 diabetes patient under hemodialysis: successful treatment with double filtration plasmapheresis and prednisolone. Ren Replace Ther 3, 14 (2017). https://doi.org/10.1186/s41100-017-0096-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41100-017-0096-9