Abstract
Exogenous insulin antibody syndrome (EIAS) has until recently been a rarely described complication of exogenous insulin therapy. EIAS results not only in hyperglycemia, but also in hypoglycemia and occasionally in ketoacidosis (DKA). The incidence of EIAS is increasing probably due to an overall increase in autoimmunity associated with the coronavirus disease 2019 (Covid-19) epidemic resulting in increasing binding of insulin by antibodies. Herein, we describe a case of EIAS occurring in an elderly patient with longstanding type 1 diabetes mellitus (T1DM) who had progressive loss of glycemic control. It responded positively, as we have previously described, to oral mycophenolate mofetil and the use of soluble regular insulin delivered by continuous subcutaneous insulin infusion (CSII). Therefore, EIAS is an increasingly frequent cause of hyperglycemia with and without DKA, and hypoglycemia in subjects with T1DM. Once diagnosed, they can be treated with mycophenolate mofetil and soluble insulin in an outpatient setting, which will decrease the rate of hospitalization and lower the expense of therapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Exogenous insulin antibody syndrome (EIAS) is being increasingly reported, which may be partially due to the increase in autoimmunity related to the Covid-19 pandemic. |
EIAS may be more likely to develop in older patients with type 1 diabetes mellitus due to the greater propensity of this group to develop autoimmunity. |
EIAS is more likely to develop with designer insulins and particularly with inhaled insulin. |
The use of mycophenolate mofetil in conjunction with soluble insulin is an economic outpatient approach to the therapy of EIAS. |
Introduction
Exogenous insulin antibody syndrome (EIAS) is a rare autoimmune syndrome characterized by the presence of high titers of anti-insulin antibodies. These antibodies bind to both exogenous and endogenous insulin, causing hyperglycemia and occasionally DKA. With saturation of the antibodies with insulin, unpredictable rises in insulin levels occur, causing hypoglycemia, which is often severe. This may occur in persons with both T1DM and T2DM [1, 2]. In T2DM, withdrawal of insulin therapy and replacement with other antidiabetic agents is the recommended treatment. If necessary, this first step is followed by diazoxide, which blocks endogenous insulin release. In both T1DM and T2DM, the use of an alpha glucosidase inhibitor to slow absorption of glucose may be helpful. [3]
In T1DM, immunosuppression or high-dose steroids may be utilized, however, high-dose steroids are associated with an increased insulin requirement sometimes associated with DKA [3]. The use of intravenous immunosuppressives requires hospitalization or in-center administration, which is costly. In severe cases, plasmapheresis may be necessary to remove antibodies, but again, this a very high-cost procedure requiring multiple treatments.
Previously, we have described successful treatment of a patient with TIDM and EIAS developing at the time of DKA following a Covid-like illness 2 weeks earlier. She developed a second episode of DKA while on > 200 units of insulin a day. Insulin antibodies were strongly positive and she was changed to CSII. She continued to require large amounts of insulin and immunotherapy was initiated using mycophenolate mofetil. The treatment plan was to use mycophenolate with rituximab to be started as an outpatient. However, she had a dramatic response to mycophenolate alone, such that a second immunosuppressive was not necessary. We documented that mycophenolate, which is oral, inexpensive and may be given on an outpatient basis, is a single immunosuppressive option for EIAS [4]. Since we published this case description, there have been other reports of using this therapy in pediatric patients. [5, 6]
In this report, we describe another patient with type 1 diabetes and EIAS who responded to mycophenolate. In this case, the patient was 84 years old and had T1DM for 34 years when EIAS was diagnosed. We suspect but cannot prove that both of these cases were associated with the Covid epidemic and represent the overall increase in autoimmunity caused by this epidemic [7,8,9]. In particular, multiple reports indicate an increased incidence of TIDM in children following Covid infection. [10,11,12]
Written informed consent to publish was provided by the patient prior to the development and production of this paper (Fig. 1).
Case Report
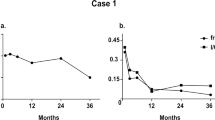
Our patient is an 84-year-old woman with past medical history significant for diabetes diagnosed at the age of 50 years. She never responded to orals and was placed on N insulin and later on N and R insulins. She was referred for endocrine consultation 2 years ago due to progressively worsening control. She was on NPH human insulin once daily and PRN aspart insulin, which was usually taken once a day. Her C-peptide was undetectable, confirming diagnosis of T1DM. Her case is complicated by retinopathy, CKD 3A, and extensive peripheral vascular disease. She initially responded to weight-based degludec and mealtime aspart with a reduction in her HgbA1C, but she began to have hypoglycemia in the late afternoon and during the night, with rapid rise in her sugar after 4 AM. This was much better defined after she was placed on a constant glucose monitoring system (CGMS). As the low glucoses were either nocturnal or after a 6-hour lag between meals, they were felt to be secondary to long-acting insulin. Therefore, her degludec was progressively decreased and her aspart was progressively increased. Her insulin requirements, especially at breakfast and lunch, continued to increase until she was receiving 44 units of aspart at breakfast without any decrease in her pre-lunch values. It seemed possible that she might not be taking her insulin, and so for 3 days all injections were observed by her family members without any change in glycemic control. Immediately prior to admission she did admit to taking her insulin at the end of her meal rather than prior to eating. Despite changing to preprandial dosing, her serum insulin level was undetectable 2 h after injection of a dose of 44 units aspart. Review of her chart indicated marked deterioration in control beginning in November 2022. This resulted in a 20-pound weight loss and consecutive HgbA1C values of greater than 10%. A fructosamine level was proportionally elevated at 501 umol/L (normal 0–285 umol/L). The presumptive diagnosis of exogenous insulin antibody syndrome was made and the patient was admitted to the hospital to test this hypothesis by using intravenous insulin. Her HgbA1C was 10.1% and her weight had declined to 130 pounds on the day of hospital admission (Table 1; Fig. 2).
Patients with EIAS respond normally to intravenous insulin [1,2,3]. On the morning of admission, the patient took 11 units of degludec and 44 units of aspart. On arrival to the hospital, her glucose was 253 mg/dl. Betahydroxybutarate was negative and the anion gap was normal. She was placed on intravenous (IV) insulin in the medical–surgical intensive care unit (MSICU) using the EndoTool glucose management system. In the first 24 h her glucose dropped into the 100–180 mg/dl range and she required only 12 units of intravenous human regular insulin. No insulin was given to cover meals during the first 24 h. She had a slight dip in glucose at 4 PM and her glucose began climbing at 2 AM but the maximal glucose on the EndoTool was 197 mg/dl after breakfast. She was requiring about 2 units of human R insulin/h intravenously. Her immunoglobulin G (IgG) insulin antibody measurement was 22 μU/mL (normal < 4 μU/mL), which is greater than 5× the upper limit of normal (ULN) and indicates a strongly positive result.
On hospital day 2, insulin drip was stopped and trial of subcutaneous human regular insulin attempted, however, patient immediately became hyperglycemic once again in the 400 mg/dl range. Patient was initiated on immunosuppression with mycophenolate mofetil 500 mg by mouth twice daily. She tolerated this medication well. Insulin drip was resumed and continued until noon of hospital day 3. On hospital day 3, the patient underwent trial of subcutaneous insulin for her mealtime coverage, and she responded to 10 units of human regular insulin at meals with post-meal glucoses of < 240 mg/dl. A loaner insulin pump was placed, loaded with human regular insulin, and programmed at a basal rate of 2 units/h. The patient was initially well controlled on 2 units basal insulin per h, however, after 8 h she became hypoglycemic with a glucose of 66 mg/dl. Basal rate and prandial insulin doses were reduced by 30%. Patient continued in ICU overnight for close blood sugar monitoring.
On hospital day 4, the patient was discharged and presented to her endocrinologist’s office, where she had a personal insulin pump placed with the same basal rate. She was instructed to give herself 6-unit boluses with meals. Her correction factor was 50 mg/dl over a target of 125 mg/dl. Unfortunately, there was a delay from the patient’s pharmacy in getting the mycophenolate, and so the patient went from the morning of her day of discharge to the night of her second day at home without the immunosuppressive drug, resulting in two doses missed. The endocrinology clinic called her daily for reports on her CGMS, and during this period of time without mycophenolate, her blood glucose once again climbed to 300 s–400 mg/dl. Patient resumed mycophenolate on the evening of day 2 following discharge. By the third day after discharge, following four doses of mycophenolate, blood glucose returned to 150 s–low 200s.
At her clinic visit 2 weeks following discharge, pump basal insulin was increased by 5% and meal time boluses were increased to 8 u. Weight was 139 pounds having, increased from 130 pounds. Lab was drawn for anti-islet cell antibodies, anti-GAD 65 antibodies, and anti-zinc transporter 8 antibodies. Result of re-measurement of insulin antibodies revealed insulin antibodies were 18 μU/mL, which represented an 18% reduction. Anti-islet cell antibodies and zinc transporter 8 antibodies were not detected. IAA2 antibodies were also not detected. Anti-GAD 65 levels were elevated at 25.2 U/mL (normal < 5), which seemed unusual after over 30 years of T1DM.
A month following her initial discharge, the patient was readmitted to the medical ICU in DKA due to interruption of her insulin pump delivery and pneumonia. She ran out of supplies for her insulin pump on a Saturday and was unable to pick up a refill. She used injections of human regular insulin with meals. She had done this in the past without any adverse sequelae prior to diagnosis and treatment of EIAS. Following management of DKA, she was resumed on insulin pump therapy and mycophenolate. Her blood glucose once again stabilized on mycophenolate and her insulin pump.
Then, 2 weeks after discharge from rehabilitation, her sensor indicated she had 55% time in range (TIR), 17% glucoses less than 70 mg/dl, which were primarily seen during her night rate, 25% above 180 mg/dl and 3% above 240 mg/dl. Her glucose management indicator (GMI) was 6.7%. Her fructosamine was 301 μmol/L (Fig. 3). Her night rates were decreased and her meal time insulin was adjusted. She was on 54 units of insulin per day, but after adjustment she is receiving 50 units a day. Insulin antibody titers and insulin requirements have continued to decline. The plan is for her to remain on mycophenolate for at least 6 months with reassessment of insulin antibodies to determine whether it needs to be continued long term.
Discussion
The development of insulin antibodies in people without diabetes (Hirata’s disease) was first described in 1970 and is also known as insulin antibody syndrome (IAS). IAS is the third leading cause of hypoglycemia in Japan behind insulinoma and nonpancreatic cancers [13,14,15,16]. The hallmark of IAS is a high titer of insulin antibodies plus a high insulin/C-peptide ratio [17]. IAS has been associated with utilization of methimazole for hyperthyroidism [18, 19] and with the use of alpha-lipoic acid [1, 20]. IAS is clearly an autoimmune disease with a common genetic haplotype, at least in Asia where it is associated with the haplotype DRB1*04:06. This haplotype is uncommon in Caucasian populations, explaining why most IAS cases are found in Asia [14]. Several cases of IAS have been reported since the beginning of the Covid-19 epidemic and are felt to be secondary to infection with the virus. [21,22,23]
EIAS, on the contrary, is not linked to drug therapy and is not associated with polyendocrine autoimmune diseases but has increased since the Covid epidemic. In addition, as in this case, autoimmune diseases may occur in older individuals, particularly older female patients. This may be because adult patients with T1DM differ genetically from younger type 1 subjects [24]. Furthermore, autoimmunity persists in older patients, with 35% having anti-GAD65 antibodies after 14 years [25]. In this case, the patient was GAD65 positive after 34 years of T1DM.
In EIAS, all forms of insulin have the ability to engender insulin antibodies. Older insulins such as regular insulin seem to be less antigenic than “designer insulins” and inhaled insulins, which are the most antigenic [26]. In a single case report, the fast-acting insulin glulisine improved diabetic control in a patient with EIAS [27]. However, in this case the use of soluble regular insulin with CSII was effective in treating EIAS.
Earlier cases of EIAS were treated with high-dose steroids, which by increasing insulin resistance, caused further hyperglycemia despite the decreasing levels of insulin antibodies. Rituximab, a CD20 specific recombinant monoclonal antibody agent approved to treat cancers, has been used successfully to treat EIAS, as has plasmapheresis. However, both treatments require hospitalization or specialized units to deliver either of these therapies, which is cost prohibitive for many patients. As we have previously reported, because of the efficacy of the oral and inexpensive mycophenolate mofetil, which can be utilized in the outpatient setting, we suggest that this should now be the drug of choice for treatment of EIAS. The ultimate goal of therapy as seen in the first case is slow weaning with serial evaluation of insulin antibody titers and discontinuation by 6 months of therapy. In addition, mycophenolate mofetil has few severe side effects with the exceptions of hepatitis and progressive multifocal leukoencephalopathy [28,29,30].. The primary mechanism of action is decrease in B cell proliferation and plasmablast formation, culminating in lower antibody production [31].
The unique feature of this case is the development of severe hyperosmolar diabetic ketoacidosis after reduction in antibodies with mycophenolate mofetil, which has not previously been reported with EIAS. In addition, this patient had not previously developed DKA during 34 years of T1DM. Undoubtedly, the DKA was due to extremely low free insulin levels that could not decrease free fatty acid release from adipocytes and the formation of acetone, betahydroxybutarate, and ketone bodies.
In conclusion, we have described the development of EIAS in an elderly established patient with T1DM, which may have been due to exposure to Covid. This is also the first report of DKA accompanying the treatment of EIAS. Use of mycophenolate mofetil in EIAS will reduce the cost of treating EIAS and avoid hospitalization.
References
Li Z, Yi D, Zheng L, et al. Analysis of the clinical characteristics of insulin autoimmune syndrome induced by exogenous insulin in diabetic patients. Diabetol Metab Syndr. 2021;13:38. https://doi.org/10.1186/s13098-021-00658-z.
Hu S, Chen F. Exogenous insulin antibody syndrome (EIAS); a clinical syndrome associated with insulin antibodies induced by exogenous insulin in diabetic patients. Endocr Connect. 2018;7:R47–55. https://doi.org/10.1530/EC-17-0309.
Church D, Cardoso L, Kay RG, Williams CL, et al. Assessment and management of anti-insulin autoantibodies in varying presentations of insulin autoimmune syndrome. JCEM. 2018;103(10):3845–55. https://doi.org/10.1210/jc.2018-00972.
Jerkins T, Bell DSH. Development of exogenous insulin antibody syndrome in a patient with newly diagnosed type 1 diabetes successfully treated with oral immunosuppressive monotherapy. Diabetes Ther. 2021;12(10):2795–9. https://doi.org/10.1007/s13300-021-01129-4. (Epub 2021 Aug 17).
Saba L, Orandi AB, Pittock S, et al. 149-LB: pediatric exogenous insulin antibody syndrome—Successful treatment with mycophenolate mofetil. Diabetes. 2022;71(Supplement 1):149-LB. https://doi.org/10.2337/db22-149-LB.
Saba L, Fatica E, Orandi A, Pittock S. Exogenous insulin antibody syndrome in a pediatric patient: successful treatment with mycophenolate mofetil. Horm Res Pediatr. 2023. https://doi.org/10.1159/000531767.
Chang R, Yen-Ting Chen T, Wang SI, Hung YM, Chen HY, Wei CJ. Risk of autoimmune diseases in patients with COVID-19: a retrospective cohort study. EClinicalMedicine. 2023;56: 101783. https://doi.org/10.1016/j.eclinm.2022.101783. (Epub 2023 Jan 10).
Liu Y, Sawalha AH, Lu Q. COVID-19 and autoimmune diseases. Curr Opin Rheumatol. 2021;33(2):155–62. https://doi.org/10.1097/BOR.0000000000000776.
Chen Y, Xu Z, Wang P, Li XM, Shuai ZW, Ye DQ, Pan HF. New-onset autoimmune phenomena post-COVID-19 vaccination. Immunology. 2022;165(4):386–401. https://doi.org/10.1111/imm.13443. (Epub 2022 Jan 7).
Weiss A, Donnachie E, Beyerlain A. Type 1 diabetes incidence and risk in children with a diagnosis of Covid-19. JAMA. 2023;329(23):2089–91. https://doi.org/10.1001/jama.2023.8674.
Clemens K, Holl RW, Rosenbauer J. Elucidating the underlying mechanisms of the marked increase in childhood type1 diabetes during the Covid-19 pandemic—The diabetes pandemic. JAMA Netw Open. 2023;6(6): e2321231. https://doi.org/10.1001//jamanetworkopen.2023.21231.
Barrett CE, Koyana AK, Alvarez P, et al. Risk for newly diagnosed diabetes > 30 days after Sars-CoV-2 infections among persons <18 years—United States March 1, 202–June 28, 2021. MMWR Morb Mortal Wkly Rep. 2022;71:59–65.
Cappellani D, Macchia E, Falorni A, Marchetti P. Insulin autoimmune syndrome (Hirata disease): a comprehensive review fifty years after its first description. Diabetes Metab Syndr Obes. 2020;13:963–78. https://doi.org/10.2147/DMSO.S219438.
Hirata Y, Ishizu H, Ouchi N. Insulin autoimmunity in a case of spontaneous hypoglycemia. J Jpn Diabetes Soc. 1970;13(3):312–20.
Ismail AA. The insulin autoantibody syndrome (IAS) as a cause of hypoglycemia: an update of pathophysiology biochemical investigations and diagnosis. Clin Chem Lab Med. 2016;54(11):1715–24. https://doi.org/10.1515/cclin-2015-1255T.
Takayama-Hasumi S, Eguchi Y, Sato A, et al. Insulin autoimmune syndrome is the third leading cause of spontaneous hypoglycemic attacks in Japan. Diabetes Res Clin Pract. 1990;10(3):211–4. https://doi.org/10.1016/0168.8227(90)90063-y.
Hua K-F, Jing B-Y, Wu Y-H. The application of the insulin to c-peptide molar ratio in primary screening for insulin antibodies in type 2 diabetes mellitus patients: a further quantitative study on the relationship between ICPR and insulin antibodies. Diabetes Metab Syndr Obes. 2023;16:1121–32. https://doi.org/10.2147/DMSO.S4004222.
Uchigata ZY, Hirata Y, Iwamoto Y. Drug induced insulin autoimmune syndrome. Diabetes Res Clin Pract. 2009;83(1):e19–20.
Hirata Y. Methimazole and insulin autoimmune with hypoglycemia in Grave’s disease. Ann Inter Med. 1974;81(2):214–8. https://doi.org/10.1016/50140-6736(83)91031-0.
Takeuchi Y, Miyamoto T, Kakizawa T, et al. Insulin autoimmune syndrome possibly caused by alpha lipoic acid. Intern Med. 2007;46(5):237–9. https://doi.org/10.2969/internalmedicine.46.1893.
Corona-Meraz FI, Quintero-Castillo BP, Henandez-Palm LA, et al. Long covid-19 and insulin autoimmune syndrome: a case report. Clin Ther. 2023;45(9):e187–92. https://doi.org/10.1016/j.clinthera.2023.06.026. (Epub 2023 Jul 29).
Sehemby MK, Lila AR, Sarathi V, Bandgar T. Insulin autoimmune hypoglycemia syndrome following coronavirus disease 2019 infection. Indian J Endocrinol Metab Case Rep. 2023;1(1):5–8. https://doi.org/10.4103/ijemcr.ijemcr_3_22.
Alabbood M, Chamberlain M, Penner H. Abstract #1602427: Post-covid-19 autoimmune hypoglycemia: a case report. Endocr Pract. 2023;29(12 supplement):S145–6.
Hughes JW, Bao YK, Salam M, Joshi P, et al. Late-onset T1DM and older age predict risk of additional autoimmune disease. Diabetes Care. 2019;42(1):32–8. https://doi.org/10.2337/dc18-1157. (Epub 2018 Oct 25).
Tridgell D, Spiekerman C, Wang RS, Greenbaum CJ. Intersection of and duration in percent of GAD and IA-2 antibody-positive in the diabetes genetics consortium database. Diabetes Care. 2011;34(4):988–93. https://doi.org/10.2337/dc10-1903.
Maneschi F, Fineberg SE, Kohner EM. Successful treatment of immune-mediated insulin resistance by human insulin (recombinant DNA). Diabetes Care. 1982;5(Supplement 2):175–9. https://doi.org/10.2337/diacare.5.2.S175.
Otoh A, Saisho Y, Mitsuishi M, et al. Insulin glulisine may ameliorate nocturnal hypoglycemia related to insulin antibody—A case report. Diabetes Res Clin Pract. 2011;94:e53–4. https://doi.org/10.1016/j.diabres.2011.04.001. (discussion e55).
Allison AC. Mechanisms of action of mycophenolate mofetil. Lupus. 2005;14(Suppl o081):s2-8. https://doi.org/10.1191/0961203305lu2109oa.
Takeuchi T, Hashimoto H, Masumoto M. Long-term safety and effectiveness of mycophenolate mofetil in adults with lupus nephritis: a real-world study in Japan. Mod Rheumatol. 2022;32(4):746–54. https://doi.org/10.1093/mr/roab076.
Saravanakumar K, et al. The safety and efficacy of mycophenolate mofetil in children and adolescents with steroid-dependent nephrotic syndrome: a single-centre study. Clin Kidney J. 2019;13(2):179–83. https://doi.org/10.1093/ckj/sfz061.
Eickenberg S, Mickholz E, Jung E, et al. Arthritis Res Therapy. 2012;14:R110. http://arthritis-research.com/content/14/3/R110.
Funding
This research was supported (in whole or in part) by HCA Healthcare and/or an HCA-Healthcare-affiliated entity. The views expressed in this publication represent those of the author(s) and do not necessarily represent the official views of HCA Healthcare or any of its affiliated entities. No funding was provided for the publication of this article.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Dr. David Bell is an editorial board member of Diabetes Therapy. Dr. Bell was not involved in the selection of peer reviewers for the manuscript nor any of the subsequent editorial decisions. He has no other conflicts of interest to declare. Dr. Terri Jerkins has received funding from NovoNordisk, Corcept, and Bayer Pharmaceuticals as a speaker, and she has research funding from AbbVie, Crinetics, and NovoNordisk Pharmaceuticals. Dr. Katherine Stockham as no conflicts of interest to declare.
Ethics Statement
Written informed consent to publish was provided by the patient prior to the development and production of this paper.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Jerkins, T., Stockham, K. & Bell, D.S.H. Exogenous Insulin Antibody Syndrome (EIAS) Presenting in an Elderly, Long-Term Patient with Type 1 Diabetes Mellitus that Resolved with Low-Cost Outpatient Therapy with Mycophenolate Mofetil and Regular Insulin by Pump. Diabetes Ther 15, 1473–1481 (2024). https://doi.org/10.1007/s13300-024-01573-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13300-024-01573-y