Abstract
Background
Spindle and kinetochore‑associated complex subunit 3 (SKA3) has recently been considered a key regulator of carcinogenesis. However, the connection between SKA3 and immune cell infiltration remains unknown.
Methods
The current study investigated the expression mode, prognostic effect, and functional role of SKA3 in different tumors, particularly bladder cancer using numerous databases, comprising TIMER, GEPIA, HPA, UALCAN, PrognoScan, and Kaplan–Meier Plotter. Differentially expressed gene and enrichment analyses were implemented on SKA3 using R packages "edgR" and "clusterProfiler". Immunohistochemistry was further used to validate the expression of SKA3 gene in bladder cancer. Following that, the relevance of SKA3 expression to immune infiltration level in bladder cancer was evaluated using TIMER.
Results
Overall, the level of SKA3 expression in tumor tissue significantly increased than in normal tissue. In bladder cancer and other tumors, patients with high SKA3 expression levels had worse overall survival (OS) (p = 0.016), disease-specific survival (DSS) (p = 0.00004), and disease-free survival (DFS) (p = 0.032). Additionally, the major molecular functions for SKA3 included nuclear division, mitotic nuclear division, mitotic sister chromatid segregation, humoral immune response, and cell chemotaxis. Additionally, SKA3 expression was found to be positively associated with enhanced M2 macrophage and T helper (Th) 2 cell infiltration in bladder cancer.
Conclusions
Our study implies that SKA3 contributes to M2 macrophage and Th2 cell polarization by acting as an oncogene in bladder cancer. SKA3 might be a novel biomarker for evaluating prognosis and immune infiltration in bladder cancer.
Similar content being viewed by others
Introduction
Bladder cancer is the tenth most common carcinoma worldwide, accounting for 3% of all cancer patients [1]. Despite novel treatment strategies, the 5-year survival rate of muscle-invasive bladder cancer does not surpass 70% in the US [2]. Tumor biomarkers are a new kind of investigator that enables clinicians to grasp tumor macroenvironment and microenvironment, allowing for early cancer diagnosis, improved outcomes, and the use of targeted therapy [3, 4]. At present, bladder cancer suffers from a shortage of valid early tumor biomarkers and diagnostic tools. Bladder urothelial carcinoma is deemed an immunogenic malignancy due to its comparatively high tumor mutational load and reactivity to Bacillus Calmette–Guerin treatment and immune checkpoint drugs. As a result, there is a pressing demand for the certification of new immune tumor biomarkers in bladder cancer.
SKA3 is an element of SKA complex, which affects maintaining kinetochore-microtubule interaction in mitosis [5,6,7]. SKA3 overexpression has been linked to the occurrence of various cancers. Research exhibited that SKA3 was somatically mutated in breast cancer, and its ectopic expression promoted tumor progression [8]. SKA3 overexpression was confirmed in renal cell carcinoma clinical specimens [9]. SKA3 expression is enhanced in liver cancer tissue compared to normal liver tissue, and inhibiting SKA3 expression considerably weakens the proliferation of liver cancer cells [10]. Moreover, SKA3 overexpression causes colorectal adenoma to progress to cancer, whereas knocking out SKA3 in colorectal adenoma cells greatly reduces cell growth rate, induces G2/M block, and reduces cell migration and invasion [11]. The above findings indicate that SKA3 develops a significant function in engendering and proliferation of tumors and is a potentially attractive anti-cancer target. However, the role of SKA3 in bladder cancer remains unknown.
We employed databases such as TIMER, GEPIA, UALCAN, PrognoScan, and Kaplan–Meier plotter for all-around assessment of SKA3 expression and its correlation with cancer patient prognosis. Additionally, SKA3 was predicted to be involved in nuclear division, mitotic nuclear division, mitotic sister chromatid segregation, humoral immune response, and cell chemotaxis.The high expression of SKA3 in bladder cancer was further verified using immunohistochemistry. In addition, we utilized TIMER to investigate the relationship between SKA3 and immune infiltration in bladder tumor microenvironments. Our findings reveal a potential practical function of SKA3 in bladder cancer and emphasize a machine-made foundation of SKA3 influences on M2 macrophage and Th2 cell polarization in tumor microenvironment.
Materials and methods
SKA3 expression analysis in Human Cancers
The expression status of SKA3 in various cancers was clarified using TIMER (http://cistrome.org/TIMER/) [12]. SKA3 expression data were obtained and compared in bladder cancer and normal tissue using GEPIA (Gene Expression Profiling Interactive Analysis, http://gepia.cancer-pku.cn/index.html). GEPIA is a dependable web server for tumors and normal gene expression profiles and interactive analyses [13]. To further judge the expression level of SKA3 in bladder cancer, the protein expression of SKA3 gene was compared between normal bladder urothelial tissue and bladder urothelial carcinoma tissue using immunohistochemistry provided by HPA database (Human Protein Atlas, https://www.proteinatlas.org/). HPA is a maximum and most detailed database aimed at assessing protein expression state in human tissues and cells, including Tissue Atlas, Pathology Atlas, and Cell Atlas [14]. UALCAN database (http://ualcan.path.uab.edu/) was employed to investigate SKA3 expression in different molecular subtypes and TP53 mutations. UALCAN is a user-friendly and interactive web resource for analyzing cancer OMICS data. It is built on PERL-CGI and provides easy access to publicly available TCGA data [15].
PrognoScan and Kaplan–Meier Plotter database analysis
The correlation between SKA3 expression and survival in pan-cancer was analyzed in PrognoScan (http://dna00.bio.kyutech.ac.jp/PrognoScan/index.html) and Kaplan–Meier plotter (https://www.kmplot.com/). Specifically, SKA3 expression level was searched in all available microarray datasets of PrognoScan to determine its relationship with prognosis [16]. The threshold was set as a Cox P-value < 0.05. Kaplan–Meier plotter is a powerful online tool that can evaluate the impact of 54,000 genes on survival in 21 cancer types from TCGA database [17]. We investigated the relevance between SKA3 expression level and OS and DFS in KIRP, BLCA, OVC, BRCA, LUAD, and COAD (Supplementary Table 1). Hazard ratios (HRs) and log-rank P-values were calculated.
DEG and enrichment analyses
Using "limma" package in R software (version 4.1.1, www.r-project.org), we divided TCGA-BLCA samples into quartiles based on SKA3 expression [18]. The group with the lowest 25% of SKA3 expression was defined as the low expression group, and the group with the highest 25% was defined as the high expression group. Then, "edgeR" package was used to screen the significant differentially expressed genes (DEGs) in low and high expression groups of SKA3 based on | log2fc |> 1 and Benjamin Hochberg adjusted P < 0.01. Gene ontology (GO) analysis was executed via EnrichGO effect in R package "clusterProfiler". Kyoto encyclopedia of genes and genomes (KEGG) analysis was executed via EnrichKEGG effect in R package "clusterProfiler".
Immunohistochemistry
Twenty-five bladder cancer tissues and 17 chronic inflammatory tissues of the bladder mucosa collected from the Affiliated Qingdao Municipal Hospital of Qingdao University were subjected to immunohistochemistry staining to detect the expression of SKA3. Immunostaining was evaluated by two pathologists from the pathology department. Staining for each of the proteins was scored using the methods of modified immunoreactive score (IRS). The intensity of staining was scored as 0 negative/weak; 1 moderate; 2 strong. The percentage of positive expression was categorized as: 1 (< 25%); 2 (25–50%); 3 (50–75%); 4 (75–100%). The scores of staining intensity and staining range were subsequently multiplied to generate IRS. Finally, the IRS of bladder cancer tissues and chronic inflammatory tissues of the baldderl mucosa was compared using an independent sample t-test using SPSS 26.0. The threshold for statistical significance was P < 0:05. The Ethics Committee of the Affiliated Qingdao Municipal Hospital of Qingdao University approved the above experiments.
Immune cell infiltration
The correlation between SKA3 expression level and immune infiltration was verified using TIMER database [12]. TIMER is a classical and authoritative database, which utilizes a deconvolution beforehand established statistical means and analyzes and visualizes the abundance of immune cell infiltration in a specific tumor type [19]. We researched the connection between SKA3 expression and immune infiltrates level, containing B cells, CD4+ T cells, CD8+ T cells, macrophages, neutrophils, and dendritic cells. In addition to the general analysis of immune infiltration, we gathered about 40 distinct immune checkpoint genes and investigated the relationship between SKA3 expression level and immune gene expression in pan-cancer. Furthermore, we investigated the link between SKA3 expression level and a few immune cell markers. These gene markers include markers of Th2 cells and M2 macrophages [20]. Scatterplots were employed to reflect the correlation between SKA3 and each immune gene marker.
Data collection and statistical analysis
RNA sequencing data, gene expression data, and corresponding clinical information of TIMER, GEPIA, UALCAN, and Kaplan–Meier plotter databases were provided by TCGA database, and Prognoscan data were provided by GEO database. The inclusion and exclusion criteria of patients were implemented using TCGA and GEO databases. Survival curves were generated using PrognoScan and Kaplan–Meier plots. The results of Kaplan–Meier plots, PrognoScan, and GEPIA are displayed with HR and P or Cox P-values from a log-rank test. In Kaplan–Meier plots, patients were divided into high and low expression groups according to the median. In PrognoScan, patients were divided into high and low expression groups according to the best cutoff, which is derived by computing all potential cutoff values between lower and higher quartiles and using the best performing threshold as a cutoff. Gene expression data (FPKM normalized) for bladder cancer tissue samples in the Cancer Genome Atlas (TCGA) were downloaded from Genome Data Commons (https:// portal.gdc.cancer.gov). For TCGA dataset, RNA sequencing data (FPKM values) were transformed into transcripts per kilobase million (TPM) values and then normalized using Z-score method. P-values < 0.05 were considered statistically significant.
Results
High SKA3 expression in bladder cancer
To investigate the distribution of SKA3 expression in different tumors, SKA3 expression in each cancer type is compared to that of normal tissue samples (reference control) in TIMER database. The discrepancy in SKA3 expression levels between tumors and corresponding normal tissues is depicted in Fig. 1A. SKA3 levels were obviously higher in BLCA, BRCA, CESC, CHOL, COAD, ESCA, GBM, HNSC, KIRC, KIRP, LIHC, LUAD, LUSC, PAAD, PCPG, PRAD, READ, STAD, THCA, and UCEC (Supplementary Table 1) than in their respective normal tissues. Interestingly, none of tumors expressed at a lower level than their normal tissues counterparts.
Pan-cancer SKA3 expression analysis. A SKA3 expression level in tumor and normal tissues in TCGA pan-cancer data using TIMER. B The comparison of RNA-seq expression levels between bladder cancer tissue (n = 404) and normal control tissue (n = 28), *indicates a significant difference (p < 0.05). C SKA3 protein level in normal urinary bladder tissues (quantity: none; intensity: negative; staining: not detected; antibody HPA03972). D SKA3 protein level in bladder urothelial carcinoma (quantity: 75%-25%; intensity: weak; staining: low; antibody: HPA03972). E SKA3 expression based on molecular subtypes on BLCA. F SKA3 expression based on TP53 mutation status of BLCA. *P < 0.05, **P < 0.01, ***P < 0.001
In addition, RNA-seq of SKA3 from TCGA bladder cancer dataset and GTEx dataset revealed that SKA3 expression was obviously higher in bladder cancer tissue (n = 404) than in normal bladder samples (n = 28) (Fig. 1B). Additionally, we confirmed SKA3 expression in bladder cancer using immunohistochemical staining in HPA database. SKA3 protein expression was enhanced in bladder urothelial carcinoma tissue than normal bladder urothelial tissue in Figs. 1C and D. In contrast to normal bladder tissue, the results confirmed that SKA3 was overexpressed in bladder cancer tissues. We further analyzed SKA3 expression in various molecular subtypes and TP53 mutation using TCGA bladder cancer dataset. The results proclaimed that SKA3 expression was much higher in neuronal type than in other four subtypes, and SKA3 expression was also higher in TP53 mutant type than in TP53 nonmutant type (Figs. 1E and F).
Correlation between cancer patient prognosis and SKA3 expression level
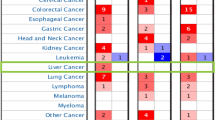
Then, we explored the prognostic value of SKA3 in various kinds of carcinomas using two powerful databases. In PrognoScan, we investigated the association between SKA3 expression level and the prognosis of multiple cancers. Notably, SKA3 expression was significantly associated with seven kinds of cancers, including bladder, lung, ovarian, colorectal, breast, brain, and skin cancers (Fig. 2). In particular, SKA3 developed a pernicious effect on six kinds of these cancers, including bladder (OS: Cox P = 0.016, HR = 1.51, gross number = 165; DSS: Cox P = 0.00004, HR = 2.41, gross number = 165), lung [OS: Cox P = 0.00019, HR = 1.53, gross number = 117; RFS (relapse-free survival): Cox P = 0.000006, HR = 3.18, gross number = 204], ovarian (OS: Cox P = 0.03, HR = 1.61, gross number = 110), breast (DSS: Cox P = 0.0087, HR = 2.04, gross number = 236),brain(OS: Cox P = 0.005, HR = 2.07, gross number = 77), and skin cancers (OS: Cox P = 0.00084, HR = 4.48, gross number = 38). Meanwhile, SKA3 developed a conservatory effect in colorectal cancer (DFS: Cox P = 0.0385, HR = 0.60, gross number = 226).
Kaplan Meier survival curve assessed the relationship between SKA3 expression level and prognosis in Prognoscan. A, B OS (overall survival) and DSS (disease-specific survival) in cohort GSE13507 of bladder cancer. C OS in cohort GSE13213 of lung cancer. D RFS (relapse-free survival) in cohort GSE31210 of lung cancer. E OS in cohort GSE31210 of ovarian cancer. F DFS (disease-free survival) in cohort GSE14333 of colorectal cancer. G DSS in cohort GSE3499-GPL97 of breast cancer. H OS in cohort GSE4271-GPL97 of brain cancer cohort. I OS in cohort GSE19234 of skin cancer
Meanwhile, Kaplan–Meier curve analysis was executed to investigate SKA3-related survival using Kaplan–Meier plotter and the Cancer Genome Atlas (TCGA) datasets because PrognoScan information is primarily extracted from Gene Expression Omnibus (GEO) database. Notably, we unexpectedly found SKA3 as a pernicious prognostic element in KIRP (OS: HR = 2.6, log-rank P = 0.0024; DFS, HR = 3, log-rank P = 0.00024) (Figs. 3A and B). For LUAD, SKA3 was identified to play a detrimental factor (OS: HR = 1.9, log-rank P = 2.1e-05). However, SKA3 had no obvious significance in LUAD (DFS: HR = 1.3, log-rank P = 0.1) (Figs. 3C and D). The findings for breast, colorectal, and ovarian cancers were inconsistent with those on PrognoScan, as high SKA3 expression levels had no discernible effect on the prognosis of three cancers, including BRCA (OS: HR = 1.2, log-rank P = 0.23) (Fig. 3F), COAD (DFS: HR = 1.1, log-rank P = 0.61) (Fig. 3G), and OVC (OS: HR = 0.95, log-rank P = 0.67) (Fig. 3H). Specifically, only BLCA (DFS: HR = 1.8, log-rank P = 0.032) (Fig. 3E) results were consistent with previous predictions using PrognoScan. Consequently, high SKA3 expression is an independent risk factor for bladder cancer patients, rather than a protective factor.
Kaplan–Meier survival curves evaluated the connection between SKA3 expression and prognosis in various cancer kinds using Kaplan–Meier plotter. A, B OS and DFS survival curves of KIRP. A, B OS and DFS survival curves of LUAD. E DFS survival curves of BLCA. F OS survival curves of BRCA. G DFS survival curves of COAD. H OS survival curves of OVC
Gene ontology and KEGG enrichment analysis
To predict SKA3 function, TCGA-BLCA samples were divided into two groups according to their SKA3 expression quartile. Differentially expressed gene analysis was performed to determine 847 differentially expressed genes (DEGs) (|log2FC|> 1, Benjamin Hochberg adjusted P < 0.01). All genes were also displayed in a volcano plot to illustrate their distribution (Fig. 4A). To explore the latent functions associated with 847 genes, they were divided into upregulated group and downregulated group, and the GO enrichment analysis was conducted using biological process, molecular function, and cellular component in bubble plots (Fig. 4B and D). The results revealed that SKA3 was primarily linked to, nuclear division,organelle fission,mitotic nuclear division,mitotic sister chromatid segregation in upregulated group; and linked to, extracellular matrix organization, extracellular structure organization, humoral immune response,cell chemotaxis in downregulated group. Following that, KEGG pathway analysis revealed that the upregulated group was enriched and interacted with in cell cycle,DNA replication,oocyte meiosis,and p53 signaling pathway (Fig. 4C); and the downregulated group was enriched and interacted with in Cytokine − cytokine receptor interaction, Viral protein interaction with cytokine and cytokine receptor, Cell adhesion molecules, and Chemokine signaling pathway (Fig. 4E). These studies confirm that SKA3 is linked to a few malignancy-related pathways that lead to bladder cancer.
Function and pathway enrichment analyses for SKA3 in bladder cancer. A A volcano plot of all DEGs from indicated TCGA microarray data. Green dots represent significantly downregulated genes, and red dots represent significantly upregulated genes. B Gene ontology analysis of upregulated group, containing biological processes (BP), molecular function (MF), and cell component (CC). C Kyoto encyclopedia of genes and genomes analyzes pathways of upregulated group. D Gene ontology analysis of downregulated group. E Kyoto encyclopedia of genes and genomes analyzes pathways of downregulated group
Confirmation of SKA3 Expression by Immunohistochemistry
To investigate the expression of SKA3 in baldder cancer, SKA3 protein levels were examined using immunohistochemistry in 25 cases of bladder cancer tissues and 17 cases of normal bladder tissues. We found negative SKA3 immunostaining in normal urothelial tissues. Increased cytoplasm SKA3 staining was found in 21 of 25 cases of bladder cancer (Fig. 5). An independent sample t-test of SPSS 26.0 was used to compare the IRS between the two groups, and the results indicated that the protein expression of SKA3 in bladder cancer tissues was significantly higher than that in noncancerous tissues (P < 0.05).
Correlation between immune cell infiltration and SKA3
Immune cell infiltration surrounding tumors was confirmed to be intimately associated with the clinical outcome of cancer patients [21, 22]. Consequently, we defined if SKA3 expression was connected to immune infiltration level by examining their correlation in bladder cancer using TIMER. This analysis revealed that SKA3 expression level in BLCA was positively correlated with the infiltration levels of CD8 + T cells, macrophages, neutrophils, and dendritic cells. Besides, it had a negative association with CD4+ T cell infiltration levels (Fig. 6A). The preceding investigation indicated that SKA3 influences patient prognosis via its interaction with immune cell infiltration in bladder cancer. We then investigated the connection between SKA3 and immune checkpoint genes in TIMER database. SKA3 was closely correlated with immune checkpoint genes in most tumors, including bladder cancer (Fig. 6B).
SKA3 expression was related to M2 macrophage and Th2 cell infiltration and polarization
The immune infiltration state in tumor microenvironment (TME) can affect patient prognosis, and previous results indicate that SKA3 overexpression was connected to deleterious prognosis in bladder cancer patients. This implies that prominent SKA3 expression promotes the proliferation and metastasis of bladder tumors, which may be tightly associated with immunosuppressive tumors. Accordingly, we further explored whether SKA3 expression was linked to immune infiltration levels of macrophages and CD4+ T cells. SKA3 expression was found to be positively associated with macrophage infiltration level using four algorithms, including EPIC (R = 0.198, P = 1.28E-04) (Fig. 7A), TIMER (R = 0.121, P = 2.00E-02) (Fig. 7B), XCELL (R = 0.109, P = 3.68E-02) (Fig. 7C), and MCPCOUNTER (R = 0.174, P = 7.92E-04) (Fig. 7D). Besides, SKA3 expression was positively linked to CD4 + memory-activated T cells infiltration level in two different algorithms, including CIBERSOFT (R = 0.177, P = 6.28E-04) (Fig. 7E) and CIBERSOFT-ABS (R = 0.176, P = 6.95E-04) (Fig. 7F). For CD4+ T cell polarization, SKA3 expression was positively linked to Th2 cells polarization (R = 0.639, P = 1.48E-43) (Fig. 7G). For macrophage polarization, SKA3 expression was positively correlated with M2 polarization (R = 0.119, P = 2.19E-02) (Fig. 7H). In addition, SKA3 expression in bladder cancer was significantly correlated with M2 macrophage markers, including MRC1 (Fig. 8A) and CD163 (Fig. 8B), and Th2 cell markers, including CCR3 (Fig. 8C) and IL-4 (Fig. 8D). The above findings support that high SKA3 expression is connected with immunosuppressive microenvironment of bladder cancer via infiltration and polarization of M2 macrophages and Th2 cells. M2 macrophages are derived from M1 activation by factors such as IL-4 and IL-13 and have the potential to suppress immune responses, promote angiogenesis, tissue repair and promote tumor growth. Tumor tissues mostly secrete Th2-like cytokines and the body is in a state of Th2 cell dominance as one of the mechanisms of tumor immune escape.
Connection among SKA3 expression with macrophage and CD4 + T cell infiltration and polarization. A–D Correlation between SKA3 expression and macrophage infiltration levels using four different algorithms: EPIC, TIMER, XCELL, and MCPCOUNTER. E, F Correlation between SKA3 expression and CD4 + memory activated T cells infiltration levels using two different algorithms: CIBERSOFT and CIBERSOFT-ABS. G Correlation between SKA3 expression and CD4 + Th2 cell infiltration levels using algorithms of XCELL. H Correlation between SKA3 expression and M2 macrophage infiltration levels using CIBERSOFT-ABS algorithms
Correlation between SKA3 expression and gene markers of M2 macrophages and TH2 cells. A Correlation among SKA3 expression with MRC1 in bladder cancer. B Correlation among SKA3 expression with CD163 in bladder cancer. C Correlation among SKA3 expression with CCR3 in bladder cancer. D Correlation among SKA3 expression with IL-4 in bladder cancer
Discussion
SKA3 is required to maintain spindle checkpoint silencing and mitotic chromosomal cohesion and ensure timely mitosis [23,24,25]. Although SKA3 has been linked to the occurrence and development of numerous diseases, it has not been widely researched in various tumors. As a result, SKA3 impact on cancer prognosis, progression, and treatment must be identified and proven urgently. Previous studies reported that SKA3 could promote PI3K/AKT pathway to accelerate cell proliferation and migration in cervical cancer [26]. Meanwhile, SKA3 expression was higher in glioblastoma than in normal tissues, and it was verified that patients with high SKA3 expression have a shorter survival time [27]. A recent study reported that high SKA3 expression is associated with poor clinical outcomes and muscle-invasive bladder cancer progression, and promotes bladder cancer cell proliferation by accelerating G2/M transition [28].
Based on our results, we employed TCGA data in TIMER to investigate SKA3 expression and its prognostic impact on various cancer types, demonstrating that SKA3 was highly expressed in 20 different tumors compared to normal tissues. In addition, in GEPIA and HPA databases, SKA3 was highly expressed in bladder cancer relative to normal bladder tissue. Additionally, using Prognoscan database, we affirmed that high SKA3 expression in tumor tissues is significantly connected with poor prognosis of patients with bladder, lung, ovarian, breast, brain, and skin cancers. On the contrary, high SKA3 expression in colorectal cancer was associated with favorable survival. Meanwhile, Kaplan–Meier Plotter analysis revealed that high SKA3 expression was correlated with inferior OS and DFS in KIRP and BLCA but had no significant impact on OS and DFS in BRCA, COAD, and OVC. In different databases, prognostic disparities in SKA3 expression levels of various cancers result from the heterogeneity of material gathering methods and the latent mechanisms underlying distinct biological characteristics. Whereas, based on research results in different databases, we determined the accordant prognostic function of SKA3 on BLCA. This finding is consistent with previous studies [28]. To investigate the expression of SKA3 in baldder cancer, SKA3 protein levels were examined using immunohistochemistry in 25 cases of bladder cancer tissues and 17 cases of normal bladder tissues. Increased cytoplasm SKA3 staining was found in 21 of 25 cases of bladder cancer, and the results indicated that the protein expression of SKA3 in bladder cancer tissues was significantly higher than that in noncancerous tissues. It is reported that molecular subtypes are associated with prognosis and immune status of bladder cancer. Based on data from 412 cases of bladder cancer RNA sequencing, TCGA research team proposed five molecular subtypes: luminal-papillary subtype, luminal-infiltrated subtype, luminal subtype, basal-squamous subtype, and neuronal subtype. The neuronal subtype showed high levels of TP53 and RB1 mutations and had the worst survival of mRNA expression subtypes [29]. Our investigation revealed that SKA3 expression in neuronal subtype was much higher than that in other four subtypes, and SKA3 expression was also higher in TP53 mutant type than in TP53 nonmutant type. Overall, the above research explicitly illustrates that SKA3 can be a latent biomarker to forecast prognosis and molecular subtypes of tumors.
Numerous bladder cancer tumor biomarkers have been identified and validated to date [30, 31]. However, with rapid development of bladder cancer immunotherapy, the discovery of tumor biomarkers or immune checkpoints pertaining to tumor immune microenvironment is critical. However, the correlation between SKA3 and immune infiltration of bladder cancer has not been studied. Another significant aspect of our research indicates that SKA3 expression is associated with various levels of immune infiltration in bladder cancer. TME is a distinct metabolic niche, which differs according to the kind of tumor and influenced organs [32, 33]. TME is produced due to well-orchestrated reciprocal interactions between cancer cells and surrounding stromal and immune cells [34]. Immune cells in tumor microenvironment are a key factor of tumor tissues. Our results illustrated that SKA3 expression correlates positively with the infiltration level of CD8+T cells, macrophages, neutrophils, and dendritic cells in BLCA. Meanwhile, significant relationships between SKA3 and immune checkpoint gene expression in pan-cancer indicate that SKA3 plays a key function in regulating tumor immunology. Tumor cells acquire strategies to evade immune surveillance during the prolonged interaction between cancer and the immune system. Tumors may abscond immunity via drawing immune suppressive cells, such as tumor-associated macrophages (TAMs). TAMs usually possess M2 phenotypes, suppress adaptive immunity, and facilitate TME that can accelerate cancer progression. Several researches have confirmed that M2 macrophages with a higher infiltration level are positively correlated with poor histopathological characteristics, including higher tumor and lymph node stage and histological grade in bladder cancer patients [35,36,37]. GO results revealed that SKA3 was strongly associated with nuclear division, mitotic nuclear division, mitotic sister chromatid segregation, humoral immune response, and cell chemotaxis. Meanwhile, KEGG analysis indicated that SKA3 was involved in the cell cycle, DNA replication, p53 signaling pathway, Cytokine − cytokine receptor interaction, and Chemokine signaling pathway. By analyzing the relationship between SKA3 and macrophage infiltration in BLCA, we discovered that macrophages in four different algorithms and M2 macrophages had significantly positive correlations with SKA3 expression levels. Moreover, SKA3 expression was positively correlated with gene markers of M2 macrophage such as MRC1 and CD163 in bladder cancer.
As immunotherapy becomes a valid treatment method for cancer, CD4+ T helper cells have received extensive attention as a crucial component of immune response [38]. Meanwhile, activated CD4+ T cells can differentiate into various T helper cells with different effector functions based on their phenotypes, cytokine profiles, and functionality, such as Th1, Th2, Th17, follicular helper T, and regulatory T cells [39,40,41]. Tumor cells and specific immune cells that differentiate towards Th2 phenotype are the best correlative immune escape mechanisms. For Th2 differentiation, IL-4 is essential for launching and sustaining Th2 phenotypes [34]. By combining with IL-4 receptors, IL-4, a typical Th2 cytokine, induces naive CD4+ T cells to develop into Th2 cells [42]. Following differentiation, TH2 cells secrete IL-4, IL-5, IL-10, IL-13, and IL-17. Additionally, IL-4, IL-5, and IL-13 have been demonstrated to accelerate tumor proliferation and metastasis [43, 44]. Our findings indicated that infiltration levels of activated CD4+ memory T cells and Th2 cells were highly linked to SKA3 expression in BLCA. Concurrently, SKA3 expression was positively correlated with the expression level of Th2 cell markers (IL-4 and CCR3) in bladder cancer.
In conclusion, SKA3 can influence the prognosis of bladder cancer patients and is associated with immune infiltration. In particular, SKA3 may promote bladder cancer growth and metastasis by influencing the differentiation of M2 macrophage and Th2 cells. Therefore, SKA3 can represent a valuable prognostic biomarker for bladder cancer.
Availability of data and materials
The datasets supporting the conclusions of this article are included within the article. Materials are available from the corresponding author on reasonable request.
Abbreviations
- DSS:
-
Disease-specific survival
- DFS:
-
Disease-free survival
- GEPIA:
-
Gene Expression Profiling Interactive Analysis
- GO:
-
Gene Ontology
- GEO:
-
Gene Expression Omnibus
- HPA:
-
Human Protein Atlas
- HRs:
-
Hazard ratios
- KEGG:
-
Kyoto Encyclopedia of Genes and Genomes
- OS:
-
Overall survival
- RFS:
-
Relapse-free survival
- SKA3 :
-
Spindle and kinetochore‑associated complex subunit 3
- Th:
-
T helper
- TCGA:
-
The Cancer Genome Atlas
- TME:
-
Tumor microenvironment
- TAMs:
-
Tumor-associated macrophages
References
Sung H, Ferlay J, Siegel RL, Leversanne M, Soerjomataram I, Jemal A , F Bray. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021; doi: https://doi.org/10.3322/caac.21660.
Saginala K, Barsouk A, Aluru JS, Rawla P, Padala SA, Barsouk A. Epidemiology of bladder cancer. Med Sci. 2020. https://doi.org/10.3390/medsci8010015.
Ruan WM, Chen X, Huang M, Wang H, Chen JX. A urine-based DNA methylation assay to facilitate early detection and risk stratification of bladder cancer. Clin Epigenetics. 2021. https://doi.org/10.1186/s13148-021-01073-x.
Chen X, Zhang JT, Ruan WM, Huang M, Wang CJ. Urine DNA methylation assay enables early detection and recurrence monitoring for bladder cancer. J Clin Invest. 2020. https://doi.org/10.1172/JCI139597.
Jeyaprakash AA, Santamaria A, Jayachandran U, Chan YW, Benda C, Nigg EA, Conti E. Structural and functional organization of the Ska complex, a key component of the kinetochore-microtubule interface. Mol Cell. 2012. https://doi.org/10.1016/j.molcel.2012.03.005.
Sivakumar S, Gorbsky GJ. Phosphatase-regulated recruitment of the spindle- and kinetochore-associated (Ska) complex to kinetochores. Biol Open. 2017. https://doi.org/10.1242/bio.026930.
Sivakumar S, Daum JR, Tipton AR, Rankin S, Gorbsky GJ. The spindle and kinetochore-associated (Ska) complex enhances binding of the anaphase-promoting complex/cyclosome (APC/C) to chromosomes and promotes mitotic exit. Mol Biol Cell. 2014. https://doi.org/10.1091/mbc.E13-07-0421.
Jiao X, Hooper SD, Djureinovic T, Larsson C, Warnberg F, Tellgren-Roth C, Botling J, Sjoblom T. Gene rearrangements in hormone receptor negative breast cancers revealed by mate pair sequencing. BMC Genom. 2013. https://doi.org/10.1186/1471-2164-14-165.
Yamada Y, Arai T, Kojima S, Sugawara S, Kato M, Okato A, Yamazaki K, Naya Y, Ichikawa T, Seki N. Anti-tumor roles of both strands of the miR-455 duplex: Their targets SKA1 and SKA3 are involved in the pathogenesis of renal cell carcinoma. Oncotarget. 2018. https://doi.org/10.18632/oncotarget.25410.
Hou YC, Wang ZM, Huang SZ, Sun CJ, Zhao JY, Shi JY, Li ZQ, Wang ZK, He XS, Tam NL, Wu LW. SKA3 Promotes tumor growth by regulating CDK2/P53 phosphorylation in hepatocellular carcinoma. Cell Death Dis. 2019. https://doi.org/10.1038/s41419-019-2163-3.
Chuang TP, Wang JY, Jao SW, Wu CC, Li LH. Over-expression of AURKA, SKA3 and DSN1 contributes to colorectal adenoma to carcinoma progression. Oncotarget. 2016. https://doi.org/10.18632/oncotarget.9960.
Li T, Fan J, Wang B, Traugh N, Chen Q, Liu JS, Li B, Liu XS. TIMER: a web server for comprehensive analysis of tumor-infiltrating immune cells. Cancer Res. 2017. https://doi.org/10.1158/0008-5472.CAN-17-0307.
Tang Z, Li C, Kang B, Gao G, Li C, Zhang Z. GEPIA: a web server for cancer and normal gene expression profiling and interactive analyses. Nucleic Acids Res. 2017. https://doi.org/10.1093/nar/gkx247.
Uhlen M, Zhang C, Lee SJ, Sjöstedt E, Fagerberg L, Bidkhori G, et al. A pathology atlas of the human cancer transcriptome. Science. 2017. https://doi.org/10.1126/science.aan2507.
Chandrashekar DS, Bashel B, Balasubramanya SAH, Creighton CJ, Rodriguez IP, Chakravarthi BVSK, Varambally S. UALCAN: A portal for facilitating tumor subgroup gene expression and survival analyses. Neoplasia. 2017. https://doi.org/10.1016/j.neo.2017.05.002.
Mizuno H, Kitada K, Nakai K, Sarai A. PrognoScan: a new database for meta-analysis of the prognostic value of genes. BMC Med Genomics. 2019. https://doi.org/10.1186/1755-8794-2-18.
Ádám N, András L, Otília M, Balázs G. Validation of miRNA prognostic power in hepatocellular carcinoma using expression data of independent datasets. Scient Rep. 2018;8:9227.
Yu G, Wang LG, Han Y, He QY. clusterProfiler: an R package for comparing biological themes among gene clusters. OMICS. 2012. https://doi.org/10.1038/s41598-018-27521-y.
Li B, Severson E, Pignon JC, Zhao HQ, Li TW, Novak J, et al. Comprehensive analyses of tumor immunity: Implications for cancer immunotherapy. Genome Biol. 2016. https://doi.org/10.1186/s13059-016-1028-7.
Danaher P, Warren S, Dennis L, Amico LD, White A, Disis ML, et al. Gene expression markers of Tumor Infiltrating Leukocytes. J Immunother Cancer. 2017. https://doi.org/10.1186/s40425-017-0215-8.
Ohtani H. Focus on TILs: Prognostic significance of tumor infiltrating lymphocytes in human colorectal cancer. Cancer Immun. 2007;7:4.
Azimi F, Scolyer RA, Rumcheva P, Moncrieff M, Murali R, McCarthy SW, et al. Tumor-Infiltrating Lymphocyte Grade Is an Independent Predictor of Sentinel Lymph Node Status and Survival in Patients With Cutaneous Melanoma. J Clin Oncol. 2012. https://doi.org/10.1200/JCO.2011.37.8539.
Daum JR, Wren JD, Daniel JJ, Sivakumar S, McAvoy JN, Potapova TA, et al. Ska3 is required for spindle checkpoint silencing and the maintenance of chromosome cohesion in mitosis. Curr Biol. 2009. https://doi.org/10.1016/j.cub.2009.07.017.
Theis M, Slabicki M, Junqueira M, Paszkowski-Rogacz M, Sontheimer J, Kittler R, et al. Comparative profiling identifies C13orf3 as a component of the Ska complex required for mammalian cell division. EMBO J. 2009. https://doi.org/10.1038/emboj.2009.114.
Zhang Q, Sivakumar S, Chen YJ, Gao H, Yang L, Yuan Z, et al. Ska3 phosphorylated by Cdk1 binds Ndc80 and recruits Ska to kinetochores to promote mitotic progression. Curr Biol. 2017. https://doi.org/10.1016/j.cub.2017.03.060.
Hu R, Wang MQ, Niu WB, Wang YJ, Liu YY, Liu LY, et al. SKA3 promotes cell proliferation and migration in cervical cancer by activating the PI3K/Akt signaling pathway. Cancer Cell Int. 2018. https://doi.org/10.1186/s12935-018-0670-4.
Li CK, Yang JY, Lei S, Wang W. SKA3 promotes glioblastoma proliferation and invasion by enhancing the activation of Wnt/β-catenin signaling via modulation of the Akt/GSK-3β axis. Brain Res. 2021. https://doi.org/10.1016/j.brainres.2021.147500.
Chaelin You, Xuan Mei Piao, Keunsoo Kang, et al. Integrative Transcriptome Profiling Reveals SKA3 as a Novel Prognostic Marker in Non-Muscle Invasive Bladder Cancer. Cancers. 2021; doi: https://doi.org/10.3390/cancers13184673.
Robertson AG, Kim J, Al-Ahmadie H, Bellmunt J, Guo GW, Cherniack AD. Comprehensive molecular characterization of muscle-invasive bladder cancer. Cell. 2017. https://doi.org/10.1016/j.cell.2017.09.007.
Xie RH, Chen X, Cheng L, Huang M, Zhou QH. NONO inhibits lymphatic metastasis of bladder cancer via alternative splicing of SETMAR. Mol Ther. 2021. https://doi.org/10.1016/j.ymthe.2020.08.018.
Zhang JT, Zhou QH, Xie KJ, Cheng L, Peng SM. Targeting WD repeat domain 5 enhances chemosensitivity and inhibits proliferation and programmed death-ligand 1 expression in bladder cancer. J Exp Clin Cancer Res. 2021. https://doi.org/10.1186/s13046-021-01989-5.
Lim AR, Rathmell WK, Rathmell JC. The tumor microenvironment as a metabolic barrier to effector T cells and immunotherapy. Elife. 2020. https://doi.org/10.7554/eLife.55185.
Reznik E, Luna A, Aksoy BA, Liu EM, La K, Ostrovnaya I, et al. A landscape of metabolic variation across tumor types. Cell Syst. 2018. https://doi.org/10.1016/j.cels.2017.12.014.
Greten FR, Grivennikov SI. Inflammation and cancer: triggers, mechanisms, and consequences. Immunity. 2019. https://doi.org/10.1016/j.immuni.2019.06.025.
Sjödahl G, Lövgren K, Lauss M, Chebil G, Patschan O, Gudjonsson S, et al. Infiltration of CD3 and CD68 cells in bladder cancer is subtype specific and affects the outcome of patients with muscle-invasive tumors. Urol Oncol. 2014. https://doi.org/10.1016/j.urolonc.2014.02.007.
Wang B, Liu H, Dong XL, Wu SX, Zeng H, Liu ZW, et al. High CD204+ tumor-infiltrating macrophage density predicts a poor prognosis in patients with urothelial cell carcinoma of the bladder. Oncotarget. 2015. https://doi.org/10.18632/oncotarget.3887.
Takeuchi H, Tanaka M, Tanaka A, Tsunemi A, Yamamoto H. Predominance of M2polarized macrophages in bladder cancer affects angiogenesis, tumor grade and invasiveness. Oncol Lett. 2016. https://doi.org/10.3892/ol.2016.4392.
Rong ET, Richardson EK, Han CT. Revisiting the role of CD4+ T cells in cancer immunotherapy—new insights into old paradigms. Cancer Gene Ther. 2020. https://doi.org/10.1038/s41417-020-0183-x.
Dobrzanski MJ. Expanding roles for CD4 T cells and their subpopulations in tumor immunity and therapy. Front Oncol. 2013. https://doi.org/10.3389/fonc.2013.00063.
Saravia J, Chapman NM, Chi H. Helper T cell differentiation. Cell Mol Immunol. 2019. https://doi.org/10.1038/s41423-019-0220-6.
Zhu JF. T helper cell differentiation, heterogeneity, and plasticity. Cold Spring Harb Perspect Biol. 2018. https://doi.org/10.1101/cshperspect.a030338.
Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008. https://doi.org/10.1038/nature07205.
Nappo G, Handle F, Santer FR, McNeill RV, Seed RI, Collins AT, et al. The Immunosuppressive Cytokine Interleukin-4 Increases the Clonogenic Potential of Prostate StemLike Cells by Activation of STAT6 Signalling. Oncogenesis. 2017. https://doi.org/10.1038/oncsis.2017.23.
Bankaitis KV, Fingleton B. Targeting IL4/IL4R for the treatment of epithelial cancer metastasi. Clin Exp Metastasis. 2015. https://doi.org/10.1007/s10585-015-9747-9.
Acknowledgements
We thank Wei Qin for his technical support.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
C W, Z Q and S L: Data extraction, analysis, and manuscript draft. X Z, Y F and F B: Data interpretation and manuscript revision. Y W and P G: Figures preparation and manuscript draft. H W, D W and S H: Concept design, funding acquisition and manuscript revision. The author(s) read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The Ethics Committee of the Affiliated Qingdao Municipal Hospital of Qingdao University approved this experiments.
Consent for publication
Not applicable.
Competing interests
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Wang , C., Liu , S., Zhang , X. et al. SKA3 is a prognostic biomarker and associated with immune infiltration in bladder cancer. Hereditas 159, 20 (2022). https://doi.org/10.1186/s41065-022-00234-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41065-022-00234-z