Abstract
Background
Staphylococcus aureus (S. aureus), especially methicillin-resistant S. aureus (MRSA), is a known disease-causing bacteria with many associated health hazards. Staphylococcal food poisoning can result from staphylococcal enterotoxins (SEs).
Methods
In this study, 50 S. aureus isolates were isolated from the gastrointestinal tract (GIT) clinical samples of patients with food poisoning in clinical laboratories at Mansoura University Hospital, Egypt. For determination their antibiogram, these isolates were tested for antimicrobial sensitivity against 12 antimicrobial agents using the agar disk diffusion test. After DNA extraction from the isolates, conventional polymerase chain reaction (PCR) was used to detect mecA and SEs genes.
Results
As a result, all isolates were ampicillin and cefoxitin-resistant, while 86% (43 of 50) of the tested isolates exhibited multidrug resistance (MDR). In contrast, the highest sensitivity was confirmed against vancomycin, linezolid and quinolones, namely ciprofloxacin and norfloxacin. Although 100% of the isolates were mecA positive, staphylococcal enterotoxin genes set-A, set-B, set-C, set-G, set-M, and set-O genes were detected in 56%, 20%, 8%, 32%, 16%, and 24%, of the tested isolates, respectively. Finally, isolates encompassing SEs genes were used to validate a microarray chip, indicating its potential for a better methodological approach for detecting and identifying SEs in human samples.
Conclusion
The genotypic findings of this study may help explain the enterotoxigenic patterns in S. aureus among Egyptian patients with food poisoning.
Similar content being viewed by others
Introduction
S. aureus, as a member of Gram-positive bacteria, could also be associated with a large-scale food poisoning outbreak, where staphylococcal enterotoxins (SEs) producing S. aureus were identified as the causative pathogen [1]. S. aureus produces many virulence factors, including proteases, enterotoxins, hemolysins, leukocidins, exfoliative toxins, and immune-modulatory factors [2]. The term ‘superantigens’ commonly refers to toxic-shock syndrome toxin-1 (TSST-1) and staphylococcal enterotoxins (SEs), produced by S.aureus activating a large population of specific T-cells at pictogram levels [3]. A large family of structurally related toxins, e.g., staphylococcal enterotoxins (SEs), are considered as one the most potent virulence factors, with the ability to stimulate T lymphocyte proliferation and induce the release of cytokines, and ultimately cause cell death [3]. Interestingly, plasmids, bacteriophages, and pathogenicity islets are capable of transporting genes encoding such toxins [4]. In staphylococcal food poisoning, a toxigenic process is triggered by a class of toxins known as SEs, primarily described in previous studies [5]. These toxins are associated with a form of gastroenteritis, characterized by vomiting and diarrhea [6]. Moreover, SEs play a role in rheumatoid arthritis [7], atopic eczema [8], and others [9]. SEs, as members of the superantigen protein family, can elicit a polyclonal immune response [10]. These toxins may also influence the host's immune response and contribute to bacterial persistence and defense [11]. S. aureus expression of particular enterotoxin (set) genes depends on the source of the host tissue and may play a role in the adaptation of S. aureus to the host environment [12]. Most SEs are heat-stable and are not affected by digestive enzymes, with the ability to induce various symptoms, such as nausea, vomiting, malaise, abdominal cramps, and diarrhea [6]. Interestingly, about 95% of “staphylococcal” food poisoning outbreaks are caused by classical types of SEs, namely A, B, C, D, and E [13]. In addition, the consumption of food contaminated with enterotoxins produced by methicillin-resistant S. aureus (MRSA) could be associated with serious complications [14, 15] such as infection of heart valves (endocarditis), gangrene or death of the soft tissues (necrotizing fasciitis), and bone or joint infections (osteomyelitis or septic arthritis) [16,17,18]. Moreover, MRSA has wider economic implications that encompass indirect expenses to the patient and society, and also hospitalization costs [19]. For instance, compared to MSSA, MRSA results in an approximately threefold increase in expenses for primary nosocomial bloodstream infections [20].
Nearly twenty-seven types of SEs have been detected and identified as single-chain proteins, with a molecular weight ranging from 19 to 29 kD. Two major toxic activities that contribute to the effects of SEs have been identified: A neurotoxic activity, which stimulates vomiting reflexes by stimulating the brain's emetic center and vagus nerve, and a superantigenic activity, which produces strong T lymphocyte activation and severe fever [21].
Various techniques have been used to identify SEs. One of the traditional methods is serological typing, which is based on antigenic detection; however, it is only semi-quantitative and lacks specificity and sensitivity [22]. Other techniques, such as PCR and DNA-DNA hybridization, have been introduced recently. Nevertheless, these techniques are limited and can only detect one or a few toxins in one experiment [22, 23]. Moreover, multiplex PCR was used to detect several genes simultaneously. To achieve unambiguous identification of set-specific amplicons, additional restriction endonuclease tests or other procedures are required [24].
Consequently, a rapid and specific method for the simultaneous detection and identification of SEs for diagnostic and epidemiological purposes are still required. The molecular basis of relationships can be studied using microarrays on a scale that is not conceivable with traditional research. [25]. The primary objective of this study was to determine the relationship between the resistance of MRSA, isolated from Egyptian patients with food poisoning, and the presence of various types of enterotoxins. By validating an enterotoxin-specific microarray, a better methodological approach for identifying several enterotoxins in one experiment could be provided.
Materials and methods
Bacterial isolation and identification
In total, 50 S. aureus isolates were collected from 157 gastrointestinal tract (GIT) clinical samples (stool samples) of patients with food poisoning in the clinical laboratories of Mansoura University Hospital, Egypt, from June to September 2021. In the next step, each stool sample needed to be put into clean, dry plastic jars with screw-top lids and had to be brought to the lab right away. All isolates were identified using standard microbiological tests [26]. Swabs were inoculated onto nutrient agar plates and incubated aerobically at 37 °C for 24–48 h. The obtained colonies were identified as S. aureus by conventional laboratory identification methods, including colonial morphology, Gram stain, catalase test, coagulase test, and growth on mannitol salt agar (MSA) [26].
The collected isolates, 50 (31.8%) isolated S. aureus from 157 stool samples included 62% from males, while 34% of the total number of cases were < 19 years old, 40% of the cases were between the ages of 19 and 40 years, 12% of cases were between the ages of 41 and 60 years, and 14% of cases were > 60 years old.
Coagulase test
Using the coagulase tube approach, 500 µl of each isolate's overnight broth culture was combined with an equal amount of human plasma in sterile glass test tubes. The tubes were then incubated at 37 °C in an incubator or water bath and observed every 30 min. The tube is retained at room temperature for overnight incubation if the test is negative after four hours at 37 °C [26].
Catalase test
The test was carried out by adding a fresh pure colony from the overnight culture with a sterile tip and 1–2 drops of 3% hydrogen peroxide (H2O2) on a dry, clean slide [26].
Antimicrobial susceptibility
Antimicrobial susceptibility was determined using the disc diffusion method, in accordance with the Clinical and Laboratory Standard Institute (CLSI) [27] for the following antibiotics: Ampicillin (AMP, 10 µg), cefoxitin (FOX, 30 µg), cefotaxime (CTX, 30 µg), ciprofloxacin (CIP, 5 µg), norfloxacin (NOR, 10 µg), tobramycin (TOB, 10 µg), gentamicin (GEN, 10 µg), doxycycline (DOX, 5 µg), amikacin (AMK, 30 µg), azithromycin (AZM, 15 µg), nitrofurantoin (NIT, 300 µg), amikacin, vancomycin (VA, 30 µg), linezolid (LZD, 30 µg) and sulfamethoxazole/trimethoprim (SXT, 1.25/23.75 µg) (Oxoid, Hampshire, England). The results were interpreted using the criteria outlined in CLSI guidelines based on the inhibition zone produced, which correlate with susceptibility levels [27]. The obtained results were used to identify the percentage of MDR among the tested isolates. As previously documented, multidrug resistance (MDR) is defined as resistance to three or more antimicrobial classes [28]. The phenotypic identification of the isolates as MRSA was performed against cefoxitin through the disk diffusion method, while the standard strain of S. aureus (ATCC 29312) was included as a control isolate.
Total DNA extraction
Pure colonies of freshly grown cells were incubated at 37 °C in Mueller Hinton Broth (MHB) (Oxoid, Hampshire, England) for 24 h. Pellets were collected after centrifugation from a 2 mL culture of each isolate. In the next step, each resulting pellet was suspended in 180 µL lysozyme (10 mg/mL), as recommended for Gram-positive bacteria (Sigma-Aldrich Co., UK), in a lysate buffer. Chromosomal DNA was extracted using the Gene JET Genomic DNA purification Kit (Thermo Scientific, Vilnius, Lithuania), in accordance with the manufacturer's guidelines.
Genomic DNA concentration and purity were determined in the prepared samples by measuring absorbance at 260 and 280 nm using nanodrop instrument (OptizenNanoQ, Daejeon, Korea).
Conventional PCR amplification for the detection of different staphylococcal enterotoxin and mecA genes
Various set and mecA genes were screened using the primers listed in Table 1. Each PCR reaction contained 12.5 µL of DreamTaq Green PCR Master Mix (Thermo Scientific, Vilnius, Lithuania), 1µL (10 µM) of each primer, 2 µL of template DNA, and up to 25 µL of nuclease-free water. Each PCR reaction started with an initial denaturation at 94 °C for 5 min, followed by 35 cycles of three-step denaturation at 94 °C for 30 s, annealing temperature (Table 1) for 30 s, and extension at 72 °C for 45 s. Finally, each PCR reaction was terminated with a final extension at 72 °C for 5 min. The resulting PCR products and the GeneRuler 100 bp plus DNA ladder (Thermo Fischer Scientific, USA) were separated on a 1.5% agarose gel and then stained with ethidium bromide (Merck, Hohenbrunn, Germany). The produced amplification bands were photographed using a UV transilluminatorUV-TM-25–230 v (Hoefer Inc.). Cluster analysis for set genes carrying isolates was performed using the un-weighted pair-group method with average linkages (UPGMA).
Oligo array printing
Microarray slides (Scienion, Berlin, Germany) were spotted in triplicate for each probe using The SpotBot microarrayer (ArrayIt, Sunnyvale, California, USA) spotting machine (Fig. 1). Each oligonucleotide solution was prepared using ArrayIt spotting buffer (ArrayIt, Sunnyvale, California, USA) in a 384-well printing plate.
Microarray testing
Genomic DNA samples were labeled (Thermo Fisher Scientific, Waltham, Massachusetts, USA) and then resuspended in the hybridization buffer (Scienion, Berlin, Germany). Hybridizations were carried out in a hybridization station (ArrayIt, Sunnyvale, California, USA). After hybridization step, microarray slides were then washed using buffers I, II, and III (Scienion, Berlin, Germany). Colorimetric observation was performed after staining using streptavidin–biotin color development system (ThermoFisher Scientific, Waltham, Massachusetts, USA), following the manufacturer’s instructions. Finally, images were acquired using the ArrayIt Microarray Scanner (ArrayIt, Sunnyvale, California, USA). Signal intensities were recorded using the Spotware software (ArrayIt, Sunnyvale, California, USA). Final intensities were calculated after subtracting the local background values from the per-sample median values.
Results
Identification of S. aureus isolates
A total of 50 (31.8%) unique S. aureus clinical isolates were separated from 157 stool samples, and identified in the current study. Most cases (62%) were males, while 34% of the total number of cases were < 19 years old, 40% of the cases were between the ages of 19 and 40 years, 12% of cases were between the ages of 41 and 60 years, and 14% of cases were > 60 years old. After growth on nutrient agar, S. aureus isolates appeared as colonies of a golden yellow hue. Gram staining indicated positive type for all isolated bacteria exhibiting a grape-like arrangement under the microscope. All the tested isolates were given immediate effervescent with 3% hydrogen peroxide. In addition, they were coagulase positive and induced gelling of the plasma. The tested isolates had the ability to ferment mannitol biochemically with yellow color produced on mannitol salt agar medium (MSA).
Antimicrobial susceptibility testing
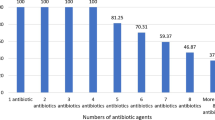
Antimicrobial susceptibility testing revealed that all isolates were ampicillin- and cefoxitin-resistant (100%), whereas 72% were cefotaxime-resistant. In contrast, the highest sensitivity was confirmed against vancomycin, linezolid, where all isolates did not exhibit any resistance to the tested antibiotics. Similarly, there was seldom any observed resistance against ciprofloxacin (8%) and norfloxacin (4%). Other antimicrobials such as azithromycin (46%), sulfamethoxazole/trimethoprim (46%), doxycycline (36%), nitrofurantoin (34%), tobramycin (30%), gentamicin (30%), and amikacin (24%) exhibited intermediate resistance (Table 2 and Fig. 1). In this study, 86% (43 of 50) of all isolates exhibited MDR, with resistance to more than 2 classes of antimicrobial agents. Patterns P1 (referred to as bacterial isolates resistant to 2 antimicrobial agents; FOX and AM) and P6 (referred to as bacterial isolates resistant to 4 antimicrobial agents; FOX, AMP, CTX, and SXT) were primarily found in S. aureus resistant isolates, 12% each (Table 2).
MRSA identification and screening of different enterotoxin genes by conventional PCR
Subsequently, mecA was amplified using PCR to confirm MRSA identification. All of our isolates tested positive for mecA, with a single band at 310 bp. Additionally, PCR was used for set genes detection, where set-A gene was detected in 56%, while set-G gene was detected in 32% of the investigated isolates. In contrast, set-C and set-B were only detected in 8% and 20% of instances, respectively. Similarly, Set-M and set-O were detected in 16% and 24% of the tested isolates, respectively (Table 3 and Fig. 2). To classify our isolates, according to the number of set genes, identified in each isolate, a correlation analysis (dendrogram) was constructed. The cluster analysis of set genes revealed that according to their frequency, they could be categorized into 20 distinct patterns 1–20. Group 1 contained the most prevalent genotype (26%), with only mecA identified, followed by group 4 (16%) carrying 2 genes (mecA and set-A) and group 11 (12%), with 3 genes (mecA, set-A, and set-G) (Fig. 3).
Validation of microarray by enterotoxin genes carrying isolates
The possibility of detecting enterotoxin genes by enterotoxin specific microarray, a more rapid method capable of detecting many different genes simultaneously, was examined. Five different isolates carrying set-A, set-B, set-C, set-G, and set-O genes were selected for microarray validation. As a result, oligonucleotides used in the microarray test were able to detect S. aureus isolates carrying the corresponding genes, indicating the possibility of using the microarray test to detect previously PCR-identified enterotoxins A, B, C, G, and O. (Figs. 4, 5).
Results of hybridization with the target food poisoning sample DNAs; positive hybridization results were obtained for SEs genes A, B, C, G, and O, on the other hand SEs genes; set-D, set-E, set-I, set-M, and set-N couldn’t be detected, the same results were obtained by PCR techniques indicating parallel results
Discussion
MRSA has been called a "superbug" due to its widespread resistance to commonly used antibiotics, making it hard to be treated. The medical complexity of S. aureus, including MRSA, arises from the extensive resistance to routinely used medications. Consequently, antibiotic resistance testing is clinically important [31]. Additionally, S. aureus is commonly associated with a broad spectrum of human tissue infections and food poisoning, with over 30 different infection causing serotypes ranging from mild to severe systemic infections and sometimes potentially fatal [32]. S aureus has the ability to produce many exotoxins, with some strains producing a family of pyrogenic toxins including enterotoxins [33].
Epidemiologically, the production of SEs is a key concern in staphylococcal food poisoning [34]. In addition to their status as superantigens, the staphylococcal enterotoxins have severe negative effects on health when ingested, as these exotoxins in food result in intoxication, violent vomiting, diarrhea, fever, and general symptoms, including headache and nausea, which are all signs of staphylococcal food poisoning [35]. These observations could provide valuable insights into the evolution of S. aureus as a pathogen for developing food safety control techniques [34].
During this study, 50 clinical GIT isolates, identified as S. aureus, were isolated. In addition, 34% of all cases were < 19 years old, 40% were between 19 and 40 years old, 12% were between 41 and 60 years old, and 14% were > 60 years old. These findings demonstrated that 19-year-olds were more susceptible to infection with S. aureus, which is consistent with a previous study in the USA [36]. In addition, SEs were detected in contaminated food, causing staphylococcal food poisoning, toxic shock, and allergic and autoimmune reaction [37].
In this study, MDR was identified in 43 (86%) isolates. The current results were consistent with those reported in China [38], where approximately 67% of the S. aureus isolates were MDR. In contrast, a lower proportion was reported in another study in China [39], where 57.5% of their tested S. aureus isolates were resistant to three or more classes of antimicrobials (MDR).
In this study, antimicrobial susceptibility testing revealed that all isolates were resistant to both ampicillin and cefoxitin (100%), followed by cefotaxime (72%). These results exceeded those previously reported in China [38], demonstrating that 92% of S. aureus isolates were penicillin-resistant, whereas only 10% were cefoxitin-resistant. Regarding two antimicrobials, i.e., nitrofurantoin and gentamicin, only 34% and 30% of the tested isolates were identified as resistant, respectively. However, much lower percentages for both nitrofurantoin (5%) and gentamicin (2%) were previously reported in China [38] (Table 2).
In this study, 36% and 8% of the tested isolates exhibited resistance to doxycycline and ciprofloxacin, respectively. However, other different values for doxycycline and ciprofloxacin were previously reported in Iran [33], where 12% and 18% of all isolates were identified as doxycycline and ciprofloxacin resistant, respectively. In this study, similar to ciprofloxacin, a very high sensitivity (100%) was confirmed against vancomycin and linezolid. In case of other antimicrobials, a higher level of resistance to cefotaxime (72%) was reported among the isolates in this study. Similarly, a close result for cefotaxime resistance (71.4%) was detected in another study in Iran [40]. For the identification of enterotoxin specificity and diversity, SE gene patterns of S. aureus isolates from patients suffering from food poisoning were analyzed. In addition, the mecA gene was detected in each of the tested isolates, indicating its contribution to their resistance [41].
Cefoxitin disc diffusion method was used to validate the phenotypic MRSA identification [42]. Interestingly, the frequency of the mecA gene among the examined S. aureus was equal to 100%. As mentioned previously, cefoxitin disc represents a useful tool for predicting methicillin resistance, with sensitivity values approaching 100% [43]. Moreover, identifying the mecA gene by PCR can be used as a positive indicator for MRSA isolates [44].
In this study, each clinical isolate tested was mecA-positive. This result was consistent with another study in Egypt [31], in which 100% of S. aureus were MRSA isolates. In the same vein, high rates of MRSA were reported in Nepal [45] and Eritrea (72%) [46]. However, moderate rates (56%) were reported in Romania [47]. In addition, the reported results from this study showed a higher prevalence of MRSA than those reported in other studies in Sweden and India, respectively [48, 49], in which variable percentages ranging from 44 to 81% were detected, respectively. This variation may be attributed to different antibiotic prescribing strategies across nations. Although methicillin was the first semisynthetic penicillinase-resistant penicillin identified, it was withdrawn from the market in the United States because of the high incidence of interstitial nephritis associated with its use [50]. In this study, mecA-positive strains demonstrated antimicrobial resistance to cefoxitin, validating the use of the cefoxitin disc as reported previously [51].
The study investigated the prevalence of various enterotoxin genes using conventional PCR. Traditional SEs, such as A, B, C, D, and E, in addition to other SEs, such as G, I, M, N, and O, commonly detected in previous studies, were selected. According to the previous results obtained, several enterotoxin genes were detected in the tested strains of S. aureus, particularly including set-A, set-B, set-C, set-D, set-E set-G, set-I, set-M, set-N, and set-O [6, 52]. In this study, set-A exhibited the highest prevalence at 56%, followed by set-G, set-O, and set-B at 32%, 24%, and 20%, respectively. Nonetheless, 16% and 8% were discovered in set M and set C, respectively. Set-D, set-E, set-I, and setN were not detected in any of the tested isolates. The study results were higher than those found in a study conducted in Tanzania, where enterotoxin C and B genes were detected in approximately 0.3% of the tested isolates, whereas set-A was completely absent [53]. Similarly, genes for both enterotoxins D and A were detected in approximately 10% of isolates in Turkey [54]. In contrast, a study in Sudan [14] reported that none of the enterotoxin genes was present in their isolates. This variation between the results of different studies may be attributable to several factors, such as the geographical origin, the source and size of the tested samples, and the type and sensitivity of the chosen method for detecting these genes.
The cluster analysis of set genes revealed that group 1 was the most prevalent genotype without any of the set genes detected, followed by group 4 carrying set-A gene. However, in the case of isolates carrying more than 2 set genes, group 11 was the most prevalent with both set-A and set-G genes. Moreover, the results obtained indicated that isolates carrying more than 2 set genes were rarely detected.
As previously reported, microarray and PCR produced similar results, making microarray testing for enterotoxins an alternative tool to be validated in this study [30]. In microarray experiments, DNA probes were immobilized at high density carrying more than one copy of each target gene in each DNA chip, which increased the possibility of carrying out each experiment in triplicates or more on the same slide. In addition, DNA microarrays were used primarily in mixed microbial community analyses based on ribosomal DNA sequence [55, 56]. Moreover, other microarray types could be used to detect some virulence factors [57].
In this study, oligonucleotides in the microarray test were able to detect the corresponding genes, indicating the possibility of using the microarray as a reliable method for detecting and identifying many SEs in replicates on the same slide.
Conclusion
In conclusion, it could be reported that the genotypic findings of this study may help distinguish the common types of enterotoxigenic S. aureus among Egyptian patients with food poisoning. They could be beneficial for the study and management of S. aureus infections in food.
Availability of data and materials
All relevant data are available upon request from the corresponding author.
References
Le HHT, Dalsgaard A, Andersen PS, Nguyen HM, Ta YT, Nguyen TT. Large-scale staphylococcus aureus foodborne disease poisoning outbreak among primary school children. Microbiol Res. 2021;12:43–52.
Oogai Y, Matsuo M, Hashimoto M, Kato F, Sugai M, Komatsuzawa H. Expression of virulence factors by Staphylococcus aureus grown in serum. Appl Environ Microbiol. 2011;77:8097–105.
Krakauer T. Staphylococcal superantigens: pyrogenic toxins induce toxic shock. Toxins. 2019;11:178.
Dinges MM, Orwin PM, Schlievert PM. Exotoxins of Staphylococcus aureus. Clin Microbiol Rev. 2000;13:16–34.
Kadariya J, Smith TC, Thapaliya D. Staphylococcus aureus and staphylococcal food-borne disease: an ongoing challenge in public health. Biomed Res Int. 2014;2014:827965.
Argudín M, Mendoza MC, Rodicio MR. Food poisoning and Staphylococcus aureus enterotoxins. Toxins. 2010;2:1751–73.
Howell MD, Diveley JP, Lundeen KA, Esty A, Winters ST, Carlo DJ, Brostoff SW. Limited t-cell receptor beta-chain heterogeneity among interleukin 2 receptor-positive synovial t cells suggests a role for superantigen in rheumatoid arthritis. Proc Natl Acad Sci. 1991;88:10921–5.
Breuer K, Wittmann M, Bösche B, Kapp A, Werfel T. Severe atopic dermatitis is associated with sensitization to Staphylococcal enterotoxin B (SEB). Allergy. 2000;55:551–5.
Lowy FD. Antimicrobial resistance: the example of Staphylococcus aureus. J Clin Investig. 2003;111:1265–73.
Balaban N, Rasooly A. Staphylococcal enterotoxins. Int J Food Microbiol. 2000;61:1–10.
Ferens WA, Bohach GA. Persistence of Staphylococcus aureus on mucosal membranes: Superantigens and internalization by host cells. J Lab. 2000;135:225–30.
Banks MC, Kamel NS, Zabriskie JB, Larone DH, Ursea D, Posnett DN. Staphylococcus aureus express unique superantigens depending on the tissue source. J Infect Dis. 2003;187:77–86.
Wang X, Meng J, Zhang J, Zhou T, Zhang Y, Yang B, Xi M, Xia X. Characterization of Staphylococcus aureus isolated from powdered infant formula milk and infant rice cereal in china. Int J Food Microbiol. 2012;153:142–7.
Ahmed YM, Ali H, Gorish MT, Ali OS, Abdalrhim SAE, Mergani HM, Elgadir AAA, Mohammed KS, Ahmed OS, Musa AN, et al. Molecular detection of staphylococcal enterotoxins and meca genes products in selected food samples collected from different areas in khartoum state. Int J Microbiol. 2021;2021:5520573.
McLauchlin J, Narayanan GL, Mithani V, O’Neill G. The detection of enterotoxins and toxic shock syndrome toxin genes in Staphylococcus aureus by polymerase chain reaction. J Food Prot. 2000;63:479–88.
Galar A, Weil AA, Dudzinski DM, Muñoz P, Siedner MJ. Methicillin-resistant Staphylococcus aureus prosthetic valve endocarditis: Pathophysiology, epidemiology, clinical presentation, diagnosis, and management. Clin Microbiol Rev. 2019. https://doi.org/10.1128/CMR.00041-18.
Kimmig A, Hagel S, Weis S, Bahrs C, Löffler B, Pletz MW. Management of Staphylococcus aureus bloodstream infections. Front Med. 2020;7:616524.
Qiao Y, Liu X, Li B, Han Y, Zheng Y, Yeung KWK, Li C, Cui Z, Liang Y, Li Z, et al. Treatment of mrsa-infected osteomyelitis using bacterial capturing, magnetically targeted composites with microwave-assisted bacterial killing. Nat Commun. 2020;11:4446.
Shorr AF. Epidemiology and economic impact of meticillin-resistant Staphylococcus aureus: review and analysis of the literature. Pharmacoeconomics. 2007;25:751–68.
Abramson MA, Sexton DJ. Nosocomial methicillin-resistant and methicillin-susceptible Staphylococcus aureus primary bacteremia: At what costs? Infect Control Hosp Epidemiol. 1999;20:408–11.
Merda D, Felten A, Vingadassalon N, Denayer S, Titouche Y, Decastelli L, Hickey B, Kourtis C, Daskalov H, Mistou M-Y. Naura: genomic tool to identify staphylococcal enterotoxins in Staphylococcus aureus strains responsible for foodborne outbreaks. Front Microbiol. 2020;11:1483.
Wu S, Duan N, Gu H, Hao L, Ye H, Gong W, Wang Z. A review of the methods for detection of Staphylococcus aureus enterotoxins. Toxins. 2016;8:176.
Abril GA, Villa TG, Barros-Velázquez J, Cañas B, Sánchez-Pérez A, Calo-Mata P, Carrera M. Staphylococcus aureus exotoxins and their detection in the dairy industry and mastitis. Toxins. 2020;12:537.
Schmitz F-J, Steiert M, Hofmann B, Verhoef J, Hadding U, Heinz H-P, Köhrer K. Development of a multiplex-PCR for direct detection of the genes for enterotoxin B and C, and toxic shock syndrome toxin-1 in Staphylococcus aureus isolates. J Med Microbiol. 1998;47:335–40.
Russo G, Zegar C, Giordano A. Advantages and limitations of microarray technology in human cancer. Oncogene. 2003;22:6497–507.
El-Nasser AM, El Salakawy AH, Mira AA, Ibrahim DF, El-Sharaky HF. Epidemiological typing of methicillin resistant Staphylococcus aureus isolated from surgical site infection following caesarean section in an Egyptian university hospital. Egypt J Hosp Med. 2019;77:5534–41.
Weinstein MP, Lewis JS. The clinical and laboratory standards institute subcommittee on antimicrobial susceptibility testing: background, organization, functions, and processes. J Clin Microbiol. 2020;58:e01864-e1819.
Gurung RR, Maharjan P, Chhetri GG. Antibiotic resistance pattern of Staphylococcus aureus with reference to MRSA isolates from pediatric patients. Future Sci. 2020;6:464.
Sun J, Yang M, Sreevatsan S, Davies PR. Prevalence and characterization of Staphylococcus aureus in growing pigs in the USA. PLoS ONE. 2015;10:e0143670.
Sergeev N, Volokhov D, Chizhikov V, Rasooly A. Simultaneous analysis of multiple staphylococcal enterotoxin genes by an oligonucleotide microarray assay. J Clin Microbiol. 2004;42:2134–43.
El-Baz AM, Yahya G, Mansour B, El-Sokkary MMA, Alshaman R, Alattar A, El-Ganiny AM. The link between occurrence of class I integron and acquired aminoglycoside resistance in clinical MRSA isolates. Antibiotics. 2021;10:488.
Wang W, Baloch Z, Jiang T, Zhang C, Peng Z, Li F, Fanning S, Ma A, Xu J. Enterotoxigenicity and antimicrobial resistance of Staphylococcus aureus isolated from retail food in China. Front Microbiol. 2017;8:2256.
Keikhaie KR, Bagheri G, Kamali A, Bazi S, Shahi Z, Javadian F. Standardization of molecular diagnostic of the entC and ent e Staphylococcus aureus isolated from human infections in zabol. Gene Cell Tissue. 2017. https://doi.org/10.5812/gct.59214.
Jørgensen H, Mørk T, Høgåsen H, Rørvik L. Enterotoxigenic Staphylococcus aureus in bulk milk in Norway. J Appl Microbiol. 2005;99:158–66.
Etter D, Schelin J, Schuppler M, Johler S. Staphylococcal enterotoxin c-an update on sec variants, their structure and properties, and their role in foodborne intoxications. Toxins. 2020;12:584.
Strassle PD, Gu W, Bruce BB, Gould LH. Sex and age distributions of persons in foodborne disease outbreaks and associations with food categories. Epidemiol Infect. 2019;147:e200.
Gonano M, Hein I, Zangerl P, Rammelmayr A, Wagner M. Phenotypic and molecular characterization of Staphylococcus aureus strains of veterinary, dairy and human origin. Epidemiol Infect. 2009;137:688–99.
Ma Y, Zhao Y, Tang J, Tang C, Chen J, Liu J. Antimicrobial susceptibility and presence of resistance & enterotoxins/enterotoxin-likes genes in Staphylococcus aureus from food. Journal of Food. 2018;16:76–84.
Wang W, Baloch Z, Jiang T, Zhang C, Peng Z, Li F, Fanning S, Ma A, Xu J. Enterotoxigenicity and antimicrobial resistance of Staphylococcus aureus isolated from retail food in china. J Infect Dis. 2017;8:2256.
Vaez H, Ghazi SK, Moradi A, Tabaraei A, Khodabakhshi B, Bazouri M, Golriz N, Ghaemi EE. Antibiotic resistance pattern of methicillin resistant Staphylococcus aureus isolated from health-educational centers of Gorgan, Iran, 2008–2009. Iran J Med Microbiol. 2010;3:31–6.
Wielders CL, Fluit AC, Brisse S, Verhoef J, Schmitz FJ. Meca gene is widely disseminated in Staphylococcus aureus population. J Clin Microbiol. 2002;40:3970–5.
Skov R, Smyth R, Larsen AR, Bolmstrôm A, Karlsson A, Mills K, Frimodt-Moller N, Kahlmeter G. Phenotypic detection of methicillin resistance in Staphylococcus aureus by disk diffusion testing and etest on mueller-hinton agar. J Clin Microbiol. 2006;44:4395–9.
Sousa Júnior FCD, Néri GDS, Silva AK, Araújo BPRCD, Guerra MJDPD, Fernandes MJDBC, Milan EP, Melo MCND. Evaluation of different methods for detecting methicillin resistance in Staphylococcus aureus isolates in a university hospital located in the northeast of brazil. Brazil J Microbiol. 2010;41:316–20.
Kadry A, Shaker G, El-Ganiny A, Youssef C. Phenotypic and genotypic detection of local mrsa isolates. Zagazig J Pharm Sci. 2016;25:39–46.
Gurung RR, Maharjan P, Chhetri GGJFSO. Antibiotic resistance pattern of Staphylococcus aureus with reference to MRSA isolates from pediatric patients. Canadian J Infect Dis Med Microbiol. 2020;6:464.
Garoy EY, Gebreab YB, Achila OO, Tekeste DG, Kesete R, Ghirmay R, Kiflay R, Tesfu T. Methicillin-resistant Staphylococcus aureus (MRSA): prevalence and antimicrobial sensitivity pattern among patients a multicenter study in Asmara, Eritrea. Canadian J Infect Dis. 2019;2019:1–9.
Hassoun A, Linden PK, Friedman B. Incidence, prevalence, and management of mrsa bacteremia across patient population a review of recent developments in MRSA management and treatment. Crit Care. 2017;21:1–10.
Schelin J, Wallin-Carlquist N, Thorup Cohn M, Lindqvist R, Barker GC. The formation of staphylococcus aureus enterotoxin in food environments and advances in risk assessment. Virulence. 2011;2:580–92.
Niveditha P, Shylaja R, Radhika M, Murali S, Harshvardhan B. A novel mpcr for the detection of prominent toxins in MRSA strains of s. Aureus recovered from diverse sources. Int J Sci Res Biol Sci. 2014;2:1–3.
Luchian I, Goriuc A, Martu MA, Covasa M. Clindamycin as an alternative option in optimizing periodontal therapy. Antibiotics. 2021;10:814.
Mashouf RY, Hosseini SM, Mousavi SM, Arabestani MR. Prevalence of enterotoxin genes and antibacterial susceptibility pattern of Staphylococcus aureus strains isolated from animal originated foods in west of iran. Oman Med J. 2015;30:283.
Omoe K, Imanishi KI, Hu DL, Kato H, Takahashi-Omoe H, Nakane A, Uchiyama T, Shinagawa K. Biological properties of staphylococcal enterotoxin-like toxin type r. Infect Immun. 2004;72(6):3664–7.
Ali HH. Isolation and identification of staphylococcus bacteria from fish of fresh water and its antibiotics sensitivity in mosul city. Basrah J Vetrinary Res. 2014;1:33–42.
Arslan S, Özdemir F. Molecular characterization and detection of enterotoxins, methicillin resistance genes and antimicrobial resistance of Staphylococcus aureus from fish and ground beef. Polish J Vet Sc. 2017;20:85–94.
El-Sokkary MMA. Microbial profiling of wound pathogens in isolates from an egyptian hospital using a microarray chip. J Appl Pharm Sci. 2021;11:139–46.
Anthony RM, Brown TJ, French GL. Rapid diagnosis of bacteremia by universal amplification of 23s ribosomal DNA followed by hybridization to an oligonucleotide array. J Clin Microbiol. 2000;38:781–8.
Chizhikov V, Rasooly A, Chumakov K, Levy DD. Microarray analysis of microbial virulence factors. Appl Environ Microbiol. 2001;67:3258–63.
Acknowledgements
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). The authors of this article express their gratitude and appreciation to the Competitive Funding Projects Postgraduate Research and Cultural Affairs Sector, Mansoura University for funding the microarray experiments in this study.
Funding
This study was financially supported by Competitive Funding Projects Postgraduate Research and Cultural Affairs Sector, Mansoura University.
Author information
Authors and Affiliations
Contributions
ME-S conceptualization, supervision, study plan, resources, software, funding acquisition; ME-S, AE-B, RG, RE-M and HR methodology, formal analysis, writing original draft, investigation, writing-review and editing; all authors read and approve the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by Delta University for Science and Technology, Egypt. Consent to participate; patients provided oral consent.
Consent for publication
The authors declare no financial interest upon publication of this study.
Competing interests
Authors declare no competing interests upon publication of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Ramadan, H.A., El-Baz, A.M., Goda, R.M. et al. Molecular characterization of enterotoxin genes in methicillin-resistant S. aureus isolated from food poisoning outbreaks in Egypt. J Health Popul Nutr 42, 86 (2023). https://doi.org/10.1186/s41043-023-00416-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41043-023-00416-z