Abstract
Background
Contemporary radiotherapy for the treatment of lung cancer is effective in targeting tumor tissue while limiting heart exposure, yet cardiac toxicity still occurs, often becoming clinically apparent years later. Cardiorespiratory fitness (CRF) is an independent predictor of cardiovascular, cancer-related, and overall mortality and may serve as a sensitive measure of subclinical cardiac toxicity following anti-cancer treatments. Prior work has demonstrated a significant relationship between reduced CRF and impaired left-ventricular (LV) diastolic reserve in cancer survivors following thoracic radiotherapy. The purpose of this study was to assess early longitudinal changes in CRF and cardiac function in patients with lung cancer following radiotherapy.
Methods
Ten patients (69 [61–76] years, 70% female) with lung cancer without known cardiovascular disease scheduled to receive radiotherapy involving a clinically-relevant heart dose (≥ 5 Gy to > 10% of heart volume) were evaluated prior to and following treatment. Changes in CRF (peak oxygen consumption [VO2peak], oxygen uptake efficiency slope [OUES]), cardiac function (LV ejection fraction [LVEF], rest and exercise diastolic function [diastolic functional reserve index (DFRI)]), cardiac biomarkers (N-terminal pro-brain natriuretic peptide [NT-proBNP], high-sensitivity C-reactive protein [hsCRP]), and health-related quality of life (HRQOL; Functional Assessment of Cancer Therapy-General-7 [FACT-G7]) were measured.
Results
The VO2peak was reduced at baseline (1.245 [0.882–1.605] L·min− 1; 70 [62–86] %-predicted) and significantly declined (1.095 [0.810–1.448] L·min− 1, P = 0.047; 62 [56–76] %-predicted, P = 0.005) at 6.0 [3.0–6.0] months post-radiotherapy. Similarly, a significant decline in the OUES was observed (1.63 [1.27–1.88] to 1.57 [1.12–1.75], P = 0.032). Systolic cardiac function was normal at baseline and did not change following radiotherapy (LVEF; 62 [56–65]% to 66 [57–68]%, P = 0.475). The DFRI significantly declined following radiotherapy (34.9 [22.7–41.6] vs. 12.8 [3.1–35.9]). The hsCRP increased significantly from 4.4 [1.4–5.8] to 6.1 [3.7–20.7] g/L, P = 0.047 with a trend towards higher levels of NT-proBNP (65 [49–125] to 121 [88–191] pg/mL, P = 0.110). Health-related quality of life significantly decreased (FACT-G7; 21.5 [18.8–25] to 15.5 [11.5–20]; P = 0.021) post-radiotherapy.
Conclusions
Patients with lung cancer receiving radiotherapy with a clinically-significant heart dose experience reductions in CRF (VO2peak, OUES) as early as six months following treatment with concurrent reductions in diastolic reserve (DFRI), HRQOL, and increases in cardiac biomarkers (NT-proBNP, hsCRP).
Similar content being viewed by others
Introduction
Radiotherapy is a standard treatment in patients with lung cancer. While it improves survival, it is associated with an increased risk of cardiovascular disease and is a leading cause of nonmalignant morbidity and mortality [1, 2]. Radiation-induced cardiac disease (RICD) is typically thought to be a late-occurring event, but studies have shown that early subclinical changes occur [2, 3]. Patients with lung cancer are at high cardiovascular risk at baseline (i.e., due to tobacco smoking history, increasing prevalence of shared cardiovascular disease (CVD) risk factors, advancing age, and comorbid chronic obstructive pulmonary disease [COPD]) which likely shortens the latency period between radiation exposure and subsequent cardiotoxicity. Commonly used tools to assess cardiac function (i.e., left ventricular ejection fraction [LVEF] with echocardiography) are known to be insensitive to minor injury and therefore subtle changes in myocardial systolic or diastolic function may go unnoticed for many years [4]. Indeed, radiation-induced cardiomyopathy typically presents more frequently with LV diastolic function abnormalities antecedent to declines in systolic function [3, 5, 6].
There is a growing body of literature that underscores the assessment of cardiorespiratory fitness (CRF) to stratify risk in the cancer patient [7,8,9,10] suggesting it may serve as an integrated functional biomarker to detect cardiotoxicity [11,12,13]. Cardiopulmonary exercise testing (CPET) is the gold standard for evaluating integrative cardiovascular function and yields an objective quantifiable measure of CRF through the measurement of peak oxygen consumption (VO2peak) and the oxygen uptake efficiency slope (OUES), surrogates for quality of life and survival in patients with lung cancer and heart failure [10, 14,15,16,17]. Previous work has demonstrated significant CRF impairment and a strong inverse relationship between CRF and survival in patients with lung cancer [18,19,20] with the mechanisms of impairment being likely multifactorial due to multiple derangements in the oxygen cascade (i.e., impairments in respiratory, cardiovascular, and musculoskeletal function).
Our previous work has demonstrated an independent association between VO2peak and the diastolic functional reserve index (DFRI), a Doppler-stress echocardiography measurement accounting for resting and exercise-induced changes in early diastolic mitral annular velocity (e’), in patients who were free of overt cardiovascular disease and had previously received chest radiotherapy, where a decreased DFRI was associated with reduced CRF [21]. Additionally, metrics of CRF were inversely associated with LV extracellular volume fraction [22], a marker of diffuse myocardial fibrosis known to play a role in the pathophysiology of RICD [23]. Furthermore, in patients receiving radiotherapy for lung cancer with reduced respiratory function, we’ve shown that diastolic dysfunction contributes to reduced CRF, and that serum levels of N-terminal pro-brain natriuretic peptide (NT-proBNP) independently predicted VO2peak [24]. These data suggest that changes in CRF and associated changes in cardiac diastolic reserve may serve as novel markers of RICD. The purpose of this study was to assess early longitudinal changes in CRF (VO2peak, OUES) and cardiac function with a focus on exercise diastolic reserve in adults with lung cancer following radiotherapy with an incidental heart dose. We hypothesized that individuals with lung cancer receiving radiotherapy would experience interval declines in CRF and cardiac diastolic reserve.
Methods
The primary aim of this pilot study was to study longitudinal changes in CRF (measured as VO2peak and the OUES) in adult patients with lung cancer undergoing radiotherapy treatment with an incidental heart dose ≥ 5 Gy (Gy) to > 10% heart volume who were able to undergo symptom-limited treadmill exercise testing. Secondary aims were to additionally study changes in cardiac diastolic reserve, blood biomarkers (high-sensitivity C-reactive protein [hsCRP], NT-proBNP), health-related quality of life, and metrics influencing the exercise response (respiratory function, physical activity levels, body habitus).
This study included patients (≥ 21 years of age) with locally advanced lung cancer within the Virginia Commonwealth University Massey Cancer Center Radiation Oncology clinics who were scheduled to receive radiotherapy with incidental heart exposure of ≥ 5 Gy to > 10% of the heart volume with no or minimal radiation dose to the heart previously (< 2 Gy mean heart dose). Exclusion criteria was contraindications to exercise testing as defined by the American Heart Association [25]. The study was approved by the local Institutional Review Board (HM20017432) and all subjects provided informed consent before enrollment. Analysis was limited to patients who completed both baseline and post-radiotherapy follow-up visits. Radiation dose was calculated based on a volumetric computed tomography data set obtained during a treatment planning session. A radiation oncologist quantified the total and heart radiation doses including %-volumes (V) of the heart receiving ≥ 5, 10, 20, 30, 40, and 50 Gy, respectively. Presence of cardiovascular disease (CVD) was defined as prior history or diagnosis of heart failure, coronary artery, cerebrovascular, or peripheral artery disease or aortic atherosclerosis. The presence of traditional CVD risk factors (smoking, hyperlipidemia, hypertension, diabetes, sedentary lifestyle, and obesity), CVD medication use (beta-blockers, angiotensin converting enzyme inhibitors/angiotensin receptor blockers, statins), comorbidities (history of COPD, prior cancers, renal disease), and cancer type, stage, and treatments were collected from medical records review and patient interview.
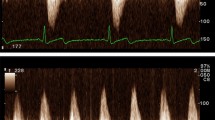
Patients underwent symptom-limited treadmill CPET according to established guidelines using a low-level (∼ 2 mL·kg− 1·min− 1 (estimated VO2)/ 30-seconds) ramping protocol coupled with Doppler-stress echocardiography before and after radiotherapy [25, 26]. Cardiorespiratory fitness was measured as VO2peak (highest average value in the final 30-seconds of exercise) and expressed in absolute (L·min− 1), relative (mL·kg− 1·min− 1), and %-predicted values using the reference values proposed by Wasserman and colleagues [27]. Impaired VO2peak was defined as < 85% of predicted values [28]. Functional disability, a threshold for the ability to independently perform activities of daily living, was defined as a VO2peak ≤ 18.0 mL·kg− 1·min− 1 [29]. The OUES, an effort-independent marker of CRF that strongly correlates with VO2peak, [30] was calculated as the quotient of VO2/log-transformed minute ventilation (VE) throughout the entire exercise period. The OUES was selected as an additional CRF measurement in this population based on its ability to assess CRF in the instance of a submaximal effort using conventional maximal test criteria [31]. The respiratory exchange ratio (RER) was calculated as the quotient of carbon dioxide production (VCO2) divided by VO2 at peak exercise. The VE/VCO2 slope was calculated from the entire exercise period. Two-dimensional transthoracic echocardiography was performed at rest and immediately post-exercise to measure cardiac structure and function according to standard recommendations [32] with focus on Doppler-derived diastolic function (early [E]/late [A] transmitral velocities, lateral and septal e’, calculation of E/e’, and DFRI [rest e’ x Δstress e’]).
Anthropometrics (body mass index [BMI]), physical activity levels, pulmonary function testing, and blood biomarkers (hsCRP, NT-proBNP, hemoglobin) were obtained pre-exercise at baseline and follow-up visits. Elevated hsCRP was defined as ≥ 1 mg/L and elevated NT-proBNP was defined as ≥ 125 pg/mL, respectively [33, 34]. Physical activity levels were assessed using the International Physical Activity Questionnaire (IPAQ), a validated questionnaire [35]. Pulmonary function testing was performed according to standard recommendations and included measurements of forced vital capacity (FVC), forced expiratory volume-1 s (FEV-1), and diffusing capacity of lung for carbon monoxide (DLCO) corrected for hemoglobin levels [36, 37]. Health-related quality of life (HRQOL) was assessed using the Functional Assessment of Cancer Therapy-General-7 (FACT-G7) instrument [38].
Continuous data are reported as median [interquartile range] based on non-normal distribution of data using tests of normality (Shapiro-Wilk) prior to data analysis or number (%) for nominal variables. All analyses were performed with non-parametric tests due to the assumption of non-normally distributed data. Spearman’s rank correlation coefficients were used to assess bivariate relationships for continuous variables. Pre/post related-samples comparisons of cardiopulmonary variables were made using the Wilcoxon signed-rank test. Post-hoc comparisons of significant pre/post related-samples testing were performed on baseline clinical characteristics (Yes/No; CVD risk factors, CV medication use, comorbidities, cancer stage, durvalumab immunotherapy use) using the Mann-Whitney U test. Statistical analysis was performed using SPSS v29.0 (IBM Corp, Armonk, NY) with a P-value < 0.05 considered significant. A formal power calculation was not completed due to the exploratory nature of the study and the lack of available data on this population to inform the calculation.
Results
Ten patients (70% White females, 80% Stage III-IV lung cancer, 69 [61–76] years of age, all Eastern Cooperative Oncology Group status 0–1) underwent assessments at 1.5 [1.0-2.5] months following lung cancer diagnosis and 6.5 [4.5–12.3] days prior to the start of radiotherapy. Post-radiotherapy assessments occurred at 7.0 [4.8–7.3] months following baseline and 6 [3.0–6.0] months following the completion of radiotherapy. None of the patients had established cardiovascular disease. Table 1 details the baseline CVD factors, cardiovascular medication use, and oncologic characteristics of the cohort. There was a high prevalence of CVD risk factors, CV medication use, and comorbid COPD at baseline. Additionally, three patients (30%) had a history of prior chest surgery of various complexity (video-assisted thoracic surgery [VATS] – no intervention; right-upper lobe lobectomy; VATS with wedge resection only). However, none of these were associated with significant interval differences (i.e., before versus after radiotherapy assessments) in CRF or echocardiogram parameters (all P-values > 0.05). All patients received concurrent chemotherapy (90% carboplatin/paclitaxel regimens). Sixty-percent also received durvalumab immunotherapy which was not associated with interval changes in cardiopulmonary variables (all P’s > 0.393). Total prescribed radiotherapy dose was 60 Gy delivered in 30 fractions (2 Gy/fraction), mean lung dose was 11.0 [8.8–13.7] Gy, mean heart dose was 8.1 [4.8–12.4] Gy, and volume of heart receiving 5 Gy was 39.0 [18.3–54.5] %. The %-volumes of the heart receiving V10, V20, V30, V40, and V50 Gy, are listed in Table 1. All patients were treated with volumetric modulated arc therapy (VMAT) using respiratory management and image guidance (IGRT) on linear accelerators (Truebeam, Varian Medical Systems, Palo Alto, CA, USA) following 4DCT-based treatment planning (Brilliance Big Bore, Philips Medical Systems, The Netherlands).
Table 2 displays the baseline and follow-up cardiopulmonary variables. Overall, the VO2peak was impaired at baseline (70 [62–86] %-predicted) with 80% of patients having values < 85% of predicted consistent with impaired CRF. The VO2peak significantly declined at post-radiotherapy follow-up assessments for absolute (Fig. 1), relative, and %-predicted VO2peak expressions (P = 0.047; P = 0.047; P = 0.005), respectively. Furthermore, the proportion of patients that met criteria for functional disability was 7/10 (70%) and 8/10 (80%) at the baseline and follow-up assessments, respectively. A significant reduction in the OUES was observed between the baseline and follow-up assessments (1.63 [1.27–1.88] to 1.57 [1.12–1.75], P = 0.032). The OUES at baseline and follow-up demonstrated a strong positive correlation with VO2peak (R = 0.818, P = 0.004 & R = 0.888, P < 0.001) reflecting its ability to track with VO2peak in the scenario of a suboptimal exercise effort.
Pre- and post-radiotherapy box-whisker plots of significant variables
Panel A: VO2peak. Panel B: FACT-G7 questionnaire scores. Panel C: Diastolic functional reserve index (DFRI). Panel D: Change in E/e’ with stress
Abbreviations: VO2 = oxygen consumption; FACT-G7 = Functional Assessment of Cancer Therapy-General-7; DFRI = diastolic functional reserve index; E/e’= early transmitral flow [E] to early diastolic mitral annular velocity [e’] ratio
We found a significant decline in the DFRI (34.9 [22.7–41.6] vs. 12.8 [3.1–35.9], P = 0.037) that was driven by an attenuated interval change in e’ after stress (4.5 [3.4–5.2] vs. 1.3 [0.3–4.5] cm/sec, P = 0.022; Fig. 1). Similarly, the change in Doppler E/e’ after stress (inverse measure of diastolic reserve) was significantly increased (-1.2 [-2.4, + 0.4] to + 2.0 [+ 0.4, + 2.3], P = 0.028; Fig. 1). Pre-treatment echocardiography revealed a LVEF of 62 [56–65] % with no significant change post-radiotherapy (66 [57–68] %).
The FACT-G7 score significantly decreased from 21.5 [18.8–25] to 15.5 [11.5–20] (P = 0.021) post-radiotherapy reflecting an interval decline in HRQOL. The hsCRP was above normal (≥ 1 mg/L) at 4.4 [1.4–5.8] in 9/10 (90%) of the subjects at baseline and increased significantly post-radiotherapy to 6.1 [3.7–20.7] mg/L, P = 0.047 with 9/10 (90%) of the subjects having elevated hsCRP levels at post-radiotherapy assessment. There was a trend toward increased levels of NT-proBNP (65 [49–125] to 121 [88–191] pg/mL, P = 0.110) at the post-radiotherapy assessment with 2/10 (20%) subjects having elevated NT-proBNP levels (≥ 125 pg/mL) at baseline and 4/10 (40%) having elevated NT-proBNP values post-radiotherapy. No significant interval changes were noted in BMI, physical activity levels, hemoglobin, pulmonary function (FVC, FEV-1, DLCO), or rest/exercise heart rates or blood pressures at post-radiotherapy follow-up assessments.
Discussion
Cardiopulmonary exercise testing is the gold standard for evaluating integrative cardiovascular function and provides the unique advantage of objective quantifiable measures of CRF that are independent predictors of lung cancer morbidity, mortality, and overall quality of life [10, 39, 40]. This longitudinal, multidisciplinary study coupling functional cardiopulmonary and cardiac imaging studies demonstrates significant early changes in CRF and diastolic reserve are evident in patients with lung cancer following radiotherapy. A 12%, 2%, and 11% decrease (absolute change: -8 [-5 to -12%]), respectively, was observed in absolute, relative, and percent-predicted VO2peak values between baseline and follow-up assessments performed 6 months following completion of radiotherapy. Furthermore, no significant differences were found between pre- and post-radiotherapy changes in body habitus (BMI), physical activity participation (IPAQ), pulmonary function results (FVC, FEV-1, DLCO), or hemoglobin levels, all of which have the potential to influence the CRF response.
In a population of apparently healthy adults, Imboden et al. described longitudinal changes in directly-measured CRF adjusted for time (mean time of 8.6 years between CRF assessments), baseline VO2peak, age, sex, and traditional risk factors and demonstrated a 1 mL·kg− 1·min− 1 change in VO2peak was inversely associated with a ∼ 11, 15, and 16% respective risk for all-cause, CVD, and cancer mortality [41]. Similarly, studies by Chiaranda et al., using a different expression of CRF demonstrated that each 1% unit change in percent-predicted VO2peak was associated with a 3% hospital admission and/or 3% mortality risk in patients with cardiovascular disease [42, 43]. These studies demonstrate that small longitudinal changes in VO2peak can have significant impact.
In this study, we demonstrated interval declines in indices of diastolic reserve following radiotherapy that were concurrent with reductions in CRF. Our findings of declines in CRF, concurrent reductions in DFRI, and increases in the change in E/e’ after stress (inverse measure of diastolic reserve) reflects impaired myocardial relaxation or elevated filling pressures with exercise may be contributing to the reductions in CRF. This corroborates previous work in patients with lung cancer following radiotherapy without established CVD or heart failure that demonstrated reduced CRF is associated with measures of diastolic function (DFRI, E/e’) and biomarkers of ventricular wall stress [21, 24]. Although the general concept of RICD presenting as a predominantly diastolic dysfunction phenotype is widely accepted, there is surprisingly sparse literature to date incorporating measurements of diastolic function into the clinical assessment or study of patients following anti-cancer radiotherapy. Abnormal diastolic function impairs exercise capacity, which is likely a main contributor to the reduced HRQOL seen in patients with diastolic dysfunction. While studies have largely focused on diastolic dysfunction in patients with reduced ejection fraction, several studies have shown reductions in HRQOL in patients with diastolic dysfunction and preserved ejection fraction, often times being attributed to elevated filling pressures, which typically presents as dyspnea and fatigue [44, 45].
We also observed a significant increase in hsCRP post radiotherapy. Systemic inflammation following radiotherapy has been previously reported and associated with cardiac dysfunction [46,47,48,49,50].
Study limitations
Limitations of this pilot study include the small sample size, single-site location, lack of a control group, confounding by the lung cancer disease process itself and potential contributions of the chemotherapeutic and immunotherapy agents, and lack of dedicated studies evaluating musculoskeletal function. Additionally, this study was limited to patients who completed both baseline and post-radiotherapy follow-up assessments, with a variable time to final assessment, and may be subject to selection/ recruitment bias. Strengths to this small study include the longitudinal study design and comprehensive assessment of non-cardiac causes of impaired CRF including respiratory function testing, hemoglobin status, and dedicated assessments of exercise diastolic function.
Conclusions
Our preliminary findings indicate that a decline in cardiorespiratory fitness can be detected within the first six months following radiotherapy in patients with lung cancer and aligns with established research in the field describing declines in cardiorespiratory fitness that occur in patients with cancer undergoing cancer-related treatment. These data suggest that serial changes in cardiorespiratory fitness and cardiac diastolic reserve may serve as early markers to evaluate the potential effects of radiation therapy. However, these findings should be regarded as hypothesis-generating only and do not infer causality. Larger confirmatory studies aimed at addressing potential confounders as well as further investigation into the pathophysiology underlying the observed changes are warranted.
Data availability
The data analyzed during this study are available from the corresponding author upon reasonable request.
Change history
18 April 2024
The presentation of author Marco Giuseppe Del Buono’s family is incorrectly tagged. This is now corrected
Abbreviations
- COPD:
-
Chronic obstructive pulmonary disease
- CPET:
-
Cardiopulmonary exercise testing
- CRF:
-
Cardiorespiratory fitness
- CVD:
-
Cardiovascular disease
- DFRI:
-
Diastolic functional reserve index
- DLCO:
-
Diffusing capacity of lung for carbon monoxide
- E/e:
-
Early transmitral flow to early diastolic mitral annular velocity ratio
- FACT-G7:
-
Functional Assessment of Cancer Therapy-General-7
- FEV-1:
-
Forced expiratory volume-1second
- FVC:
-
Forced vital capacity
- HRQOL:
-
Health-related quality of life
- hsCRP:
-
High-sensitivity C-reactive protein
- LVEF:
-
Left ventricular ejection fraction
- NT-proBNP:
-
N-terminal pro-brain natriuretic peptide
- OUES:
-
Oxygen uptake efficiency slope
- RICD:
-
Radiation-induced cardiac disease
- V:
-
Volume
- VO2 :
-
Oxygen consumption
References
Chang HM, Okwuosa TM, Scarabelli T, Moudgil R, Yeh ETH. Cardiovascular complications of Cancer Therapy: best practices in diagnosis, Prevention, and management: part 2. J Am Coll Cardiol. 2017;70(20):2552–65. https://doi.org/10.1016/j.jacc.2017.09.1095
Atkins KM, Rawal B, Chaunzwa TL, et al. Cardiac Radiation Dose, Cardiac Disease, and mortality in patients with Lung Cancer. J Am Coll Cardiol. 2019;73(23):2976–87. https://doi.org/10.1016/j.jacc.2019.03.500
Groarke JD, Nguyen PL, Nohria A, Ferrari R, Cheng S, Moslehi J. Cardiovascular complications of radiation therapy for thoracic malignancies: the role for non-invasive imaging for detection of cardiovascular disease. Eur Heart J. 2014;35(10):612–23. https://doi.org/10.1093/eurheartj/eht114
Makavos G, Ikonomidis I, Palios J, et al. Cardiac imaging in cardiotoxicity: a focus on clinical practice. Heart Fail Rev. 2021;26(5):1175–87. https://doi.org/10.1007/s10741-020-09952-w
Belzile-Dugas E, Eisenberg MJ. Radiation-Induced Cardiovascular Disease: review of an Underrecognized Pathology. J Am Heart Assoc. 2021;10(18):e021686. https://doi.org/10.1161/JAHA.121.021686
Tuohinen SS, Skyttä T, Huhtala H, et al. 3-Year Follow-Up of Radiation-Associated changes in diastolic function by Speckle Tracking Echocardiography. JACC CardioOncol. 2021;3(2):277–89. https://doi.org/10.1016/j.jaccao.2021.03.005
Nadruz W Jr, West E, Sengeløv M, et al. Cardiovascular phenotype and prognosis of patients with heart failure induced by cancer therapy. Heart. 2019;105(1):34–41. https://doi.org/10.1136/heartjnl-2018-313234
Lakoski SG, Willis BL, Barlow CE, et al. Midlife Cardiorespiratory Fitness, Incident Cancer, and Survival after Cancer in men: the Cooper Center Longitudinal Study. JAMA Oncol. 2015;1(2):231–7. https://doi.org/10.1001/jamaoncol.2015.0226
Groarke JD, Payne DL, Claggett B, et al. Association of Post-diagnosis cardiorespiratory fitness with cause-specific mortality in cancer. Eur Heart J Qual Care Clin Outcomes. 2020;6(4):315–22. https://doi.org/10.1093/ehjqcco/qcaa015
Jones LW, Watson D, Herndon JE 2, et al. Peak oxygen consumption and long-term all-cause mortality in nonsmall cell lung cancer. Cancer. 2010;116(20):4825–32. https://doi.org/10.1002/cncr.25396
Howden EJ, Foulkes S, Dillon HT, et al. Traditional markers of cardiac toxicity fail to detect marked reductions in cardiorespiratory fitness among cancer patients undergoing anti-cancer treatment. Eur Heart J Cardiovasc Imaging. 2021;22(4):451–8. https://doi.org/10.1093/ehjci/jeaa421
Foulkes S, Claessen G, Howden EJ, Daly RM, Fraser SF, La Gerche A. The Utility of Cardiac Reserve for the early detection of Cancer Treatment-Related Cardiac Dysfunction: a comprehensive overview. Front Cardiovasc Med. 2020;7:32. https://doi.org/10.3389/fcvm.2020.00032
Howden EJ, Bigaran A, Beaudry R, et al. Exercise as a diagnostic and therapeutic tool for the prevention of cardiovascular dysfunction in breast cancer patients. Eur J Prev Cardiol. 2019;26(3):305–15. https://doi.org/10.1177/2047487318811181
Arena R, Canada JM, Popovic D, et al. Cardiopulmonary exercise testing – refining the clinical perspective by combining assessments. Expert Rev Cardiovasc Ther. 2020;18(9):563–76. https://doi.org/10.1080/14779072.2020.1806057
Yakal S, Sofyalı S, Özkan B, Yıldız S, Toker A, Kasikcioglu E. Oxygen uptake efficiency slope and prediction of post-operative morbidity and mortality in patients with Lung Cancer. Lung. 2018;196(2):255–62. https://doi.org/10.1007/s00408-018-0085-y
Kasikcioglu E, Toker A, Tanju S, et al. Oxygen uptake kinetics during cardiopulmonary exercise testing and postoperative complications in patients with lung cancer. Lung Cancer. 2009;66(1):85–8. https://doi.org/10.1016/j.lungcan.2008.12.024
Weemaes ATR, Weijenberg MP, Lenssen AF, Beelen M. Exercise training as part of multidisciplinary rehabilitation in cancer survivors: an observational study on changes in physical performance and patient-reported outcomes. Support Care Cancer. 2022;30(11):9255–66. https://doi.org/10.1007/s00520-022-07351-5
Ezzatvar Y, Ramírez-Vélez R, de Asteasu ML, et al. Cardiorespiratory fitness and all-cause mortality in adults diagnosed with cancer systematic review and meta-analysis. Scand J Med Sci Sports. 2021;31(9):1745–52. https://doi.org/10.1111/sms.13980
Lee JC, Fitness P, Activity W, Speed. Lack of participation in leisure activities, and Lung Cancer Mortality: a systematic review and Meta-analysis of prospective cohort studies. Cancer Nurs. 2021;44(6):453–64. https://doi.org/10.1097/NCC.0000000000000847
Vainshelboim B, Lima RM, Edvardsen E, Myers J. Cardiorespiratory fitness, incidence and mortality of lung cancer in men: a prospective cohort study. J Sci Med Sport. 2019;22(4):403–7. https://doi.org/10.1016/j.jsams.2018.10.002
Canada JM, Trankle CR, Carbone S, et al. Determinants of Cardiorespiratory Fitness following thoracic radiotherapy in lung or breast Cancer survivors. Am J Cardiol. 2020;125(6):988–96. https://doi.org/10.1016/j.amjcard.2019.12.019
Canada J, Weiss E, Grizzard J, et al. Influence of extracellular volume fraction on peak exercise oxygen pulse following thoracic radiotherapy. Cardio-Oncology. 2022;8(1). https://doi.org/10.1186/s40959-021-00127-6
Quintero-Martinez JA, Cordova-Madera SN, Villarraga HR. Radiation-Induced Heart Disease. J Clin Med. 2021;11(1). https://doi.org/10.3390/jcm11010146
Thomas GK, Trankle CR, Carbone S, et al. Diastolic dysfunction contributes to impaired Cardiorespiratory Fitness in patients with Lung Cancer and reduced lung function following chest Radiation. Lung. 2021;199(4):403–7. https://doi.org/10.1007/s00408-021-00454-6
Fletcher GF, Ades PA, Kligfield P, et al. Exercise standards for testing and training: a scientific statement from the American Heart Association. Circulation. 2013;128(8):873–934. https://doi.org/10.1161/CIR.0b013e31829b5b44
Arena R, Humphrey R, Peberdy MA, Madigan M. Predicting peak oxygen consumption during a conservative ramping protocol: implications for the heart failure population. J Cardiopulm Rehabil. 2003;23(3):183–9. https://doi.org/10.1097/00008483-200305000-00004
Wasserman K, Hansen JE, Sue DY, Stringer WW, Whipp BJ. Principles of Exercise Testing and Interpretation: including pathophysiology and clinical applications. 4th ed. Lippincott Williams & Wilkins; 2005.
American Thoracic Society. American College of Chest Physicians. ATS/ACCP Statement on cardiopulmonary exercise testing. Am J Respir Crit Care Med. 2003;167(2):211–77. https://doi.org/10.1164/rccm.167.2.211
Morey MC, Pieper CF, Cornoni-Huntley J. Is there a threshold between peak oxygen uptake and self-reported physical functioning in older adults? Med Sci Sports Exerc. 1998;30(8):1223–9.
Davies LC, Wensel R, Georgiadou P, et al. Enhanced prognostic value from cardiopulmonary exercise testing in chronic heart failure by non-linear analysis: oxygen uptake efficiency slope. Eur Heart J. 2006;27(6):684–90. https://doi.org/10.1093/eurheartj/ehi672
Balady GJ, Arena R, Sietsema K, et al. Clinician’s guide to cardiopulmonary exercise testing in adults: a scientific statement from the American Heart Association. Circulation. 2010;122(2):191–225. https://doi.org/10.1161/CIR.0b013e3181e52e69
Nagueh SF, Smiseth OA, Appleton CP, et al. Recommendations for the evaluation of left ventricular diastolic function by Echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2016;29(4):277–314. https://doi.org/10.1016/j.echo.2016.01.011
Ridker PM. Clinical application of C-reactive protein for cardiovascular disease detection and prevention. Circulation. 2003;107(3):363–9. https://doi.org/10.1161/01.cir.0000053730.47739.3c
Lyon AR, López-Fernández T, Couch LS, et al. 2022 ESC guidelines on cardio-oncology developed in collaboration with the European Hematology Association (EHA), the European Society for Therapeutic Radiology and Oncology (ESTRO) and the International Cardio-Oncology Society (IC-OS). Eur Heart J. 2022;43(41):4229–361. https://doi.org/10.1093/eurheartj/ehac244
Craig C, Marshall A, Sjostrom M, et al. International Physical Activity Questionnaire: 12-Country reliability and validity. Med Sci Sports Exerc. 2003;35(8):1381–95. https://doi.org/10.1249/01.MSS.0000078924.61453.FB
Graham BL, Steenbruggen I, Miller MR, et al. Standardization of Spirometry 2019 Update. An official American Thoracic Society and European Respiratory Society Technical Statement. Am J Respir Crit Care Med. 2019;200(8):e70–e88. https://doi.org/10.1164/rccm.201908-1590ST
Graham BL, Brusasco V, Burgos F, et al. 2017 ERS/ATS standards for single-breath carbon monoxide uptake in the lung. Eur Respir J. 2017;49(1):1600016. https://doi.org/10.1183/13993003.00016-2016
Yanez B, Pearman T, Lis CG, Beaumont JL, Cella D. The FACT-G7: a rapid version of the functional assessment of cancer therapy-general (FACT-G) for monitoring symptoms and concerns in oncology practice and research. Ann Oncol. 2013;24(4):1073–8. https://doi.org/10.1093/annonc/mds539
Peddle-McIntyre CJ, Singh F, Thomas R, Newton RU, Galvão DA, Cavalheri V. Exercise training for advanced lung cancer. Cochrane Database Syst Rev. 2019;2(2):CD012685. https://doi.org/10.1002/14651858.CD012685.pub2
Voorn MJJ, Franssen RFW, Verlinden JMWF, et al. Associations between pretreatment physical performance tests and treatment complications in patients with non-small cell lung cancer: a systematic review. Crit Rev Oncol Hematol. 2021;158:103207. https://doi.org/10.1016/j.critrevonc.2020.103207
Imboden MT, Harber MP, Whaley MH, et al. The Association between the change in directly measured Cardiorespiratory Fitness across Time and Mortality Risk. Prog Cardiovasc Dis. 2019;62(2):157–62. https://doi.org/10.1016/j.pcad.2018.12.003
Chiaranda G, Myers J, Arena R, et al. Improved percent-predicted peak VO(2) is associated with lower risk of hospitalization in patients with coronary heart disease. Analysis from the FRIEND registry. Int J Cardiol. 2020;310:138–44. https://doi.org/10.1016/j.ijcard.2020.02.057
Chiaranda G, Myers J, Arena R, et al. Prognostic comparison of the FRIEND and Wasserman/Hansen peak VO2 equations applied to a submaximal walking test in outpatients with cardiovascular disease. Eur J Prev Cardiol. 2021;28(3):287–92. https://doi.org/10.1177/2047487319871728
Edelmann F, Stahrenberg R, Polzin F, et al. Impaired physical quality of life in patients with diastolic dysfunction associates more strongly with neurohumoral activation than with echocardiographic parameters: quality of life in diastolic dysfunction. Am Heart J. 2011;161(4):797–804. https://doi.org/10.1016/j.ahj.2011.01.003
Ohno Y, Okura Y, Ramadan MM, et al. Health-related quality of life of outpatients with systolic and isolated diastolic dysfunction: Sado Heart failure study. Circ J. 2008;72(9):1436–42. https://doi.org/10.1253/circj.cj-07-0842
Tao Y, Li P, Zhao C, et al. Plasma markers for early prediction of Radiation-Induced myocardial damage. J Interferon Cytokine Res. 2023;43(4):173–81. https://doi.org/10.1089/jir.2022.0226
Boopathi E, Thangavel C. Dark side of Cancer Therapy: Cancer Treatment-Induced cardiopulmonary inflammation, fibrosis, and Immune Modulation. Int J Mol Sci. 2021;22(18). https://doi.org/10.3390/ijms221810126
Mauro AG, Mezzaroma E, Toldo S, et al. NLRP3-mediated inflammation in cardio-oncology: sterile yet harmful. Translational Res. 2023;252:9–20. https://doi.org/10.1016/j.trsl.2022.08.004
Mezzaroma E, Mikkelsen RB, Toldo S, et al. Role of Interleukin-1 in Radiation-Induced Cardiomyopathy. Mol Med. 2015;21:210–8. https://doi.org/10.2119/molmed.2014.00243
Canada JM, Thomas GK, Trankle CR, et al. Increased C-reactive protein is associated with the severity of thoracic radiotherapy-induced cardiomyopathy. Cardio-Oncology. 2020;6(1):2. https://doi.org/10.1186/s40959-020-0058-1
Acknowledgements
Not applicable.
Funding
This work was supported by a Virginia Commonwealth University Department of Internal Medicine Pilot Project grant and a VCU Massey Cancer Center - Pauley Heart Center Pilot Project grant (P30CA016059, Richmond, VA).
Author information
Authors and Affiliations
Contributions
GT wrote the manuscript. GT, JC, AA critically revised the manuscript. FM, MGD, EW, EK provided editing suggestions during the writing of the manuscript. AA, MGD, FM, MG performed and reviewed the echocardiographic data. JC, JW, and RM performed the cardiopulmonary exercise testing. AA, MGD, FM, JC analyzed the cardiopulmonary exercise data. EW recruited participants and analyzed/reviewed radiation treatment doses. All authors read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Data were collected as part of participation in this prospective pilot study. The study was approved by the Virginia Commonwealth University Institutional Review Board and all participants provided informed consent prior to study participation.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Thomas, G., Weiss, E., Del Buono, M.G. et al. Early reduction in cardiorespiratory fitness and diastolic reserve following radiation therapy for lung cancer. Cardio-Oncology 10, 15 (2024). https://doi.org/10.1186/s40959-024-00216-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40959-024-00216-2