Abstract
Purpose
To describe changes in physical performance and patient-reported outcomes in cancer survivors who participated in an exercise program as part of usual-care multidisciplinary rehabilitation and the influence of training adaptations during the coronavirus-19 (COVID-19) pandemic.
Methods
In an observational cohort study, cancer survivors underwent usual-care multidisciplinary rehabilitation including a 10-week exercise program. During the COVID-19 pandemic, the exercise program was adapted with reduced training time and frequency. Mean changes and 95% confidence intervals in physical performance (peak oxygen uptake (VO2peak), peak work rate during a steep ramp test (SRT-WRpeak), 6-min walking distance, muscle strength) and patient-reported outcomes (health-related quality of life, fatigue, anxiety, and depression) were assessed between the start and the end of the exercise program. Linear regression analysis, adjusting for baseline levels of outcomes, was used to investigate differences in changes in outcomes between participants who underwent the original and the adapted program.
Results
All outcomes statistically significantly improved over time, regardless of adaptations in the exercise program. VO2peak increased with 9.6% and 7.7% in the original and adapted program, respectively. Significant smaller improvements were observed in SRT-WRpeak (− 3.9%) and upper body muscle strength (− 10.8%) after participation in the adapted compared to the original program. No significant between-group differences were observed for other outcomes.
Conclusion
Physical performance and patient-reported outcomes statistically and clinically significantly improved in cancer survivors who participated in an exercise program as part of usual-care multidisciplinary rehabilitation. Improvements of performance outcomes were smaller since the training adaptations, though only significant for SRT-WRpeak and upper body strength.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Over the last decades, aging and improved diagnostics and treatment modalities have led to an increased number of cancer survivors. In 2018, approximately 50 million people worldwide were living with or beyond cancer [1]. Cancer survivors are often confronted with disease- and treatment-related side effects, like fatigue, declined aerobic capacity and muscle strength, anxiety and depression, and a diminished health-related quality of life (HRQoL) [2,3,4,5,6].
Research in the field of cancer survivorship care expanded in the past decades, and growing evidence emerged for the positive effects of exercise on the aforementioned side effects [7,8,9,10]. For this reason, international guidelines of the American College of Sports Medicine (ACSM) have emphasized the importance of the integration of exercise in cancer survivorship care [11].
While most studies focus on exercise interventions alone, multidisciplinary rehabilitation programs may better address the complex needs of patients with cancer [12]. Dutch cancer guidelines advocate prescription of a supervised, exercise-based multidisciplinary rehabilitation program to cancer survivors who experience combined physical and psychosocial problems [13]. Multidisciplinary rehabilitation commonly contains exercise training, supplemented by a range of treatments to improve mental health, chronic fatigue, work reintegration, body composition, and nutritional intake. Recently, two systematic reviews were published about the effects of multidisciplinary oncology rehabilitation in cancer survivors. Overall, rehabilitation resulted in positive effects on physical and psychosocial state, but the effects varied across studies [12, 14].
In recent exercise guidelines, the majority of available evidence on the efficacy of oncology rehabilitation is derived from randomized controlled trials (RCTs). RCTs have strengthened the body of proof for the efficacy of exercise in cancer rehabilitation, but have been reported to lack generalizability to the clinical setting [15, 16]. Patients have to meet pre-specified criteria (e.g., diagnosis, disease stage, age) in order to be eligible for enrollment in RCTs and have to give consent to participate. This might result in a healthier, fitter, and more motivated population, which may not be comparable to a broader population of cancer survivors [11]. While RCTs have the most powerful study design to investigate the efficacy of rehabilitation in a specific population under ideal circumstances, observational studies may be more appropriate to evaluate interventions in daily practice and in more heterogeneous populations with complex, chronic diseases such as cancer [15, 16].
In this observational study, we present data about physical performance and patient-reported outcomes in cancer survivors who participated in an exercise program as part of multidisciplinary rehabilitation between February 2019 and March 2021. Due to the coronavirus 2019 (COVID-19) pandemic, social distance policies and disruption of outpatient clinic care led to changes in the content of the training and a reduction in the training time and frequency. Therefore, this study had the following objectives:
The primary objective of this study was to describe changes in aerobic capacity, muscle strength, HRQoL, fatigue, and anxiety and depression in cancer survivors who participated in a 10-week exercise program as part of usual-care multidisciplinary rehabilitation.
The secondary objective was to compare changes in outcomes between the group of participants that followed the original program and the group of participants that followed an adapted exercise program, due to COVID-19 measures.
Methods
Participants
Participants were recruited from the multidisciplinary rehabilitation program at the Department of Physical Therapy of the Maastricht University Medical Centre (MUMC +) between February 2019 and March 2021. Patients participating in the program were asked for consent to use their exercise rehabilitation data. Patients who signed informed consent were included in the study. Participants were excluded if they were unable to follow the training program as intended. Patients were eligible for the program when they were ≥ 18 years, completed active medical treatment (i.e., surgery, chemotherapy, radiotherapy, stem cell transplantation) and were suffering from physical, and/or psychosocial complaints and/or chronic fatigue. Contraindications for participation in the rehabilitation program were the inability to perform basic activities of daily living and the presence of disabling comorbidities that seriously hamper physical exercise.
Study design
This study was a prospective, longitudinal observational cohort study and all data were collected during usual-care multidisciplinary oncology rehabilitation at the MUMC + . Study procedures complied with the Declaration of Helsinki and were approved by the Medical Ethics Committee of the Maastricht UMC + with registration number 2018–0648.
Rehabilitation program
Participants completed a 10-week supervised, group-based exercise program. The exercise program consisted of two training sessions weekly, both starting with 1 h of combined resistance and endurance training, followed by a 30-min break and, subsequently, 30 min of varying sports activities in the gym or swimming pool. In addition, participants took part in at least one of the following interventions: a psychoeducational intervention (seven individual or group-based sessions) guided by the psychologist or the social worker; fatigue management courses (six individual or group-based sessions) guided by the occupational therapist; return-to-work counselling (three individual sessions) guided by the occupational therapist; and dietary counselling (three individual sessions) delivered by the dietician. These additional programs were provided (partly) in parallel with the exercise program. Participants completed exercise tests and questionnaires before the start of the exercise program (T = 0) and in the week after completing the exercise program (T = 1). Of note is that some of the other interventions were not finished yet at T = 1.
Measurements
Performance outcomes
A cardiopulmonary exercise test (CPET) was performed as part of usual-care, to screen for cardiopulmonary contraindications to exercise and to determine aerobic capacity. The CPET was conducted on a cycle ergometer (Lode Corival, Lode BV) as described previously [17]. After a warm-up phase, the work rate (WR) increased gradually according to an incremental ramp protocol, which was determined based on the participants’ self-reported physical activity level, aiming at a test duration of 8–12 min. The WR increased until the patient stopped cycling or pedaling frequency fell below 60 rpm. This point was defined as peak WR (CPET-WRpeak). Continuous breath-by-breath analysis was obtained using a spirometry system calibrated for respiratory gas and breathing volume measurements (Vyntus CPX, CareFusion Netherlands).
CPET results were analyzed by a trained researcher who was blinded for the moment of testing (T = 0 or T = 1), using a standardized protocol. Values of oxygen uptake (VO2) and the respiratory exchange rate at WRpeak (VO2peak and RERpeak, respectively) were averaged over 30 s. An improvement in VO2peak of 1.0 mL/min/kg was found to be associated with a 9% risk reduction in all-cause mortality and was therefore considered a clinically relevant improvement [18]. Submaximal outcomes of CPET were determined as well. The VO2 at the ventilatory anaerobic threshold (VO2VAT) was determined using the “V-slope” method [19] and the ventilatory equivalents method [20]. The VO2 at the respiratory compensation point (VO2RCP) was determined using the ventilatory equivalents method and the minute ventilation/carbon dioxide production (VE/VCO2) slope [21]. The interrater reliability for determination of VO2VAT and VO2RCP was determined previously, when two researchers at Maastricht UMC + both analyzed 48 tests (partly from the current study) independently. This resulted in an intraclass correlation coefficient (ICC) of 0.95 (95% confidence interval (CI) 0.90–0.97) for determination of the VO2VAT and an ICC of 0.99 (95% CI 0.98–0.99) for determination of the VO2RCP, which indicates an excellent interrater reliability. The oxygen uptake efficiency slope (OUES) was derived from the relation between VO2 and minute ventilation, using the following formula: VO2 = (a × Log VE) + b, where a is the OUES [22].
In addition, patients performed a 6-min walk test (6MWT) and a steep ramp test (SRT). These tests also gave an indication of aerobic capacity and were performed as part of the usual-care rehabilitation program, to determine baseline training intensity. During the 6MWT, participants were instructed to walk as many meters as possible, on a marked 44-m course, within 6 min. Based on the minimal clinically important difference (MCID) of the 6MWT in adults with pathology, a change in the maximal walking distance of 30.5 m was considered clinically relevant [23]. The SRT was performed on a cycle ergometer (Lode Corival, Lode BV) as described previously [17]. After warming up, the WR increased with 25 W/10 s in a ramp-like manner, until the participant stopped cycling or pedaling frequency fell below 60 rpm. This point was defined as peak WR (SRT-WRpeak). The minimal detectable change in SRT-WRpeak was recently determined in survivors of cancer who participated in exercise-based multidisciplinary rehabilitation in MUMC +. Based on the findings of this study, an increase of 0.26 W/kg in SRT-WRpeak was seen as a true improvement [17].
Lower and upper body muscle strength was measured during submaximal repetition maximum (RM) tests on the leg press and chest press machine, respectively. The 5-RM was estimated for both exercises and the participant was asked to perform the maximum achievable number of repetitions up to five repetitions with this weight. When five repetitions were reached, the weight was increased and participants repeated the exercise after a 1-min pause until they no longer reached 5 repetitions. True 1-RM values were calculated afterwards using the Brzycki equation [24]. In March 2020, the gym at Maastricht UMC + was updated and the exercise machines were replaced by comparable new ones. Each participant performed strength tests at T = 0 and T = 1 on the same machines. A change in 1-RM chest press of 6.25 kg was considered clinically relevant, as determined in a study to the MCID of RM testing in patients with chronic obstructive pulmonary disease [25].
Patient-reported outcomes
HRQoL was measured using the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire Core-30 (EORTC QLQ-C30). Each of the 30 item has to be rated on a scale from 1 to 4 and for two items from 1 to 7. This questionnaire distinguishes 15 subscales. The functioning scales (physical, role, emotional, social, and cognitive functioning), the global QoL scale, and a functioning sum score (averaged across the 15 items that belong to the functioning scales) were calculated. Sub-scores as well as sum scores were linearly transformed on a 100-point scale, with higher scores indicating higher levels of HRQoL [26, 27]. A change of 10 points on each subscale or the sum score was considered clinically relevant [28, 29].
Fatigue was assessed using the Multidimensional Fatigue Inventory-20 (MFI-20), which is a 20-item questionnaire with a five-dimensional structure (general, physical and mental fatigue, reduced motivation and activity). Each item is scored on a 5-point Likert scale. The sub-scores range from 4 to 20, with lower scores indicating lower levels of fatigue. The sum score was calculated by adding up the sub-scores [30]. Changes on the MFI-20 subscales that exceeded MCIDs as determined in a cohort of patients with cancer receiving radiotherapy (ranging from 3.18 to 3.80 for different subscales) were considered clinically relevant [31].
Anxiety and depression were assessed using the 14-item Hospital Anxiety and Depression Scale (HADS). Items are scored on a 4-point scale and sub-scores for anxiety and for depression range from 0 to 21, with lower scores indicating lower levels of anxiety and depression. The sum score was calculated by adding up the sub-scores [32]. A change of 1.7 points for each sub-score was considered clinically relevant, as assessed in patients with cardiovascular disease in the study of Lemay et al. [33].
Other measurements
Age, cancer type, presence of metastasis and comorbidities, treatment type, and time since treatment at T = 0 were extracted from medical records. Height and weight were measured at T = 0 and T = 1, after which body mass index (BMI) was calculated. The training compliance (%) was calculated by dividing the number of training sessions that participants attended, by the number of planned training sessions, multiplied by 100. Indication for other interventions in the rehabilitation program and completion rates of these therapies at T = 1 were reported as well.
Exercise protocol
Participants performed four strengthening exercises each session, targeting large muscle groups of the upper and lower body, and core. Resistance training consisted of three sets of 8–12 repetitions and training intensity was set at 60% of the participant’s initial 1-RM. Endurance training in the first training session of the week consisted of 20-min walking on a treadmill, with a walking speed of 80% of their mean speed in the baseline 6-MWT. In the other training session, participants performed two sets of 10 min of interval training on a cycle ergometer, one set before and one after the resistance training program. Intervals were performed for 30 and 60 s at 65% and 30% of the participant’s SRT-WRpeak, respectively [34]. The training load was adjusted in a personalized manner, according to the 0–10 Borg rating of perceived exertion and weekly increase in load was aimed for in order to reach overload. A moderate- to high-intensity exercise was pursued for all training components, corresponding with a Borg score of 4–6 [35].
COVID-19
The rehabilitation program was interrupted between March 2020 and July 2020, because all outpatient activities were cancelled in that time frame, due to COVID-19 measures. Rehabilitation data of participants who were enrolled in the exercise program prior to this period and did not finish yet were excluded from this study because measurements at T = 1 were cancelled or postponed. In July 2020, national guidelines permitted resumption of the rehabilitation program. Because of the social distancing policies, exercise training took place in smaller groups of four instead of eight patients. In order to avoid a long waiting list, the training frequency was reduced to once weekly. Because there was only one training session weekly, endurance training was changed to 10-min walking and 10-min cycling in one session. Intensity of the endurance training remained the same. Contact sports and swimming were not allowed, so patients could only perform the endurance and resistance training program. For participants who were recruited from July 2020 onwards, the frequency, time, and type of exercise training changed. However, we encouraged all participants to be physically active on other weekdays and to perform body weight strengthening exercises at home once to twice weekly. Online instructions for a home-based program with strengthening exercises were offered to all participants. Other interventions of the rehabilitation program took place in smaller groups as well, or via phone calls.
Statistical analysis
Continuous variables were checked for normality using histograms and Q-Q plots and are presented as mean ± SD or median and interquartile ranges, as appropriate. Categorical variables are presented as frequencies (n) and percentages (%). Performance- and patient-reported outcomes are reported for the group of participants that completed the original exercise program and the participants who completed the adapted program since COVID-19. Outcomes are reported for measurements at T = 0 and at T = 1. Mean changes between T = 0 and T = 1 in outcome variables within individuals are reported with 95% CI. Mean differences (and 95% CI) in change scores between participants who underwent the original program and the adapted program were estimated using linear regression analysis, with the change scores as outcome, a group variable (indicating whether individuals followed the original or adapted exercise program) as dependent variable, and additional adjustment for the absolute values of the outcomes at T = 0. Differences between changes in muscle strength were reported as percentages as well, to account for possible differences in baseline values due to the change of the exercise machine during the study. If the confidence intervals did not include zero, the mean change or difference in change was considered statistically significant. MCIDs are reported when they were available in literature. Changes were considered clinically relevant when they exceeded MCIDs. Statistical analyses were performed using SPSS version 25.0 (SPSS Statistics for Windows, version 25.0, IBM Corp.).
Results
Participants
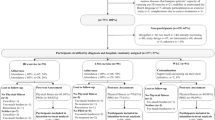
A total of 196 patients participated in the multidisciplinary oncology rehabilitation program at MUMC + between February 2019 and March 2021.Three of them gave no informed consent for the use of their data resulting in a participation rate of 98.4%. Eight participants were excluded because they were unable to follow the exercise training as intended (i.e., because of physical impairments, absence for longer periods). This resulted in a final sample size of 185 subjects. Seventy-four and 62 participants completed the original exercise program and the adapted program since the COVID-19 pandemic, respectively. Twelve (11.0%) and 14 (18.4%) participants were unable to complete the original and the adapted exercise program due to medical or other reasons, respectively. Twenty-three out of 109 (21.1%) participants were lost to follow-up due to COVID-19 (Fig. 1).
Of the total population, 143 participants (77.3%) were women. Mean age was 55.7 ± 11.5 years and mean BMI was 27.9 ± 5.3 kg/m2. Breast cancer was the most common diagnosis (46.5%). Subjects started with the exercise program on average 4.7 ± 4.4 months after completing active medical treatment. Patient characteristics were not significantly different between groups (Table 1).
Rehabilitation program
The training compliance rate was 93.7 ± 7.7% and 91.37 ± 11.8% in participants who completed the original and the adapted program, respectively. The percentage of indication for other interventions and their completion rates at T = 1 did not differ notably between the groups, but the other interventions were often not completed yet at T = 1 (Table 2).
Changes in physical performance and patient-reported outcomes
All measures of aerobic capacity and muscle strength improved statistically and clinically significantly after 10 weeks of exercise training in both groups. An increase of 1.9 mL/kg/min (9.6%) and 1.4 mL/kg/min (7.2%) in VO2peak was observed after participation in the original and the adapted program, respectively (Table 3). Patient-reported outcomes for HRQoL, fatigue, and anxiety and depression improved statistically significantly after 10 weeks of exercise training, both before and after the changes in the program due to COVID-19. Clinically relevant improvements in HRQoL were reached in four out of six subscales of the EORTC-QLQ-C30 in the original program and five out of six subscales in the adapted program. A clinically relevant decrease in general and physical fatigue on the MFI was observed for both groups. Clinical relevant improvements on the HADS were seen only in the depression subscale before the adaptations in the program and in the anxiety and the depression scale after the adaptations (Table 4).
The influence of training adaptations
For nearly all performance outcomes, changes over time were more pronounced before the adaptations in the program, albeit only statistically significantly different for SRT-WRpeak and upper body strength. Mean upper body strength improved with 48.5% in participants who took part in the original program and with 21.5% in participants who took part in the adapted program. Mean improvement in SRT-WRpeak improved with 0.36 W/kg (CI 0.28–0.44) or 11.5% in participants in the original program and 0.23 (CI 0.14–0.32) W/kg or 7.6% in participants in the adapted program since COVID-19 (Table 3). In contrast with results of the performance tests, improvements in patient-reported outcomes were not different between the groups that participated before or since training adaptations (Table 4).
Discussion
The results of this study showed significant improvements in aerobic capacity, muscle strength, HRQoL, fatigue, anxiety, and depression in cancer survivors following a 10-week exercise program as part of usual-care multidisciplinary oncology rehabilitation. Changes were clinically relevant for nearly all outcomes; MCIDs were not available in literature for 1-RM leg press, submaximal outcomes of CPET, and for the sum score of the HADS. For SRT-WRpeak, only the minimal detectable change was available, which was therefore used to compare our study results with. A significant and clinically relevant improvement in VO2peak of 1.9 mL/min/kg and 1.4 mL/min/kg was seen after participation in the original program and the adapted program since COVID-19, respectively. In a meta-analysis by Scott et al. on the effects of exercise therapy on aerobic capacity in cancer survivors, a larger improvement of 2.8 mL/min/kg (CI weighted mean difference 1.58–2.67 mL/min/kg) in mean VO2peak was observed [9]. Current guidelines of ACSM prescribe an 8–12-week combined aerobic and resistance exercise program with moderate intensity three times weekly to improve physical function [11]. The lower improvements in VO2peak found in the current study might be explained by a lower training frequency. Moreover, the moderate- to high-intensity training prescribed in the current study was potentially not always reached due to limited adherence of training intensity. Finally, the training intensity in the current study was based on baseline performance tests and perceived exertion. Training frequency, type, and time were equal for all participants and could be more personalized in the future.
Another plausible explanation for this inconsistency is the fact that Scott et al. only included RCTs in the meta analyses, which might have resulted in a fitter population [15, 16]. Besides, this meta-analysis focused on the effects of exercise alone, while the current study investigated the effects of exercise as part of multidisciplinary rehabilitation, in patients with more complex care needs. Surprisingly, a systematic review of Dennett et al. showed no significant effects for supervised exercise-based, multidisciplinary rehabilitation on VO2peak, which was attributed to issues with exercise prescriptions, which are often not well-reported in trials [14]. In both reviews, a large heterogeneity between studies was seen.
In our study, a significant improvement in HRQoL was seen after participation in the original program (sum score EORTC-QLQ-C30 + 12.48) and the adapted program (sum score EORTC-QLQ-C30 + 16.00). Comparable improvements were seen in a study on the effectiveness of a 12-week, multidisciplinary rehabilitation program in breast cancer patients (sum score EORTC-QLQ-C30 + 11.67) [36]. However, since most of our participants did not yet complete the other interventions at T = 1, improvements in patient-reported outcomes probably could have been larger. Studies on oncology rehabilitation vary a lot in content, duration, and timing of the programs and in reported outcome measures. Therefore, more extensive comparison of our study results with existing literature was not possible.
In this study, we also compared changes in outcomes between participants who exercised twice weekly in the original program and participants who exercised once weekly due to changes in the program since the COVID-19 pandemic. Significant between-group differences were observed for SRT-WRpeak and upper body muscle strength, with larger improvements for the group that participated in the original program. This is not surprising, because participants in this group attended the exercise training twice weekly. When looking at changes in the other performance outcomes, non-significant differences were seen between groups, with larger improvements for the group in the original program. Attention for habitual physical activity guidelines may have increased when training frequency and time were diminished since COVID-19. Consequently, participants may have been more active outside the training program since the training adaptations, which could have reduced the expected difference in training improvements. No significant between-group differences were seen for patient-reported outcomes. Unexpectedly, improvements in HRQoL did not decrease under the circumstances of the COVID-19 pandemic (e.g., social isolation, anxiety). This could have been due to a “response shift”, referring to changes in internal standards and values during a crisis [37]. This study was not originally designed to investigate the differences between groups; therefore, caution is warranted when drawing conclusions from this study based on significance testing alone.
A strength of this study was the observational design and the fact that data was collected during daily practice. The results might give a more realistic reflection of the physical and psychosocial changes after an oncology rehabilitation program, when compared to RCTs with strict inclusion criteria, in an experimental setting [15, 16]. Another strength was the fact that this study investigated a multidisciplinary rehabilitation program, which is best suited in this population with complex care needs, but has not often been studied before. Furthermore, data on different outcome measures were collected, covering not only physical but also psychosocial issues and fatigue. The observational design was not only a strength, but also a limitation of this study, because it is more challenging to draw firm conclusions about the changes in outcomes without a control group and random group assignment. It is likely that the natural course of improvement in physical performance and patient-reported outcomes after cancer treatment has played a role in the observed changes in outcomes over time. However, in the meta-analysis of Scott et al., a negligible mean improvement in VO2peak of 0.2 mL/kg/min was seen in patients with cancer who received no exercise intervention. The fact that participants took part in interventions other than the exercise training during this study could be seen as a limitation as well. Although patient-reported outcomes may have been influenced by other interventions than the exercise interventions alone (e.g., psychoeducational intervention, fatigue- and return-to-work counseling), these interventions were less likely to have influenced performance outcomes since they did not contain exercise elements. Further of note is that this study was aimed at investigating cancer patients with both physical and psychosocial complaints and/or chronic fatigue. Therefore, the findings of the current study cannot be generalized to all cancer survivors.
Conclusion
The results of this study indicate that cancer survivors with both physical and psychosocial complaints significantly improve in aerobic capacity, muscle strength, HRQoL, fatigue, anxiety, and depression during a 10-week supervised, group-based exercise program as part of usual-care multidisciplinary oncology rehabilitation. Reductions in frequency, time, and type of training during the COVID-19 pandemic still resulted in significant improvements of all outcomes. However, improvements of most performance outcomes appeared to be smaller since the training adaptations, though only significant for SRT-WRpeak and upper body strength.
Data availability
Study data are available on request from the corresponding author.
Code availability
Not applicable.
References
World Health Organization (2020) WHO report on cancer: setting priorities, investing wisely and providing care for all. Geneva. Licence. CC BY-NC-5A 3.0 IGO
Jones LW, Courneya KS, Mackey JR, Muss HB, Pituskin EN, Scott JM et al (2012) Cardiopulmonary function and age-related decline across the breast cancer survivorship continuum. J Clin Oncol 30(20):2530–2537. https://doi.org/10.1200/JCO.2011.39.90144
Nayak MG, George A, Vidyasagar MS, Mathew S, Nayak S, Nayak BS et al (2017) Quality of life among cancer patients. Indian J Palliat Care 23(4):445–450. https://doi.org/10.4103/IJPC.IJPC_82_17
Ebede CC, Jang Y, Escalante CP (2017) Cancer-related fatigue in cancer survivorship. Med Clin North Am 101(6):1085–1097. https://doi.org/10.1016/j.mcna.2017.06.007
Jean CY, Syrjala KL (2017) Anxiety and depression in cancer survivors. Medical Clinics 101(6):1099–1113. https://doi.org/10.1016/j.mcna.2017.06.005
Marques VA, Ferreira-Junior JB, Lemos TV, Moraes RF, Junior JRS, Alves RR et al (2020) Effects of chemotherapy treatment on muscle strength, quality of life, fatigue, and anxiety in women with breast cancer. Int J Environ Res Public Health 17(19). https://doi.org/10.3390/ijerph17197289
Stout NL, Alfano CM, Belter CW, Nitkin R, Cernich A, Lohmann Siegel K et al (2018) A bibliometric analysis of the landscape of cancer rehabilitation research (1992–2016). J Natl Cancer Inst 110(8):815–824. https://doi.org/10.1093/jnci/djy108
Buffart LM, Kalter J, Sweegers MG, Courneya KS, Newton RU, Aaronson NK et al (2017) Effects and moderators of exercise on quality of life and physical function in patients with cancer: an individual patient data meta-analysis of 34 RCTs. Cancer Treat Rev 52:91–104. https://doi.org/10.1016/j.ctrv.2016.11.010
Scott JM, Zabor EC, Schwitzer E, Koelwyn GJ, Adams SC, Nilsen TS et al (2018) Efficacy of exercise therapy on cardiorespiratory fitness in patients with cancer: a systematic review and meta-analysis. J Clin Oncol 36(22):2297–2305. https://doi.org/10.1200/JCO.2017.77.5809
Campbell KL, Winters-Stone KM, Patel AV, Gerber LH, Matthews CE, May AM et al (2019) An executive summary of reports from an international multidisciplinary roundtable on exercise and cancer: evidence, guidelines, and implementation. Rehabilitation Oncology 37(4):144–152. https://doi.org/10.1097/01.REO.0000000000000186
Campbell KL, Winters-Stone KM, Wiskemann J, May AM, Schwartz AL, Courneya KS et al (2019) Exercise guidelines for cancer survivors: consensus statement from international multidisciplinary roundtable. Med Sci Sports Exerc 51(11):2375–2390. https://doi.org/10.1249/MSS.0000000000002116
Campbell KL, Winters-Stone KM, Wiskemann J, May AM, Schwartz AL, Courneya KS et al (2020) Multidisciplinary outpatient cancer rehabilitation can improve cancer patients’ physical and psychosocial status-a systematic review. Curr Oncol Rep 22(12):122. https://doi.org/10.1007/s11912-020-00979-8
IKNL, Medisch specialistische revalidatie bij oncologie (2016) IKNL: Utrecht
Dennett AM, Sarkies M, Shields N, Peiris CL, Williams C, Taylor NF et al (2021) Multidisciplinary, exercise-based oncology rehabilitation programs improve patient outcomes but their effects on healthcare service-level outcomes remain uncertain: a systematic review. J Physiother 67(1):12–26. https://doi.org/10.1016/j.jphys.2020.12.008
Faraoni D, Schaefer ST (2016) Randomized controlled trials vs. observational studies: why not just live together? BMC Anesthesiol 16(1):102. https://doi.org/10.1186/s12871-016-0265-3
Ligthelm RJ, Borzi V, Gumprecht J, Kawamori R, Wenying Y, Valensi P (2007) Importance of observational studies in clinical practice. Clin Ther 29:1284–92. https://doi.org/10.1016/j.clinthera.2007.07.004
Weemaes ATR, Beelen M, Bongers BC, Weijenberg MP, Lenssen AF (2021) Criterion validity and responsiveness of the steep ramp test to evaluate aerobic capacity in survivors of cancer participating in a supervised exercise rehabilitation program. Arch Phys Med Rehabil. https://doi.org/10.1016/j.apmr.2021.04.016
Laukkanen JA, Zaccardi F, Khan H, Kurl S, Jae SY, Rauramaa R (2016) Long-term change in cardiorespiratory fitness and all-cause mortality: a population-based follow-up study. Mayo Clin Proc 91(9):1183–1188. https://doi.org/10.1016/j.mayocp.2016.05.014
Beaver WL, Wasserman K, Whipp BJ (1986) A new method for detecting anaerobic threshold by gas exchange. J Appl Physiol (1985) 60(6):2020–7. https://doi.org/10.1152/jappl.1986.60.6.2020
Reinhard U, Muller PH, Schmulling RM (1979) Determination of anaerobic threshold by the ventilation equivalent in normal individuals. Respiration 38(1):36–42. https://doi.org/10.1159/000194056
Simonton CA, Higginbotham MB, Cobb FR (1988) The ventilatory threshold: quantitative analysis of reproducibility and relation to arterial lactate concentration in normal subjects and in patients with chronic congestive heart failure. Am J Cardiol 62(1):100–107. https://doi.org/10.1016/0002-9149(88)91372-0
Baba R, Nagashima M, Goto M, Nagano Y, Yokota M, Tauchi N et al (1996) Oxygen uptake efficiency slope: a new index of cardiorespiratory functional reserve derived from the relation between oxygen uptake and minute ventilation during incremental exercise. J Am Coll Cardiol 28(6):1567–1572
Bohannon RW, Crouch R (2017) Minimal clinically important difference for change in 6-minute walk test distance of adults with pathology: a systematic review. J Eval Clin Pract 23(2):377–381. https://doi.org/10.1111/jep.12629
Brzycki M (1993) Strength testing—predicting a one-rep max from reps-to-fatigue. J Phys Educ Recreat Dance 64(1):88–90. https://doi.org/10.1080/07303084.1993.10606684
Araújo Oliveira AL, Rebelo P, Paixão C, Jácome C, Cruz J, Valente C et al (2019) Minimal clinically important difference using one-repetition maximum in COPD. Eur Respir J 54(63):1205. https://doi.org/10.1183/13993003.congress-2019.PA12055
Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ et al (1993) The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. JNCI: J Natl Cancer Inst 85(5):365–376. https://doi.org/10.1093/jnci/85.5.365
Hinz A et al (2012) Is it useful to calculate sum scores of the quality of life questionnaire EORTC QLQ-C30? Eur J Cancer Care (Engl) 21(5):677–683. https://doi.org/10.1111/j.1365-2354.2012.01367.x
Osoba D (1999) What has been learned from measuring health-related quality of life in clinical oncology. Eur J Cancer 35(11):1565–1570. https://doi.org/10.1016/s0959-8049(99)00192-6
Hinz A et al (2018) Quality of life in cancer patients—a comparison of inpatient, outpatient, and rehabilitation settings. Support Care Cancer 26(10):3533–3541. https://doi.org/10.1007/s00520-018-4211-4
Hinz A, Einenkel J, Briest S, Stolzenburg JU, Papsdorf K, Singer S (2020) Fatigue in cancer patients: comparison with the general population and prognostic factors. Support Care Cancer 28(9):4517–4526. https://doi.org/10.1007/s00520-019-05260-8
Purcell A et al (2010) Determining the minimal clinically important difference criteria for the Multidimensional Fatigue Inventory in a radiotherapy population. Support Care Cancer 18(3):307–315. https://doi.org/10.1007/s00520-009-0653-z
Bjelland I, Dahl AA, Haug TT, Neckelmann D (2002) The validity of the Hospital Anxiety and Depression Scale. An updated literature review. J Psychosom Res 52(2):69–77. https://doi.org/10.1016/s0022-3999(01)00296-3
Lemay KR et al (2019) Establishing the minimal clinically important difference for the Hospital Anxiety and Depression Scale in patients with cardiovascular disease. J Cardiopulm Rehabil Prev 39(6):E6–E11. https://doi.org/10.1097/hcr.0000000000000379
De Backer IC, Schep G, Hoogeveen A, Vreugdenhil G, Kester AD, van Breda E (2007) Exercise testing and training in a cancer rehabilitation program: the advantage of the steep ramp test. Arch Phys Med Rehabil 88(5):610–616. https://doi.org/10.1016/j.apmr.2007.02.013
Borg G (1998) Borg’s perceived exertion and pain scales. Borg’s perceived exertion and pain scales. Human Kinetics. viii, 104-viii, 104, Champaign
Leclerc AF, Foidart-Dessalle M, Tomasella M, Coucke P, Devos M, Bruyere O et al (2017) Multidisciplinary rehabilitation program after breast cancer: benefits on physical function, anthropometry and quality of life. Eur J Phys Rehabil Med 53(5):633–642. https://doi.org/10.23736/s1973-9087.17.04551-8
Sprangers MA, Schwartz CE (1999) Integrating response shift into health-related quality of life research: a theoretical model. Soc Sci Med 48(11):1507–1515. https://doi.org/10.1016/s0277-9536(99)00045-3
Funding
This study is part of a PhD trajectory, which was funded by Maastricht University Medical Center + .
Author information
Authors and Affiliations
Contributions
All authors contributed to the conception and the design of the study. Data collection was carried out by AW and MB. Data analysis was performed by AW. The article was written by AW, under supervision of MW, TL, and MB. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
Study procedures were in compliance with the Declaration of Helsinki and were approved by the Medical Ethics Committee of the Maastricht UMC + with registration number 2018–0648.
Consent to participate
Participants gave written informed consent for participation in this study.
Consent to publish
Participants gave written informed consent for the publication of their data.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Weemaes, A.T.R., Weijenberg, M.P., Lenssen, A.F. et al. Exercise training as part of multidisciplinary rehabilitation in cancer survivors: an observational study on changes in physical performance and patient-reported outcomes. Support Care Cancer 30, 9255–9266 (2022). https://doi.org/10.1007/s00520-022-07351-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-022-07351-5