Abstract
Background
Invasive micropapillary carcinoma (IMPC) is an uncommon subtype of breast cancer. Previous studies of this subtype demonstrated a higher propensity for lymph node metastases as compared with invasive ductal carcinoma (IDC). The purpose of the present study was to determine the clinical characteristics, outcomes, and propensity for lymph node metastasis of patients with IMPC of the breast recorded in the National Cancer Database (NCDB).
Methods
Records of patients with IMPC diagnosed between 2004 and 2014 were retrieved from the NCDB. Log-rank test was performed to evaluate associations of clinical characteristics with overall survival (OS). Cox proportional hazards model was used to determine variables associated with OS.
Results
Overall, 2660 patients with IMPC met the selection criteria; the 5-year OS rate was 87.5% and 24.9% of patients had nodal involvement at presentation. Patients with ≥ 4 positive lymph nodes had shorter OS than node-negative patients, whereas patients with 1–3 positive nodes had similar OS to node-negative patients. Age < 65 years, receipt of radiotherapy, and estrogen receptor positivity were also associated with prolonged OS. The benefit of radiotherapy was limited to IMPC patients undergoing lumpectomy; there was no benefit for the patients undergoing mastectomy (regardless of nodal positivity/negativity).
Conclusions
Favorable prognostic factors of IMPC patients included age < 65 years, < 4 positive lymph nodes, receipt of radiotherapy, and estrogen receptor positivity. The results presented herein suggest a survival benefit associated with radiotherapy in IMPC treatment, though this may be limited to the patients treated with lumpectomy.
Similar content being viewed by others
Background
Invasive micropapillary carcinoma (IMPC) of the breast is an uncommon variant of breast cancer that was first described in 1980 [1]. Histologically, this subtype appears as tumor cells arranged in small solid fragments or tubules with small or obliterated lumina, which appear as micropapillae without central fibrovascular cores [2]. These micropapillae are surrounded by clear stromal spaces not lined by endothelial cells, giving it an appearance similar to retraction artifact [3]. IMPC constitutes less than 2% of all invasive breast cancers, although 3%–6% of invasive breast cancers were reported to have a focal micropapillary growth pattern [4].
Previous studies demonstrated that IMPC was associated with lymphovascular invasion and a higher propensity for lymph node metastases than invasive ductal carcinoma (IDC) and other invasive subtypes of breast cancer [5,6,7,8]. It has been thought that, due to the lymphotropic nature of IMPC, these patients experience worse overall outcomes than those with IDC. The National Cancer Database (NCDB) is a national hospital-based cancer registry that is co-sponsored by the American College of Surgeons (ACoS) and the American Cancer Society. It houses data from more than 1500 hospitals with ACoS-accredited cancer treatment programs, accounting for almost 70% of all newly diagnosed cancer cases in the United States [9,10,11,12,13,14]. In this study, we aimed to analyze the survival outcomes of IMPC patients recorded in the NCDB.
Patients and methods
Patient selection
Records of patients with biopsy-proven IMPC diagnosed between January 2004 and December 2014 were retrieved from the NCDB. Diagnosis was made according to the International Classification of Disease for Oncology, third edition (ICD-O-3), code 8507. This study only included patients with American Joint Committee on Cancer (AJCC, 7th edition) stage cT1-4N0-3M0 pure IMPC and complete records regarding surgical therapy and radiotherapy.
Prognosis analysis
Data of patient’s age, race, sex, Charlson–Deyo comorbidity score, histologic grade, estrogen receptor (ER) status, progesterone receptor (PR) status, human epidermal growth factor receptor 2 (HER2) status (only available for patients diagnosed between 2010 and 2014), TNM stage, number of positive lymph nodes, type of surgical resection, and the receipt of external beam radiotherapy (EBRT), chemotherapy, and hormonal therapy were collected.
Univariate analysis evaluated factors associated with overall survival (OS); subsequently, Cox multivariate analysis included variables that were statistically significant with a P value of < 0.05. OS was defined as the duration from the date of diagnosis to the date of last follow-up and was assessed using the Kaplan–Meier method. Patients were censored at the data of either death or the last follow-up. Only patients with complete data for the parameters of interest were included in the final analysis. Statistical analyses were performed using Stata/SE version 10 for Windows (StataCorp, College Station, TX, USA). A P value < 0.05 was considered significant.
Results
Patient characteristics
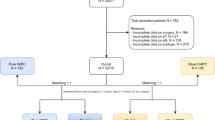
Overall, as shown in Fig. 1, a total of 2660 patients met the selection criteria. Median follow-up was 40 months (range 0.5–137 months). The median age of diagnosis was 60 years (range 19–90 years). Complete patient characteristics are summarized in Table 1.
At presentation, 662 (24.9%) patients had nodal involvement. In terms of histologic grade, 2304 (86.6%) patients had grade 2 or 3 disease, only 196 (7.4%) patients had grade 1 disease. In terms of biomarker status, 2327 (87.5%) had ER-positive disease, 2112 (79.4%) had PR-positive disease, and 397 (14.9%) had HER2-positive disease. Unfortunately, 765 (28.8%) patients had unknown HER2 status. Of the patients with complete biomarker status, a majority had hormone receptor-positive, HER2-negative disease.
In terms of surgery, 1281 (48.2%) patients underwent lumpectomy, and 1379 (51.8%) underwent mastectomy. Overall, 1592 (59.9%) patients received EBRT, 1979 (74.4%) received hormonal therapy, and 1273 (47.9%) received chemotherapy.
Outcomes and prognostic factors
At a median follow-up of 4 years (interquartile range 3.2–7.4 years), the 5-year OS rate was 87.5% (95% confidence interval [CI] 85.6%–89.4%). Univariate analysis showed that patients with ≥ 4 positive lymph nodes had shorter OS than patients with node-negative disease (hazard ratio [HR], 2.44; 95% CI 1.75–3.40; P < 0.001). However, those with 1–3 positive nodes had an OS similar to patients with node-negative disease (Fig. 2). As presented in Table 2, other indicators of poor prognosis on univariate analysis included age ≥ 65 years (HR, 1.93; 95% CI 1.33–2.81; P = 0.001), Charlson–Deyo comorbidity score = 1 (HR, 1.87; 95% CI 1.31–2.67; P = 0.001), Charlson–Deyo comorbidity score ≥ 2 (HR, 3.35; 95% CI 1.90–5.91; P < 0.001), omission of radiotherapy (HR, 1.76; P < 0.001), mastectomy (HR, 1.37; 95% CI 1.04–1.81; P = 0.025), ER-negative disease (HR, 2.24; 95% CI 1.63–3.11; P < 0.001), and lack of hormonal therapy use (HR, 1.53; 95% CI 1.13–2.05; P = 0.005). On multivariate analysis, factors associated with short OS included age > 65 years, Charlson–Deyo comorbidity score of 1 or ≥ 2, stage T2–4, stage N2, omission of radiation therapy, ER-negative disease, PR-negative disease, or ≥ 4 metastatic lymph nodes (P < 0.05 for all).
Figure 3 represents Kaplan–Meier curves comparing overall survival of patients who underwent surgery either with or without radiotherapy. A longer OS was associated with radiotherapy among patients receiving lumpectomy, but such association was not observed among patients with either positive or negative nodal disease receiving mastectomy.
Discussion
IMPC is a rare variant of breast cancer, making it difficult to study. As a result, using a large national database such as the NCDB allows for analysis using a large number of patients to help inform treatment management decisions. Our findings indicated that EBRT was associated with prolonged OS in IMPC patients undergoing lumpectomy but not for patients undergoing mastectomy. Additional poor prognostic factors for OS included older age, extensive lymph node involvement, and ER-positive disease.
The findings of this study are in accordance with those in the literature (Table 3) in that there is a higher rate of lymph node involvement seen in IMPC compared to the rate seen in IDC in previous studies. Because a higher rate of lymph node involvement and/or higher number of metastatic lymph nodes confers a higher N stage, it has been presumed that IMPC patients have worse survival outcomes than IDC patients. However, despite this higher propensity for lymph node involvement with IMPC than with IDC, we found that the 5-year OS rate of IMPC patients in our analysis was similar to the historical 5-year OS rate of patients with IDC reported in previous literature, which is in accordance with an analysis of a large group of IMPC patients using the Surveillance, Epidemiology, and End Results (SEER) database [3, 15].
The IMPC patient characteristics in the present study differ in some ways from the IMPC patient characteristics reported in the literature. For example, the median age of presentation for IMPC patients, while similar to the SEER database analysis [3, 15], was older than the age at presentation reported in other IMPC patient series [2, 3, 5, 6]. In addition, we found higher rates of hormone receptor positivity than the rates in those series. As we know, ER positivity is associated with older age and longer OS of breast cancer patients as a whole [16, 17], which may explain the favorable survival outcomes for IMPC patients in the present study.
On multivariate analysis, ER positivity was associated with improved prognosis. This finding speaks to the growing focus in oncology on the molecular and biologic characteristics of disease rather than the clinical presentations and stage. The majority of patients in our analysis fall under the luminal A/B molecular subtypes (hormone receptor-positive, HER2-negative), which are associated with better outcomes than HER2-positive or triple-negative disease [18,19,20].
It is interesting to note that age < 50 years was associated with prolonged OS, as it has been previously observed that breast cancer patients who present at a younger age tend to have worse outcomes [21,22,23]. This finding may be unique to this particular subtype of breast cancer although several contributing and confounding factors may also be at play. In breast cancer as a whole, patients who present at a younger age are more likely to have more aggressive molecular subtypes, higher grade disease, and present at a more advanced stage than those at an older age [24,25,26]. As noted previously, the large majority of IMPC patients in our analysis had high rates of ER and PR positivity and therefore fall under the luminal A and B molecular subtypes, which may explain why the younger patients in our study did not have a worse prognosis. In addition, because the NCDB tracks only OS and not cause-specific or disease-specific survival, it is possible that patients older than 50 years had other comorbidities that affected the survival outcomes. Indeed, a Charlson–Deyo comorbidity score ≥ 1 was associated with short OS of IMPC patients on both univariate and multivariate analyses, which is consistent with the observations on breast cancer as a whole [27,28,29].
Another important and interesting finding of our analysis is that EBRT was associated with prolonged OS on univariate analysis. Importantly, the OS benefit was limited to patients receiving lumpectomy, and no OS benefit was observed among patients receiving mastectomy. Radiotherapy is well known to improve locoregional control and OS after breast-conserving surgery and mastectomy [30,31,32,33], but has not been studied specifically in IMPC. It is possible that, due to the high propensity of lymph node involvement in IMPC, EBRT may be important to provide good locoregional control and, subsequently, OS.
For breast cancer as a whole, the role of EBRT in nodal disease has evolved over time [28]. Given IMPC’s lymphotrophic nature, whether regional nodes should be included along with the standard whole-breast irradiation field is an important issue. Recent trials have highlighted prolonged disease-free survival with regional nodal irradiation (RNI) in patients with early-stage breast cancer [34, 35]. In addition, a meta-analysis conducted by the Early Breast Cancer Trialists’ Collaborative Group demonstrated that the survival benefit of postmastectomy radiotherapy with comprehensive lymph node coverage is not limited to patients with ≥ 4 positive lymph nodes, but also extended to patients with 1–3 positive lymph nodes [36]. Although EBRT likely plays an important part in the treatment of IMPC, the specific role of RNI in this subtype is still unclear.
Our study has several limitations due to its reliance on the NCDB. First, the retrospective nature of the study and all associated inherent biases must be acknowledged. The lack of central review of pathology specimens is another limitation; it is not clear what threshold level of micropapillary involvement was required for the samples to be flagged as IMPC in the database. However, previous studies have failed to find an association between the degree of micropapillary involvement and OS or lymph node involvement, suggesting that the presence of IMPC involvement (not the degree of involvement) is the most important factor in determining outcomes [2, 37]. The NCDB also does not include information of the receipt of targeted therapy. Finally, although the NCDB has information regarding the treatment delivered, it does not have information regarding the reasons for the delivery of each treatment. It is possible that patients who did not receive radiotherapy may have had a low Eastern Cooperative Oncology Group (ECOG) or Karnofsky Performance Status (KPS) score, and that the observed short OS in these patients was likely due to their underlying poor performance status and not the omission of radiotherapy.
Conclusions
Although IMPC has a high propensity for lymph node metastasis, patients’ OS is comparable to the historical OS of IDC reported in literature. On univariate analysis, ≥ 4 positive lymph nodes, a Charlson–Deyo comorbidity score ≥ 1, and age > 65 years were associated with short OS. In contrast, receipt of EBRT and ER positivity were associated with prolonged OS. This study demonstrated a survival benefit of IMPC patients associated with EBRT, though this may be limited to patients receiving lumpectomy.
Availability of data and materials
The data that support the findings of this study are available from the NCDB but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are however available from the authors upon reasonable request and with permission of the NCDB.
Abbreviations
- IMPC:
-
invasive micropapillary carcinoma
- IDC:
-
invasive ductal carcinoma
- NCDB:
-
National Cancer Database
- OS:
-
overall survival
- ACoS:
-
American College of Surgeons
- ER:
-
estrogen receptor
- PR:
-
progesterone receptor
- HER2:
-
human epidermal growth factor receptor 2
- EBRT:
-
external beam radiotherapy
- HR:
-
hazard ratio
- SEER:
-
Surveillance, Epidemiology, and End Results
- RNI:
-
regional nodal irradiation
- ECOG:
-
Eastern Cooperative Oncology Group
- KPS:
-
Karnofsky Performance Status
References
Fisher ER, Palekar AS, Redmond C, Barton B, Fisher B. Pathologic Findings from the National Surgical Adjuvant Breast Project (Protocol No. 4): VI. Invasive papillary cancer. Am J Clin Pathol. 1980;73(3):313–22. https://doi.org/10.1093/ajcp/73.3.313.
Kim MJ, Gong G, Joo HJ, Ahn SH, Ro JY. Immunohistochemical and clinicopathologic characteristics of invasive ductal carcinoma of breast with micropapillary carcinoma component. Arch Pathol Lab Med. 2005;129(10):1277–82.
Chen AC, Paulino AC, Schwartz MR, Rodriguez AA, Bass BL, Chang JC, et al. Prognostic markers for invasive micropapillary carcinoma of the breast: a population-based analysis. Clin Breast Cancer. 2013;13(2):133–9.
Tavassoli FA, Devilee P. Pathology and genetics of tumours of the breast and female genital organs. New York: IARC; 2003.
Yu JI, Choi DH, Huh SJ, Cho EY, Kim K, Chie EK, et al. Differences in prognostic factors and failure patterns between invasive micropapillary carcinoma and carcinoma with micropapillary component versus invasive ductal carcinoma of the breast: retrospective multicenter case–control study (KROG 13-06). Clin Breast Cancer. 2015;15(5):352–3.
Chen H, Ding A. Comparison of invasive micropapillary and triple negative invasive ductal carcinoma of the breast. Breast. 2015;24(6):723–31.
Shi WB, Yang LJ, Hu X, Zhou J, Zhang Q, Shao ZM. Clinico-pathological features and prognosis of invasive micropapillary carcinoma compared to invasive ductal carcinoma: a population-based study from China. PLoS ONE. 2014;9(6):e101390.
Cui ZQ, Feng JH, Zhao YJ. Clinicopathological features of invasive micropapillary carcinoma of the breast. Oncol Lett. 2015;9(3):1163–6. https://doi.org/10.3892/ol.2014.2806.
Lerro CC, Robbins AS, Phillips JL, Stewart AK. Comparison of cases captured in the national cancer data base with those in population-based central cancer registries. Ann Surg Oncol. 2013;20(6):1759–65. https://doi.org/10.1245/s10434-013-2901-1.
Haque W, Lewis GD, Verma V, Darcourt JG, Butler EB, Teh BS. The role of adjuvant chemotherapy in locally advanced bladder cancer. Acta Oncol (Madr). 2018;57(4):509–15. https://doi.org/10.1080/0284186X.2017.1415461.
Haque W, Verma V, Lewis GD, Lo SS, Butler EB, Teh BS. Utilization of radiotherapy and stereotactic body radiation therapy for renal cell cancer in the USA. Future Oncol. 2018;14(9):819–27. https://doi.org/10.2217/fon-2017-0536.
Lewis GD, Haque W, Butler EB, Teh BS. Survival outcomes and patterns of management for anal adenocarcinoma. Ann Surg Oncol. 2019;26(5):1351–7. https://doi.org/10.1245/s10434-019-07202-4.
Lewis GD, Dalwadi SM, Farach A, Butler EB, Teh BS. The role of adjuvant radiotherapy in the treatment of pleural mesothelioma. Ann Surg Oncol. 2019;26(6):1879–85. https://doi.org/10.1245/s10434-019-07235-9.
Lewis GD, Haque W, Verma V, Butler EB, Teh BS. The role of adjuvant radiation therapy in locally advanced bladder cancer. Bl Cancer. 2018;4(2):205–13.
Chen AC, Paulino AC, Schwartz MR, Rodriguez AA, Bass BL, Chang JC, et al. Population-based comparison of prognostic factors in invasive micropapillary and invasive ductal carcinoma of the breast. Br J Cancer. 2014;111(3):619–22.
Clark GM, Osborne CK, McGuire WL. Correlations between estrogen receptor, progesterone receptor, and patient characteristics in human breast cancer. J Clin Oncol. 1984;2(10):1102–9.
Osborne CK, Fisher E, Redmond C, Knight WA, Yochmowitz MG, McGuire WL. Estrogen receptor, a marker for human breast cancer differentiation and patient prognosis. Boston: Springer; 1982. p. 377–85. https://doi.org/10.1007/978-1-4615-7192-6_23.
Nguyen PL, Taghian AG, Katz MS, Niemierko A, Abi Raad RF, Boon WL, et al. Breast cancer subtype approximated by estrogen receptor, progesterone receptor, and HER-2 is associated with local and distant recurrence after breast-conserving therapy. J Clin Oncol. 2008;26(14):2373–8. https://doi.org/10.1200/JCO.2007.14.4287.
Haffty BG, Yang Q, Reiss M, Kearney T, Higgins SA, Weidhaas J, et al. Locoregional relapse and distant metastasis in conservatively managed triple negative early-stage breast cancer. J Clin Oncol. 2006;24(36):5652–7.
Zuo T, Zeng H, Li H, Liu S, Yang L, Xia C, et al. The influence of stage at diagnosis and molecular subtype on breast cancer patient survival: a hospital-based multi-center study. Chin J Cancer. 2017;36(1):84.
de la Rochefordière A, Campana F, Fenton J, Vilcoq JR, Fourquet A, Asselain B, et al. Age as prognostic factor in premenopausal breast carcinoma. Lancet. 1993;341(8852):1039–43.
Fredholm H, Magnusson K, Lindström LS, Garmo H, Fält SE, Lindman H, et al. Long-term outcome in young women with breast cancer: a population-based study. Breast Cancer Res Treat. 2016;160(1):131–43. https://doi.org/10.1007/s10549-016-3983-9.
Wong FY, Tham WY, Nei WL, Lim C, Miao H. Age exerts a continuous effect in the outcomes of Asian breast cancer patients treated with breast-conserving therapy. Cancer Commun (London, England). 2018;38(1):39.
Bonnier P, Romain S, Charpin C, Lejeune C, Tubiana N, Martin P-M, et al. Age as a prognostic factor in breast cancer: relationship to pathologic and biologic features. Int J Cancer. 1995;62(2):138–44. https://doi.org/10.1002/ijc.2910620205.
Sabiani L, Houvenaeghel G, Heinemann M, Reyal F, Classe JM, Cohen M, et al. Breast cancer in young women: pathologic features and molecular phenotype. Breast. 2016;29:109–16.
Liedtke C, Rody A, Gluz O, Baumann K, Beyer D, Kohls E-B, et al. The prognostic impact of age in different molecular subtypes of breast cancer. Breast Cancer Res Treat. 2015;152(3):667–73. https://doi.org/10.1007/s10549-015-3491-3.
Griffiths RI, Gleeson ML, Valderas JM, Danese MD. Impact of undetected comorbidity on treatment and outcomes of breast cancer. Int J Breast Cancer. 2014;2014:970780.
Braithwaite D, Moore DH, Satariano WA, Kwan ML, Hiatt RA, Kroenke C, et al. Prognostic impact of comorbidity among long-term breast cancer survivors: results from the LACE study. Cancer Epidemiol Biomarkers Prev. 2012;21(7):1115–25.
Land LH, Dalton SO, Jørgensen TL, Ewertz M. Comorbidity and survival after early breast cancer: a review. Crit Rev Oncol Hematol. 2012;81(2):196–205.
Clarke M, Collins R, et al. Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;366(9503):2087–106.
Overgaard M, Nielsen HM, Overgaard J. Is the benefit of postmastectomy irradiation limited to patients with four or more positive nodes, as recommended in international consensus reports? A subgroup analysis of the DBCG 82 b&c randomized trials. Radiother Oncol. 2007;82(3):247–53.
Ragaz J, Olivotto IA, Spinelli JJ, Phillips N, Jackson SM, Wilson KS, et al. Locoregional radiation therapy in patients with high-risk breast cancer receiving adjuvant chemotherapy: 20-year results of the British Columbia randomized trial. JNCI J Natl Cancer Inst. 2005;97(2):116–26. https://doi.org/10.1093/jnci/djh297.
Haffty BG, Mahmoud O. The evolution of regional nodal irradiation in breast cancer. Breast J. 2015;21(1):32–41. https://doi.org/10.1111/tbj.12351.
Whelan TJ, Olivotto IA, Parulekar WR, Ackerman I, Chua BH, Nabid A, et al. Regional nodal irradiation in early-stage breast cancer. N Engl J Med. 2015;373(4):307–16. https://doi.org/10.1056/NEJMoa1415340.
Poortmans PM, Collette S, Kirkove C, Van Limbergen E, Budach V, Struikmans H, et al. Internal mammary and medial supraclavicular irradiation in breast cancer. N Engl J Med. 2015;373(4):317–27. https://doi.org/10.1056/NEJMoa1415369.
EBCTCG (Early Breast Cancer Trialists’ Collaborative Group) P, McGale P, Taylor C, Correa C, Cutter D, Duane F, et al. Effect of radiotherapy after mastectomy and axillary surgery on 10-year recurrence and 20-year breast cancer mortality: meta-analysis of individual patient data for 8135 women in 22 randomised trials. Lancet (London, England). 2014;383(9935):2127–35.
Walsh MM, Bleiweiss IJ. Invasive micropapillary carcinoma of the breast: eighty cases of an underrecognized entity. Hum Pathol. 2001;32(6):583–9.
Vingiani A, Maisonneuve P, Dell’Orto P, Farante G, Rotmensz N, Lissidini G, et al. The clinical relevance of micropapillary carcinoma of the breast: a case–control study. Histopathology. 2013;63(2):217–24. https://doi.org/10.1111/his.12147.
Adrada B, Arribas E, Gilcrease M, Yang WT. Invasive micropapillary carcinoma of the breast: mammographic, sonographic, and MRI features. Am J Roentgenol. 2009;193(1):W58–63. https://doi.org/10.2214/AJR.08.1537.
Chen L, Fan Y, Lang R, Guo X, Sun Y, Cui L, et al. Breast carcinoma with micropapillary features: clinicopathologic study and long-term follow-up of 100 cases. Int J Surg Pathol. 2008;16(2):155–63. https://doi.org/10.1177/1066896907307047.
De La Cruz C, Moriya T, Endoh M, Watanabe M, Takeyama J, Yang M, et al. Invasive micropapillary carcinoma of the breast: clinicopathological and immunohistochemical study. Pathol Int. 2004;54(2):90–6. https://doi.org/10.1111/j.1440-1827.2004.01590.x.
Luna-Moré S, Casquero S, Pérez-Mellado A, Rius F, Weill B, Gornemann I. Importance of estrogen receptors for the behavior of invasive micropapillary carcinoma of the breast. Review of 68 cases with follow-up of 54. Pathol Res Pract. 2000;196(1):35–9.
Middleton LP, Tressera F, Sobel ME, Bryant BR, Alburquerque A, Grases P, et al. Infiltrating micropapillary carcinoma of the breast. Mod Pathol. 1999;12(5):499–504.
Nassar H, Wallis T, Andea A, Dey J, Adsay V, Visscher D. Clinicopathologic analysis of invasive micropapillary differentiation in breast carcinoma. Mod Pathol. 2001;14(9):836–41.
Paterakos M, Watkin WG, Edgerton SM, Moore DH, Thor AD. Invasive micropapillary carcinoma of the breast: a prognostic study. Hum Pathol. 1999;30(12):1459–63.
Pettinato G, Manivel CJ, Panico L, Sparano L, Petrella G. Invasive micropapillary carcinoma of the breast. Am J Clin Pathol. 2004;121(6):857–66. https://doi.org/10.1309/XTJ7VHB49UD78X60.
Yamaguchi R, Tanaka M, Kondo K, Yokoyama T, Kaneko Y, Yamaguchi M, et al. Characteristic morphology of invasive micropapillary carcinoma of the breast: an immunohistochemical analysis. Jpn J Clin Oncol. 2010;40(8):781–7.
Zekioglu O, Erhan Y, Ciris M, Bayramoglu H, Ozdemir N. Invasive micropapillary carcinoma of the breast: high incidence of lymph node metastasis with extranodal extension and its immunohistochemical profile compared with invasive ductal carcinoma. Histopathology. 2004;44(1):18–23. https://doi.org/10.1111/j.1365-2559.2004.01757.x.
Acknowledgements
There are no acknowledgements.
Funding
There was no funding for this study.
Author information
Authors and Affiliations
Contributions
GL was the major contributor to the writing of this manuscript and involved in the design of this study. YX and WH were involved with the statistical analysis and data collection process. TP, MS, AC, AF, SH, EBB, and JC were involved with the writing of the manuscript. BST was significantly involved in the design of the study and the writing of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
As all patient information in the NCDB database is de-identified, this study was exempt from institutional review board evaluation.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Lewis, G.D., Xing, Y., Haque, W. et al. Prognosis of lymphotropic invasive micropapillary breast carcinoma analyzed by using data from the National Cancer Database. Cancer Commun 39, 60 (2019). https://doi.org/10.1186/s40880-019-0406-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40880-019-0406-4