Abstract
Background
Wedelia chinensis (W. chinensis) is a beneficial medicinal herb used in folk medicine to treat many chronic diseases. The present study is aimed to evaluate the antidiabetic property of methanolic extract of W. chinensis leaf (MEWL) in alloxan induced Swiss albino diabetic mice.
Methods
Methanol was used as a solvent to obtain W. chinensis leaf extract. In vitro antidiabetic property of MEWL was revealed by α-amylase and α-glucosidase inhibition assays. To explore in vivo antidiabetic properties of MEWL, diabetes was induced in Swiss albino mice by intraperitoneal injection of alloxan at dose of 80 mg/kg body weight (BW). All Swiss albino mice (normal mice and diabetic mice) were divided into five groups and orally treated with normal pellet diet and water (normal control and diabetic control), glibenclamide 5 mg/kg BW and MEWL (100 and 200 mg/kg BW). Effects of MEWL on blood glucose levels, activity of liver function enzymes associated with diabetes and serum levels of lipid parameters were evaluated.
Results
In α-amylase inhibition assay, 48.39% inhibition in the activity of α-amylase was observed at 500 μg/ml concentration of MEWL. Moreover, 39.37% inhibition of α-glucosidase activity was observed at 10 μg/ml concentration of MEWL. The results of in vivo antidiabetic assays showed that MEWL significantly (p < 0.01) decreased blood glucose level and ameliorated parameters of lipid profile (TG, TC, LDL, VLDL and HDL) in diabetic mice. MEWL treatments for 21 days also reduced the activity of SGPT and SGOT enzymes, and CRP levels in the serum of diabetic mice significantly (p < 0.01) compared to that of untreated diabetic mice.
Conclusion
The present study demonstrated that W. chinensis leaf extract effectively subsidized the hyperglycemic effect along with restoring the lipid profile parameters in diabetic mice and might be used as an alternative medicine for the management of diabetes.
Similar content being viewed by others
Introduction
Nowadays, diabetes mellitus (DM) is considered as one of the most common health problems in the world [1]. In 2019, it was estimated that 463 million people had DM throughout the world, and it is projected that total number of diabetic patients will be 578 million by 2030 [2].With the increasing prevalence of DM, death associated with DM and DM related chronic diseases increased by 66% and 33%, respectively [3]. Instead of this high prevalence of DM, there is no treatment available to cure DM yet.
DM is a chronic disease that occurs due to lack of insulin production by pancreas or when the body can not use insulin properly produced by it. Therefore, elevated levels of blood glucose are found in DM over a prolong period [4]. This increased blood glucose level affects protein, carbohydrate and lipid metabolism in DM patients [5]. Insulin deficiency and insulin resistance affect enzymes and pathways of lipid metabolism. For that reason, increased levels of triglycerides (TG), low density lipoprotein (LDL), very low density lipoprotein (VLDL), and a decreased levels of high density lipoprotein (HDL) are very common in DM [5, 6]. DM may increase the risk of liver diseases. In DM, abnormal activity of liver enzymes, such as serum glutamate-pyruvate transaminase (SGPT) and serum glutamic oxaloacetic transaminase (SGOT), non-alcoholic liver steatosis, liver cirrhosis and liver cancer were reported previously [7, 8]. Moreover, many other complications are associated with chronic DM including neuropathy, retinopathy, atherosclerosis, hypertension, cardiovascular diseases, diabetic foot, periodontal diseases, dry mouth, dental caries, oral cancer, arthritis, and many more [9, 10]. Alpha-glucosidase and α-amylase catalyze the conversion of polysaccharides into monosaccharide and disaccharide, and inhibitors of these two enzymes are also used to decrease blood glucose levels in type I and type II DM [11]. Changing food habit, daily exercise, lifelong use of insulin (for type I DM), herbal medicine, and synthetic hypoglycemic agents such as; sulfonylureas, biguanides, thiazolidinediones and meglitinides are common treatments for DM, but these synthetic drugs have many adverse effects [12,13,14,15]. Transplantation of artificial pancreas is a new hope to cure type I DM, but it is highly expensive for common people [16]. Therefore, search for new antidiabetic drugs least or no side effect remains a challenge.
Medicinal plants have attracted the attention of researchers for the treatment of DM and other diseases due to their fewer side effects. According to previous study, plants are the major sources of different drugs, and almost 800 plants may process antidiabetic activity [17,18,19,20]. Many plants are already being used for the treatment of DM in traditional medicine [19, 21]. To find out polysaccharide hydrolyzing natural inhibitor, we under took an unexplored Asteraceae family medicinal plant, W. chinensis. It is commonly known as Bhimra or Bhringraj which is rich in flavonoids and polyphenols [22]. It is traditionally used for the treatment of chin cough, diarrhea, diphtheria and jaundice [23]. Previous studies suggested that polyphenols and flavonoids are prominent sources of natural antioxidants and exhibit conspicuous biological actions [24, 25]. This study implies to the evaluation of therapeutic potentiality in treatment of diabetic animal model by evaluating through the alpha-amylase and alpha-glucosidase inhibition effects.
Materials and method
Chemicals and reagents
Sodium chloride, α-amylase, iodine reagent, HCl, starch, p-nitrophenol α-D-glucopyranoside (PNPG), sodium azide, 0.1 M Sodium phosphate buffer, α-glucosidase, acarbose and alloxan were purchased from Sigma-Aldrich (St. Louis, MO, USA). Reagent kit for glucose for the estimation of TC, TG, LDL, VLDL, HDL, CRP, SGPT and SGOT were purchased from Linear chemicals (Barcelona, Spain). Glibenclamide was purchased from Advanced Chemical Industries (ACI) Limited, Bangladesh. All other chemicals were used in analytical grade.
Collection of plant material and authentication
W. chinensis leaves were collected from Rajshahi University, Rajshahi-6205, Bangladesh at July, 2019 and authenticated by Dr. A.H.M. Mahbubur Rahman, Professor, Department of Botany University of Rajshahi, Bangladesh.
Preparation of extract
At first, W. chinensis leaves were washed with clean water to remove adhering dirt and sorted to collect fresh and mature leaves. Then those leaves were chopped and shed dried at 25–28 °C for 7 days. After complete drying, the entire portions were grinded into a coarse powder by a grinding machine (FFC-15, China) and stored in an airtight container at room temperature for further use. About 80 g of the powdered material was taken into a clean, round bottomed glass bottle and soaked in 400 ml of solvent (methanol). The container with its content was sealed by cotton plug and aluminum foil, and kept for 15 days accompanied with occasional shaking and stirring. After maceration, the resulting extracts were filtered through Whatman No.1 filter paper. Afterwards, the solvents were evaporated under reduced pressure at 39 °C using a rotary evaporator. Finally, the residues were kept in small sterile bottles at 4 °C until used for further experiments.
α-amylase inhibition assay
Screenings for α-amylase inhibition by extracts were carried out according to the method described by Xiao et al. [26] with slight modification. Different concentrations of 500 μl extracts were added to 500 μl of 0.02 M sodium phosphate buffer (6 mM sodium chloride; pH 6.9) containing 0.5 mg/ml of α-amylase solution, and incubated at 37 °C for 10 min. Then 500 μl soluble starch (1%, w/v) was added to each reaction test tube and incubated at 37 °C for 15 min. After that, 1 M HCl (20 μl) was added to stop the enzymatic reaction followed by the addition of 100 μl of iodine reagent (5 mM I2 and 5 mM KI). The color change was noted and the absorbance was read at 620 nm. Acarbose was used as positive control. The results were expressed as % inhibition calculated using the formula:
Where A1 is the absorbance of the test sample, A2 is the absorbance of product control (sample without α-amylase solution) and A0 is the absorbance of negative control (α-amylase without extract).
α-glucosidase inhibition assay
The α-glucosidase inhibition assay was performed following the method described by Schmidt et al. [27]. In a 96 well microplate, 10 μl of extract at various concentrations and 90 μl of 0.1 M sodium phosphate buffer (SPB) pH containing 0.02% sodium azide. After that, 80 μl of α-glucosidase solution (2.0 U/ml) in SPB were added in each well, and the mixture was incubated at 28 °C for 10 min. Acarbose was used as a positive control. After the incubation, 20 μl of PNPG (0.4 mM, dissolved in SPB) was mixed into the solution to initiate the reaction. The rate of PNPG conversion to p-nitrophenol was determined by the measurement of absorbance of p-nitrophenol at 405 nm using a Multiscan FC microplate photometer (Thermo Fisher Scientific, Waltham, MA, USA). Acarbose was used as standard. The percentage of α-glucosidase inhibition was calculated by the following equation:
Animal care
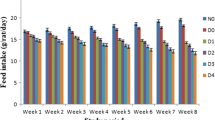
Swiss albino mice weighting about 20–28 g were collected from the International Cholera and Dysentery Disease Research, Bangladesh (ICDDRB). They were individually housed in polypropylene cages in well-ventilated rooms (temperature 25 ± 2 °C; humidity 55 ± 5% with 12 h light/dark cycle) under hygienic conditions. Mice were allowed free access to standard dry pellet diet and water. Food intake was withdrawn before 16 to 18 h to start the experiments.
Induction of diabetes
Diabetes was induced in overnight fasting mice by a single intraperitoneal injection of alloxan (80 mg/kg body weight; BW) in a 0.1 M sodium citrate buffer (pH 4.5). The age-matched control mice received an equivalent amount of citrate buffer. Food and water intake were closely monitored daily after alloxan administration. The development of hyperglycemia in mice was confirmed by measuring fasting blood glucose level (tail vein blood) after 48 h of alloxan administration with a portable glucometer (Accu-Chek, Roche, Germany). The animals with fasting blood glucose level ≥ 11.0 mmol/l were considered as diabetic mice.
Experimental groups
After 1 week of acclimatization period, all mice were divided into following five groups:
-
Group-1 (Normal control): Mice feed with standard pellet diet and water.
-
Group-2 (Diabetic control): Diabetic mice without treatment.
-
Group-3 (Positive control): Diabetic mice were treated by glibenclamide at dose of 5 mg/kg BW [28, 29].
-
Group-4 (MEWL 100 mg/kg BW): The diabetic mice treated with MEWL at a dose of 100 mg/kg BW for 21 days [30].
-
Group-5 (MEWL 200 mg/kg BW): The diabetic mice treated with MEWL at a dose of 200 mg/kg BW for 21 days [30].
Oral glucose tolerance test (OGTT)
This test was performed according to a previously described protocol with slight modifications [31]. All mice were kept fasting for 16 h prior to the experiment. Mice of group 4 and group 5 were orally administrated with MEWL 100 mg/kg and 200 mg/kg BW, respectively, followed by oral administration of glucose solution (1 g/kg BW). Mice from group 3 were treated with the antihyperglycemic agent glibenclamide at a dose of 5 mg/kg body weight, followed by oral glucose administration (1 g/kg BW), while the of control group (Group I) did not receive glucose solution. Blood samples were subsequently collected from tail veins at 0, 30, 60, 90 and 120 min following oral glucose administration and glucose levels were measured using a portable glucometer (Accu-Chek, Roche, Germany) by vein puncture [32].
Collection of blood
Blood samples from all mice were collected on day 1, 5, 10, 15 and 21 in a fasting state from the tail vein by 26 G needle and syringe. At final stage of experiment period (21 days), mice were anesthetized with chloroform, and sacrificed after overnight fasting. After that, blood was taken from the artery of the heart by syringe. The serum was separated by allowing blood samples left for 10 min at a temperature of 25 °C, then centrifuged at 3000 rpm for 20 min, after centrifugation, serum was collected and kept in plastic vial at − 80 °C until further experiments.
Measurement of biochemical parameters
Plasma concentrations of TG, TC, HDL, LDL, VLDL, CRP, SGPT and SGOT were measured using commercially available kit (Linear chemicals, Barcelona, Spain) using an automatic Bio-analyzer (Hitachi 7180, Hitachi, Tokyo, Japan).
Statistical analysis
All statistical analyses were performed using SPSS software of (version 16). All values were expressed as mean ± standard deviation (SD), and considered statistically significant at p < 0.05 (one-way ANOVA followed by Dunnett’s t test).
Results
Effect of MEWL on α-amylase inhibitory activity
α-amylase inhibitory activity of W. chinensis leaf extract is shown in (Fig. 1). In this assay, dose-dependent increase in the percentage inhibitory activity against α-amylase enzyme was noted. The percent of inhibition of α-amylase activity was 48.39 ± 0.277% by MEWL at a concentration of 500 μg/ml, and standard acarbose showed 57.98 ± 1.492% inhibition in α-amylase activity at the same concentration.
Effect of MEWL on -glucosidase inhibition assay
α-glucosidase inhibitory activity of W. chinensis leaf is shown in (Fig. 2). In this assay, the percentage inhibitory activity of MEWL and acarbose against α-glucosidase enzyme was gradually increased with concentration. At 10 μg/ml concentration, MEWL showed 39.37% inhibition in the activity of α-glucosidase and acarbose showed 51.45% inhibition in the activity of α-glucosidase.
Effect of MEWL on oral glucose tolerance test in diabetic mice
OGTT has been performed in fasting mice after oral administration of methanolic extract. The results of the OGTT are summarized in Table 1. At the ultimate 120 min interval following oral glucose intake, the mice treated with MEWL (100 and 200 mg/kg BW) were able to metabolize glucose significantly compared to the mice of diabetic control group.
Effects of MEWL on blood glucose level
Effects of MEWL treatment in the blood glucose levels of alloxan induced diabetic mice are represented in Fig. 3. Intraperitoneal administration of alloxan (80 mg/kg BW) significantly increased the blood glucose levels of diabetic control mice compared to normal control mice. MEWL (100 and 200 mg/kg BW) treatment significantly reduced the blood glucose levels of diabetic mice compared to that of diabetic control mice. This level of reduction was as near as glibenclamide administered subject. In 5th to 21stdays, MEWL at both doses (100 mg/kg and 200 mg/kg BW) lowered the glucose level by 9.9% - 39.72% and 14.5% - 50.96%, respectively, compared to diabetic control mice.
Effects of MEWL on lipid profile
Figure 4 represents the effects of 21 days supplementation with MEWL on the levels of serum TC, TG, HDL, LDL and VLDL in experimental mice. Serum levels of TG, TC, LDL and VLDL were increased, and HDL level was decreased significantly in alloxan induced diabetic mice compared to that of normal control mice. Oral administration of MEWL at both doses (100 and 200 mg/kg BW) regulated those parameters of lipid profile in diabetic mice towards the normal level significantly (p < 0.01) compared to the diabetic control mice. After 21 days treatment with MEWL, serum levels of TC, TG, LDL and VLDL decreased significantly compared to that of diabetic control mice. MEWL treatment also increased serum HDL levels in diabetic mice significantly.
Effects of MEWL on serum SGPT, SGOT and CRP level
Effects of MEWL supplementation in the activity of serum SGPT and SGOT in diabetic mice are represented in Fig. 5. Increased activity of serum SGPT and SGOT in diabetic mice was reduced significantly by supplementation of MEWL for 21 days. Compared to diabetic control mice, MEWL decreased SGPT activity by 19.87% (100 mg/kg BW) and 27.03% (200 mg/kg BW), whereas glibenclamide reduced SGPT activity by 36.03%. The reduction of SGOT activity with the treatment of MEWL were 12.72% (100 mg/kg BW) and 19.75% (200 mg/kg BW), whereas glibenclamide lowered SGOT activity was 32.005%.
Alloxan induced diabetic mice also showed increased levels of serum CRP compared to normal control mice (Fig. 6). The administration of MEWL also reduced the CRP levels significantly compared to that of diabetic control mice at both doses.
Discussion
In this study, we have explored in vitro and in vivo antidiabetic properties of W. chinensis leaf extract (MEWL) for first time. MEWL treatments also showed promising beneficial effects to lipid profile, liver function enzymes activity and serum CRP levels in alloxan induced diabetic mice.
In this study, in vitro antidiabetic activity of MEWL were evaluated using α-amylase inhibition assay and α-glucosidase inhibition assay. Alpha-amylase catalyzes the breakdown of starch into maltose and α-glucosidase catalyzes the conversion of polysaccharides into monosaccharides, such as glucose. Therefore, these two assays are used largely for the screening of drugs having antidiabetic activity. In this present study, we observed α-amylase and α-glucosidase inhibitory properties of MEWL.
Increased blood glucose level or hyperglycemia due to the lack of insulin production by pancreas or insulin resistance is the most common characteristics of DM [4, 33]. MEWL supplementation decreased blood glucose levels significantly in diabetic mice compared to that of diabetic control mice. According to previous study, W. chinensis leaf contains several antioxidants and possesses antidiabetic activity (in vitro) [34, 35]. Therefore, these results are consistent with previous study.
Increased levels of serum TC, TG, LDL and VLDL, and decreased level HDL are also very common in DM patients due to the altered metabolism of carbohydrates and lipids [6, 36, 37]. With the progression of DM, these abnormalities are associated with the development of cardiovascular diseases in DM patients [6]. Significantly decreased levels of serum TC, TG, LDL and VLDL, and increased levels of HDL in MEWL treated diabetic mice compared to diabetic control mice were noted in this study. These data represent possible potential effects to W. chinensis leaf to reduce or prevent DM associated complication in lipid metabolism.
Liver damage is also very common in patients having chronic DM [38]. Chronic DM can lead to non-alcoholic fatty liver disease by affecting the metabolism of lipids, carbohydrates and proteins, which can further progress to non-alcoholic steatohepatitis, liver cirrhosis, and finally hepatocellular carcinomas promoting oxidative stress inflammatory response [38]. Due to liver damage, increased activity or levels of liver function enzymes, such as SGPT and SGOT are found in patients with DM [39]. Additionally, increased levels of CRP in the serum of patients having DM has been reported previously [40]. It is a potent marker of systemic inflammation and important risk factor of cardiovascular diseases [40, 41]. MEWL treatment for 21 days significantly restored the levels of SGPT, SGOT and CRP in diabetic mice compared to that of diabetic control mice. Therefore, this study also reveals the potentiality of W. chinensis leaf to reduce DM associated risk of liver damage and cardiovascular damages.
Conclusion
In summary, W. chinensis leaf has potential in vitro and in vivo antidiabetic properties. Furthermore, supplementation of W. chinensis leaf extract can ameliorate altered levels of TC, TG, LDL, VLDL, HDL, SGPT, SGOT and CRP in diabetic mice. Therefore, this study suggests the possible beneficial effects of W. chinensis leaf in the treatment and prevention of DM and DM associated complications. However, further study should be done to explore specific antidiabetic compounds present W. chinensis leaf and their mechanism of action.
Abbreviations
- ANOVA:
-
Analysis of variance
- TC:
-
Total cholesterol
- TG:
-
Triglyceride
- HDL:
-
High density lipoprotein
- LDL:
-
Low density lipoprotein
- VLDL:
-
Very low density lipoprotein
- SGPT:
-
Serum alanine aminotransferase
- SGOT:
-
Serum aspartate aminotransferase
- OGTT:
-
Oral glucose tolerance test
- CRP:
-
C Reactive protein
- SD:
-
Standard deviation
References
Wild SH, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030: response to Rathman and Giani. Diabetes Care. 2004;27(10):2569.
Saeedi P, Petersohn I, Salpea P, Malanda B, Karuranga S, Unwin N, et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas. Diabetes Res Clin Pract. 2019;157:107843.
Liu M, Liu S-W, Wang L-J, Bai Y-M, Zeng X-Y, Guo H-B, et al. Burden of diabetes, hyperglycaemia in China from to 2016: findings from the 1990 to 2016, global burden of disease study. Diabetes Metab. 2019;45(3):286–93.
Triplitt C, Solis-Herrera C, Cersosimo E, Abdul-Ghani M, Defronzo RA. Empagliflozin and linagliptin combination therapy for treatment of patients with type 2 diabetes mellitus. Expert Opin Pharmacother. 2015;16(18):2819–33.
Saltiel AR, Kahn CR. Insulin signalling and the regulation of glucose and lipid metabolism. Nature. 2001;414(6865):799–806.
Laakso M, Pyörälä K, Sarlund H, Voutilainen E. Lipid and lipoprotein abnormalities associated with coronary heart disease in patients with insulin-dependent diabetes mellitus. Arteriosclerosis. 1986;6(6):679–84.
Goyal A, Singh NP. Consumer perception about fast food in India: an exploratory study. Br Food J. 2007;109(2):182–95.
Beckman JA, Creager MA, Libby P. Diabetes and atherosclerosis: epidemiology, pathophysiology, and management. Jama. 2002;287(19):2570–81.
Akintola AA, van Heemst D. Insulin, aging, and the brain: mechanisms and implications. Front Endocrinol. 2015;6:13.
Nathan DM, Bayless M, Cleary P, Genuth S, Gubitosi-Klug R, Lachin JM, et al. Diabetes control and complications trial/epidemiology of diabetes interventions and complications study at 30 years: advances and contributions. Diabetes. 2013;62(12):3976–86.
Tundis R, Loizzo M, Menichini F. Natural products as α-amylase and α-glucosidase inhibitors and their hypoglycaemic potential in the treatment of diabetes: an update. Mini Rev Med Chem. 2010;10(4):315–31.
Kasole R, Martin HD, Kimiywe J. Traditional medicine and its role in the management of diabetes mellitus:“patients’ and herbalists’ perspectives”. Evid Based Complement Alternat Med. 2019;2019:2835691.
Chen Z, Zhang S, Yan L, Wu M, Chen L, Ji L. Association between side effects of oral anti-diabetic drugs and self-reported mental health and quality of life among patients with type 2 diabetes. Zhonghua Yi Xue Za Zhi. 2011;91(4):229–33.
Pickup J, Keen H. Continuous subcutaneous insulin infusion at 25 years: evidence base for the expanding use of insulin pump therapy in type 1 diabetes. Diabetes Care. 2002;25(3):593–8.
Bahmani M, Eftekhari Z, Saki K, Fazeli-Moghadam E, Jelodari M, Rafieian-Kopaei M. Obesity phytotherapy: review of native herbs used in traditional medicine for obesity. J Evid-Based Complement Altern Med. 2016;21(3):228–34.
Bekiari E, Kitsios K, Thabit H, Tauschmann M, Athanasiadou E, Karagiannis T, et al. Artificial pancreas treatment for outpatients with type 1 diabetes: systematic review and meta-analysis. Bmj. 2018;361:k1310.
Alarcon-Aguilara F, Roman-Ramos R, Perez-Gutierrez S, Aguilar-Contreras A, Contreras-Weber C, Flores-Saenz J. Study of the anti-hyperglycemic effect of plants used as antidiabetics. J Ethnopharmacol. 1998;61(2):101–10.
Hasan N, Shirin F, Khan MAJ, Al Mamun M, Belal MH, Hasan MM, et al. Hypoglycemic, hypolipidemic and antibacterial activity of Ficus racemosa fruit extract. J Pharmaceut Res Int. 2017;16(1):1–9.
Li W, Zheng H, Bukuru J, De Kimpe N. Natural medicines used in the traditional Chinese medical system for therapy of diabetes mellitus. J Ethnopharmacol. 2004;92(1):1–21.
Newman DJ, Cragg GM. Natural products as sources of new drugs over the last 25 years. J Nat Prod. 2007;70(3):461–77.
Harlev E, Nevo E, Mirsky N, Ofir R. Antidiabetic attributes of desert and steppic plants: a review. Planta Med. 2013;79(06):425–36.
Bora KS, Pant A. Pharmacognostic standardization of Wedelia chinensis Merrill Leaf. Pharmacologia. 2017;8(83):89.
Cai C, Zhang Y, Yang D, Hao X, Li S. Two new kaurane-type diterpenoids from Wedelia chinensis (Osbeck.) Merr. Nat Prod Res. 2017;31(21):2531–6.
Quijada-Morín N, Hernández-Hierro JM, Rivas-Gonzalo JC, Escribano-Bailón MT. Extractability of low molecular mass flavanols and flavonols from red grape skins. Relationship to cell wall composition at different ripeness stages. J Agric Food Chem. 2015;63(35):7654–62.
Chen J, Mangelinckx S, Adams A, Wang Z-t, Li W-l, De Kimpe N. Natural flavonoids as potential herbal medication for the treatment of diabetes mellitus and its complications. Nat Prod Commun. 2015;10(1):187–200.
Xiao Z, Storms R, Tsang A. A quantitative starch? Iodine method for measuring alpha-amylase and glucoamylase activities. Anal Biochem. 2006;351(1):146–8.
Schmidt JS, Lauridsen MB, Dragsted LO, Nielsen J, Staerk D. Development of a bioassay-coupled HPLC-SPE-ttNMR platform for identification of α-glucosidase inhibitors in apple peel (Malus× domestica Borkh.). Food Chem. 2012;135(3):1692–9.
Tasnin MN, Islam A, Islam M, Hossain MI, Matiar M. A study on the antidiabetic property of a mixed herbal food. World J of Pharm and Pharm Sci. 2019;8(6):70-83.
Patel D, Kumar R, Laloo D, Hemalatha S. Diabetes mellitus: an overview on its pharmacological aspects and reported medicinal plants having antidiabetic activity. Asian Pac J Trop Biomed. 2012;2(5):411–20.
Huang Y-T, Wen C-C, Chen Y-H, Huang W-C, Huang L-T, Lin W-C, et al. Dietary uptake of Wedelia chinensis extract attenuates dextran sulfate sodium-induced colitis in mice. PLoS One. 2013;8(5):e64152.
Chung I-M, Kim E-H, Yeo M-A, Kim S-J, Seo MC, Moon H-I. Antidiabetic effects of three Korean sorghum phenolic extracts in normal and streptozotocin-induced diabetic rats. Food Res Int. 2011;44(1):127–32.
Ahangarpour A, Shabani R, Farbood Y. The effect of betulinic acid on leptin, adiponectin, hepatic enzyme levels and lipid profiles in streptozotocin–nicotinamide-induced diabetic mice. Res Pharmaceut Sci. 2018;13(2):142.
Amos AF, McCarty DJ, Zimmet P. The rising global burden of diabetes and its complications: estimates and projections to the year 2010. Diabet Med. 1997;14(S5):S7–S85.
Senthilkumar R, Ahmedjohn S, Archunan G, Manoharan N. Antioxidant activity of Wedelia chinensis in alloxan induced diabetic rats. Pharmacologyonline. 2008;2:640–51.
Thao NP, Binh PT, Luyen NT, Hung TM, Dang NH, Dat NT. α-Amylase and α-glucosidase inhibitory activities of chemical constituents from Wedelia chinensis (Osbeck.) Merr. leaves. J Anal Methods Chem. 2018;2018:2794904.
Renard CB, Kramer F, Johansson F, Lamharzi N, Tannock LR, von Herrath MG, et al. Diabetes and diabetes-associated lipid abnormalities have distinct effects on initiation and progression of atherosclerotic lesions. J Clin Invest. 2004;114(5):659–68.
Parhofer KG. Interaction between glucose and lipid metabolism: more than diabetic dyslipidemia. Diabetes Metab J. 2015;39(5):353–62.
Mohamed J, Nafizah AN, Zariyantey A, Budin SB. Mechanisms of diabetes-induced liver damage: the role of oxidative stress and inflammation. Sultan Qaboos Univ Med J. 2016;16(2):e132.
Mandal A, Bhattarai B, Kafle P, Khalid M, Jonnadula SK, Lamicchane J, et al. Elevated liver enzymes in patients with type 2 diabetes mellitus and non-alcoholic fatty liver disease. Cureus. 2018;10(11):e3626.
Mugabo Y, Li L, Renier G. The connection between C-reactive protein (CRP) and diabetic vasculopathy. Focus on preclinical findings. Curr Diabetes Rev. 2010;6(1):27–34.
Ridker PM, Buring JE, Shih J, Matias M, Hennekens CH. Prospective study of C-reactive protein and the risk of future cardiovascular events among apparently healthy women. Circulation. 1998;98(8):731–3.
Acknowledgements
The authors are thankful to Department of Biochemistry and molecular biology of Rajshahi University and also to ICDDRB, for supplying the Swiss albino mice.
Declarations
All authors have approved this manuscript. The content of this manuscript or any portion thereof, has not been published or submitted for publication elsewhere.
Funding
This research did not receive any grant.
Author information
Authors and Affiliations
Contributions
MWB, MAI, MK, MIH, MMR designed the experiment. MWB, MMI, MJS, RA collected plant material, prepared extract and performed in vivo and in vitro experiments. MWB, MMI, MK, AI, MIH, MMR, MAI conducted data analysis and data interpretation. MWB, AI, MAI prepared draft copy of that manuscript. MWB, MMI, MK, MJS, RA, AI, MIH, MMR, MAI substantively revised drafted manuscript. All authors read and approved the final draft of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This research work was approved by the Institutional Animal, Medical Ethics, Bio-Safety and Bio-Security Committee (IAMEBBC) for Experimentations on Animal, Human, Microbes, and Living Natural Sources, memo no. 118/320-IAMEBBC/IBSc. Institute of Biological Sciences, University of Rajshahi, Bangladesh.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bari, M.W., Islam, M.M., Khatun, M. et al. Antidiabetic effect of Wedelia chinensis leaf extract in alloxan induced Swiss albino diabetic mice. Clin Phytosci 6, 58 (2020). https://doi.org/10.1186/s40816-020-00197-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40816-020-00197-6