Abstract
Background
Men with metastatic castrate-resistant prostate cancer can experience an array of treatment-related side effects. Accumulating evidence suggests exercise may alleviate some of these adversities and assist in disease management. However, empirical evidence in advanced prostate cancer patients remains limited. The purpose of this study is to determine whether men with metastatic prostate cancer, who are ineligible for high-intensity exercise, can partake in a home-based, moderate-intensity exercise program and the impact of doing so on quality of life and physical fitness parameters.
Methods
Thirty men with adenocarcinoma of the prostate and progressive systemic, metastatic disease will be recruited. Clinicians will screen patients against inclusion criteria to determine eligibility. All men enrolled will be prescribed a tailored, home-based, moderate-intensity exercise intervention consisting of aerobic and strengthening components for 12 weeks. Patients will receive supplementary education materials and weekly behavioural change consultations throughout the intervention. The primary outcome will be the feasibility of delivering such an intervention in men with metastatic disease. Secondary endpoints including skeletal events will be monitored for safety, as will the feasibility of patient-reported outcome measures and the sampling time points, generating data pertaining to completion rates and potential effect in future trials. General physical fitness will be assessed during these visits, using timed sit-to-stand testing and a 6-min walking test. Prior to each visit, objective physical activity levels will be captured for 7 days using an accelerometer, to determine the feasibility of this technology and the quality of data obtained. In parallel with the feasibility aspects of the trial, changes compared to baseline will be reported. Direct regular contact will also serve as a feedback loop, should any issues arise. This study has received ethical approval from the Office for Research Ethics Committees Northern Ireland.
Conclusions
This study aims to determine the potential utility of a home-based exercise intervention in managing side effects associated with advanced prostate cancer and its treatment. This feasibility trial will inform the design and implementation of a larger randomised control trial to determine the efficacy of moderate aerobic and strengthening exercise as an adjuvant therapy in men with metastatic prostate cancer. Collecting such evidence provides further support for exercise in this paradigm and potential for its inclusion as a low-toxicity therapy in standard cancer care, in the longer term.
Trial registration
ClinicalTrials.gov, NCT03658486
Trial sponsor: Queen’s University Belfast (Reference: B18/15). Contact: Dr. Paula Tighe, Research and Enterprise, Queen’s University Belfast. Telephone: 02890 973,296. Email: p.tighe@qub.ac.uk. The sponsor reviewed the protocol and ethical application prior to submission.
Protocol issue: Version 1 (18th May 2018). Authors: MB, MM, SJ and GP.
Similar content being viewed by others
Introduction
The annual incidence rate for prostate cancer (PCa) in Northern Ireland is approximately 1100, making it the most prevalent cancer in males [1]. Of those diagnosed, 20% are advanced cases, characterised by metastatic progression to secondary sites and the ultimate development of resistance to hormone therapy [2]. Some patients enter the advanced disease state having initially failed primary treatment with radiotherapy or surgery. Commonly, metastatic PCa patients receive castration therapy, usually in the form of luteinising hormone-releasing hormone agonist (LHRHa) therapy. Despite significant developments, such methods can cause a number of adverse effects [3]. Numerous physical problems are presented (e.g. sexual dysfunction, urinary incontinence, reduced bone mineral density, increased fat mass and reduced muscle mass) while psychological issues such as anxiety and depression also arise [4]. Metastatic spread to the bone is common, subsequently increasing bone pain and fracture risk and shortening survival [5, 6]. Men with castrate-resistant, bone metastatic PCa may also receive chemotherapy, which can also cause adverse effects including severe fatigue [4]. Thus, the adverse effects of PCa and its treatment present a considerable clinical issue.
Accumulating evidence suggests that regular exercise can induce a host of physiological and psychological benefits, which may alleviate certain treatment-related toxicities and improve disease outcomes [7, 8]. Previous exercise research in cancer populations adds further credibility, to the extent that regular aerobic exercise may confer a risk reduction of fatality [9]. An initial study, in men receiving androgen deprivation therapy (ADT) for localised PCa, reported progressive resistance training increased muscular strength, endurance and physical function [10]. A follow-up combined exercise program reported improved lean muscle mass, muscular strength, aerobic fitness and quality of life (QOL) while ameliorating fatigue and inflammation [11]. Other clinical exercise studies also report lower risks in progression, mortality, cancer-related fatigue and pain [9, 12]. Consequently, tailored exercise has developed into a promising and effective therapy particularly in localised PCa [13]. However, the potential mechanism(s) remains a topic of debate but may be founded in endocrine regulation (insulin-like growth factor (IGF)-1 or testosterone signalling), metabolism, reactive oxygen species and antioxidant signalling, epigenetics or cytokine signalling among potential others [14, 15].
Despite early findings, advanced prostate patients are normally omitted due to their increased risk of fracture and hence do not avail of such health benefits. Cormie et al. [16] conducted a preliminary supervised resistance exercise program in metastatic patients and reported high retention rates alongside improved physical function and lean muscle mass compared to the control. These beneficial changes persisted at 6-month follow-up along with improvements in QOL [17]. The exercise volume, including intensity, appears a key mediator in accruing such health benefits and enhancing survival [9, 18]. This recent progress and international recognition has resulted in several position stands and expert statements endorsing exercise in cancer care including advanced cancers [19,20,21,22,23].
Though awareness is increasing, clinical and empirical evidence in PCa patients with bone metastases remains limited [16]. Appropriately designed and supervised training programs have proved feasible and clinically meaningful, but the chronic effects are yet to be determined [16]. The Movember GAP4 INTERVAL trial is seeking to determine such effects through a collaborative, randomised control trial [8]. We anticipate that some men with advanced disease may be ineligible or unable to tolerate the exercise component (i.e. high-intensity training), so we aim to provide a parallel intervention to ensure these men have the opportunity to capitalise on the benefits of partaking in regular exercise, albeit at moderate intensity. Our proposed study is the first to examine the effects of a progressive, home-based, combined moderate program, with continual behavioural support in this population. Research to date tends to utilise supervised exercise with good reason and success, but at this time an intensive model of exercise is potentially unsustainable in the UK National Health Service (i.e. cost and resource). Home-based exercise is emerging as a feasible and effective strategy for post-therapy cancer survivors [24] and a key component in future cancer trials [25]. Further, a recent small study of cancer survivors (n = 20 Australian adults) expressed a preference for this exercise option [26]; thus, the need to evaluate a remotely supervised regime in this population is necessary. This study aims to provide meaningful clinical information with the hope of directly impacting future care.
Our primary objective is to establish the feasibility of delivering an individualised, home-based walking and strengthening exercise intervention to men with advanced PCa, who are unable or ineligible for high-intensity exercise. The feasibility evaluation will address the accessibility and acceptability of the intervention as well as recruitment, attrition and exercise adherence rates. The secondary objectives will (1) determine the feasibility on QOL, functional ability and patient-reported assessments and any changes from baseline and (2) collect preliminary data on health-related QOL and health resource usage to inform cost-effectiveness. All men recruited will receive the intervention to refine and optimise the methodology. Whilst a randomised control trial (RCT) is the ‘gold standard’ for garnering evidence, supporting the putative role of exercise in cancer care, given how resource-intensive and challenging exercise trials are, we first aim to determine the efficacy and acceptability in a smaller feasibility study, to determine if and how the full trial should be conducted [27, 28]. Feasibility trials also improve the chances of conducting a high-quality RCT and inform methodological design and resource requirements, to reduce the likelihood of research waste [28, 29]. We propose this exercise intervention will measurably impact QOL in men with advanced PCa.
Methods
Study design
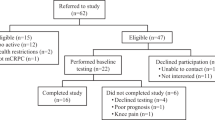
This study is a single-arm, feasibility trial coordinated by Queen’s University Belfast in conjunction with the Northern Ireland Cancer Centre (Belfast Health and Social Care Trust). All patients will be assigned to the exercise intervention to establish feasibility and provide justification for a randomised control trial. This trial was registered on 5 September 2018 (https://clinicaltrials.gov/ct2/show/NCT03658486) and has since opened for recruitment. A chart detailing study procedures can be found in Fig. 1. This protocol has been developed using the Standard Protocol Items: Recommendations for Interventional Trials (SPIRIT) guidelines. Figure 2 shows the SPIRIT figure.
Study objectives
The primary objective of this study is to establish the feasibility of delivering a prescribed exercise intervention to men with advanced prostate cancer, who are ineligible for high-intensity exercise (i.e. the Movember GAP4 INTERVAL study). As part of this primary objective, we will collect data:
-
To determine patient eligibility and recruitment rates.
-
To assess adherence to the programme and attrition rates.
-
To determine the rate of exercise-induced adverse events (if any).
-
To ascertain the extent to which the intervention might be integrated into clinical practice (and the cost implications of doing so).
-
To refine methodological variables, optimising the study design and data collection processes.
-
To explore the perceptions, acceptability and experiences of men with advanced prostate cancer undertaking the intervention and those men eligible that declined participation.
-
To explore the perception and experience of those recruiting to and/or delivering the intervention (e.g. Exercise Physiologist/Clinical Oncologists/Research Nurses).
Secondary objectives will focus on the feasibility of data collection processes, mainly collecting changes in body composition, functional ability, physical fitness, physical activity levels and patient-reported outcome measures (cancer-related fatigue, pain and quality of life). Additionally, we will use this feasibility trial to collect preliminary data on health resource usage to inform cost-effectiveness of future trials and refine statistical considerations (e.g. ascertain sample size estimates).
Progression criteria
Our methods for determining progression to a definitive RCT are based on recent recommendations [30]. Trial progression will primarily depend on the feasibility trial satisfying recruitment targets and secondarily protocol adherence targets, pre-determined by the trial steering committee. For example, 75–100% recruitment of the target sample size will enable progression to a main trial application, with little or no changes to relevant aspects of the protocol, while < 25% recruitment will prevent the trial from progressing. Recruitment of 50–74% will enable progression following a review of patients deemed ineligible or who declined the study invite, recruitment barriers and possible changes to relevant aspects of the protocol. Recruitment of 25–49% will only progress if a ‘rescue plan’ can be developed by the trial team and may involve identifying additional sites or changes to the protocol. Protocol adherence targets will be held to similar standards. Objectives relating to the feasibility of data collection will support the design of a definitive trial, but we have not assigned progression criteria.
Participants
Men with adenocarcinoma of the prostate and progressive mCRPC will be recruited from the Northern Ireland Cancer Centre, Belfast. Patients must be on androgen deprivation therapy (ADT) with a gonadotropin-releasing hormone (GnRH) agonist/antagonist or have undergone bilateral orchiectomy. All patients must remain castrated throughout the study period. At enrolment, patients must be receiving abiraterone or enzalutamide, with no evidence of progression. Patients must also be ≥ 4 weeks since last surgery and fully recovered. At enrolment, patients must have no known contraindications to moderate-intensity exercise, including, but not limited to, acute congestive heart failure, unstable angina, recent myocardial infarction and peripheral neuropathy greater than or equal to grade 3. Men will be included if they have an Eastern Cooperative Oncology Group performance status of 0–2. Fluency and an understanding of English language is also a requirement due to the qualitative interviewing component. Lastly, men will be 18 years of age with no upper age limit for entry, and medical clearance to partake will be sought from their treating clinician. Exclusion criteria can be found in Table 1.
Sample size calculation
As this is a feasibility study, a formal sample size calculation has not been performed. We aim to recruit 30 patients to the study which is standard practice for feasibility trials [31,32,33]. Assuming an 80% adherence rate, 25 patients should complete the intervention and subsequent evaluation.
Screening
The clinical care team will screen clinic lists to determine eligibility and introduce the study researchers who can provide further information. While attending treatment clinics, clinical research nurses and members of the research team will screen each patient against the inclusion criteria. Following full disclosure of the study, informed consent will be obtained from each patient. Participants meeting current physical activity guidelines (≥ 150 min of moderate-intensity or ≥ 75 min of high-intensity exercise per week) at screening (determined via International Physical Activity Questionnaire and in consultation with an Exercise Physiologist) will be deemed suitably active and excluded. The remit of this study is to progressively accrue 150 min of moderate-intensity exercise weekly, so conceivably those already doing so have a higher exercise tolerance gained through a process of adaptation and are unlikely to gain the intended health benefits. Following medical clearance from a clinical oncologist, patients will be required to complete a series of baseline questionnaires and will undertake basic physical fitness and anthropometric assessments.
Exercise prescription
This multicomponent, self-managed, home-based exercise strategy has been previously trialled in colorectal cancer patients (NCT02607787). The program will consist of 12 weeks of moderate-intensity walking (55–70% max HR; 12–14 Borg scale) and strengthening exercises (predominantly body weight; e.g. wall press, sit-to-stand; lateral raise and bicep curls, using household items for resistance). Brisk walking was selected as the mode of exercise for this feasibility trial as it poses a low risk of injury and due to its popularity, accessibility, cost-effectiveness and ease in adjusting exercise intensity/demand. Prior to each exercise session, participants will complete a warm up and at the end a cool down. Participants will receive a pedometer and an exercise booklet detailing the desired exercise prescription, allowing space to record daily exertion, particularly the mode, duration and intensity. Information to enable safe exercise, injury avoidance and overcoming barriers, as well as lifestyle strategies to incorporate exercise, will also be provided. The exercise program will be individually tailored and progressed over the 12-week duration, aiming to achieve recommended ACSM guidelines upon completion (Table 2). The individualised, self-managed nature of the program will allow for a level of autoregulation allowing modifications based on readiness and flexibility to complete certain exercises at a later date. A further advantage of autoregulation is that once patients become more active, they can work beyond their set program (e.g. increase walking distance or intensity). In the event that metastatic lesions pose a contraindication to certain exercises, the program will be modified and adapted. Participants will be required to attend the NICC to obtain outcome measures (QOL questionnaires, anthropometric and physical fitness testing) on three occasions (Table 3). The week prior to each of these visits, patients will wear an accelerometer to objectively record 7-day physical activity levels (at baseline, 12 and 24 weeks). Anthropometric measures will comprise height, weight, hip and waist circumferences while strength and cardiovascular endurance will be determined via a timed sit-to-stand test and 6-min walking test respectively. At 12 weeks, participants, in consultation with the exercise professional, will discuss a suitable exit strategy to enable exercise maintenance. All participants will be followed up at 24 weeks. Though the intervention is predominantly completed at home, weekly telephone contact permits a degree of remote supervision. An example of the exercise prescription, for an inactive patient at baseline, can be found in Table 4.
Behavioural support
The behaviour change component of this particular programme is based on the COM-B method [34]. Behavioural support will comprise a behavioural change consultation at baseline and weekly, structured telephone contact. The behavioural support component will follow a scripted protocol of defined questioning, to maintain intervention fidelity. The same member of the research team will lead the behavioural consultations throughout, to maintain reliability. Information pertaining to weekly exercise adherence, perceived barriers and potential solutions, weekly goal setting and self-confidence will be recorded. Behavioural support sessions provide the opportunity to reinforce key aspects of the trial and a chance to provide positive feedback on progress. Records of call durations and patient availability will be stored. The behavioural support programme will ensure continuity in the exercise prescribed, so all patients receive equal support to meet the desired exercise volume by study cessation. The purpose of this communication is to maintain a close relationship with the patient and identify additional support required. Accurate recording of physical activity data is essential to enable associations with treatment-related adversities and the outcome markers. Participants will specify their preferred communication type (if not telephone), to ensure regular contact is maintained.
Primary endpoint
The primary endpoint is to determine the feasibility of delivering a tailored, home-based, 12-week exercise intervention in men with metastatic castrate-resistant prostate cancer. Recruitment and attrition rates, exercise adherence (number of sessions completed), general safety/adverse events and the patient experience will be a focus of determining feasibility.
Secondary endpoints
Secondary endpoints will be assessed at baseline, 12 and 24 weeks accounting for pain, cancer-related fatigue, skeletal-related events, physical fitness and QOL. Direct contact throughout the study will also serve as a feedback loop for certain measures should they occur outside each visit. Secondary outcome measures will be assessed mainly for completeness in accordance with the feasibility theme of the study. Although the sample size is small and perhaps underpowered to definitely report causation, changes from baseline will be analysed, to provide an indication of efficacy and detect any differences. It should be noted that all secondary outcome measures are obtained principally for testing the ability to undertake and complete measures and not to detect differences from baseline. Processes relating to data handling and analysis are described in greater detail in the “Data analysis” section.
Pain progression and opiate use
Pain and analgesic management using opiates will be assessed using the Brief Pain Inventory Short Form (BPI-SF) [35] and a review of medical records at baseline, 12 and 24 weeks. The BPI was originally developed to assess pain severity and impact in cancer patients, with reported good internal consistency and validity [36, 37]. The BPI-SF is now widely recommended as a core outcome measure in clinical trials.
Skeletal-related events
Symptomatic skeletal-related events (SSEs) will be determined at treatment clinics and reviewing patient medical records, continuously throughout the 24-week intervention.
Fatigue and QOL measures
Cancer-related fatigue will be determined using the FACIT fatigue scale at baseline, 12 and 24 weeks [38]. The Functional Assessment of Cancer Therapy—Prostate (FACT-P) and EuroQOL five-dimensional questionnaire (EQ-5D-5 L) will be measured at the same time points to assess QOL. Both questionnaires are internationally recognised validated measures for all stages of prostate cancer and are sensitive to changes in indicators of treatment efficacy [39,40,41]. The change in score for the FACT-P and FACIT fatigue questionnaires can be calculated for each participant and categorised according to pre-established minimally important differences (MIDs). The EQ-5D-5 L will be used as a general index of health status and to inform the cost-effectiveness of future trials across the five dimensions.
Physical activity levels
Each patient’s level of physical activity will be assessed subjectively at baseline, 12 and 24 weeks using the International Physical Activity Questionnaire Short Form (IPAQ-SF). Craig et al. [42] reported the IPAQ-SF produced repeatable data and has acceptable measurement properties for self-report physical activity. Lee and colleagues [43] have reported the IPAQ-SF typically overestimates physical activity and thus should be supplemented with objective measures. In order to prevent this potential overestimation, this questionnaire will be completed in consultation with an Exercise Physiologist. Further, to complement the subjective assessment, objective physical activity will be captured the week prior to each outcome visit, using an accelerometer (ActiGraph GT3X). The accelerometer will be worn for 7 days, capturing data at 10-s Epoch intervals. The analysis will be completed at 60-s Epoch intervals, in accordance with recommendations [44, 45]. Moderate to vigorous physical activity and step counts will be assessed for each patient.
Physical fitness
Physical fitness will be assessed at baseline, 12 and 24 weeks using a timed sit-to-stand test (30 s) and 6-min walking test. The number of repetitions will be recorded during the sit-to-stand test, while the distance covered will determine aerobic fitness.
Qualitative evaluation
Upon study completion (week 24), the impact and experiences of key stakeholders including active participants, eligible patients that declined participation and the research staff delivering the intervention will be collected, via face-to-face semi-structured interview. Men who declined to participate will be given the opportunity to discuss their decision and gather information on suitable alternatives. Study staff interviews will be conducted by an impartial colleague to reduce bias.
The feasibility, acceptability, potential utility of the program, approaches to optimise and appropriateness of the timing and outcome measures will be a priority during interviewing. The semi-structured interviews will explore perceived facilitators and barriers to exercise and experiences of exercise in managing treatment-related symptoms, with common themes extracted during content analysis. Information relating to the number screened and eligible, retention and compliance, follow-up rates and satisfaction will be recorded on a discussed weekly but revisited during interviewing, followed by a descriptive analysis. Interviews will be tape-recorded and transcribed verbatim.
Ethical approval
Ethical approval to conduct this study was obtained from the Office for Research Ethics Committees Northern Ireland (ORECNI) in 2018 (IRAS project ID: 248301). Any subsequent protocol modifications will be reviewed by ORECNI. Following approval, a memorandum, detailing the amendment, will be issued to all members of the trial steering committee.
Data management and monitoring
All data collected will be stored securely in accordance with General Data Protection Regulations (within a key card accessed building, on a password-protected computer and locked filing cabinet). Only members of the study management team will have access to this data. A trial steering committee (comprising MB, MM, LM, HM, JMO, SJ, GP and NICRCF advisory group members) will oversee the trial and convene regularly to discuss pertinent aspects of the study. As clinical oncologists sit on this trial steering committee, we felt a trial data monitoring committee was not required. Spontaneous adverse events will be captured during regular, weekly contact with patients. Any adverse events will be expedited to the trial oncologists, upon report, for an immediate resolution and further reviewed at regular committee meetings. This trial will be subject to audit from the study sponsor, who has the power to terminate the trial if necessary. Trial results will be submitted for publication and communicated in a relevant medical or scientific journal. Anonymity will be maintained and unique identifiers will be removed in any subsequent outputs.
Data analysis
From a feasibility perspective, data analysis will account for the number of patients screened, numbers participating in the intervention and the numbers unwilling to participate, after eligibility is confirmed, with reasons for non-participation. This data will be examined and scrutinised using descriptive analysis to identify differences between participants and non-participants. Patient compliance, utilisation and satisfaction with the intervention will be assessed, as will completion rates for the intervention and the outcome measures. The acceptability of the physical outcome measures and questionnaires used in determining health resource use will also be reported. Additionally, the feasibility of the accelerometer data (e.g. volume and quality) will be analysed. All measures will be scored according to standard practice and analysed for mean changes from baseline, using a one-way repeated measures ANOVA. For each outcome measure, change will be calculated as follow-up (at both 12 and 24 weeks) minus baseline and presented alongside 95% CIs. We will also calculate effect size (Cohen’s d) for each outcome measure, at 12 and 24 weeks, using the following formula: (mean post-test − mean baseline)/(baseline standard deviation). Conventions of small (d = 0.20), medium (d = 0.50) and large (d = 0.80) will be used. Preliminary feasibility results will inform the future RCT sample size calculation and the parameters for investigation to determine potential clinical meaningfulness.
Transcriptions of audio recorded interviews will be analysed using thematic analysis [46]. At each stage, findings will be verified and discussed in order to assess the accuracy of the interpretation, promote reliability and ensure rigour [47]. NVivo qualitative data analysis software will be used to aid data management. Data generated from interviewing will assist in determining the safety of the intervention as well as the health economics moving forward.
Discussion
Accumulating evidence supports the putative synergistic role of exercise in managing the myriad of treatment-related adversities associated with prostate cancer [2]. Data from recent epidemiological studies provides further recognition of the association between exercise and a reduced risk of prostate cancer mortality [18, 48, 49]. Collectively, we now have level 1 evidence that exercise is effective in improving QOL, fatigue and exercise tolerance in men with prostate cancer, with higher quality results observed in advanced cases treated with ADT [7]. Commonly, due to the high-risk nature of advanced prostate cancer (i.e. heightened risk of fractures) as well as poor retention and compliance, supervised exercise is advocated and has proved effective. Yet, despite the reported benefits such a regime, at this time, is economically unsustainable in current NHS settings, due to the limited number of specialist exercise professionals, equipment and resources, highlighting a necessity for establishing an alternative. Culos-Reed and colleagues [50] successfully implemented a tailored, partly home-based intervention and reported an attenuation of the side effects associated with treatment. Recently, Hardcastle et al. [26] presented several key variables that influence exercise participation in cancer populations including availability, access, time, cost and confidence. A notable finding identified a keen interest in home-based, remotely supervised exercise alongside professional exercise counselling [26].
Implementing a remotely supervised walking and strengthening programme with sufficient, ongoing behavioural support is appealing and can overcome many perceived barriers, in terms of its practicality (incorporating into daily living) and cost-effectiveness (home-based with little investment in equipment or memberships). We hope this intervention caters for perceived patient preferences and will assist in uptake and adherence, but admittedly may prove resource intensive, with dedicated researcher time invested in weekly contact. Perhaps exercise provision for mCRPC patients could eventually be embedded in the standard of care follow-up consultations between patients and trained, specialist nurses/physiotherapists, if feasible, to alleviate some of this time burden, while resulting in a coordinated effort to facilitate behavioural change. This study will investigate whether men with advanced prostate cancer can take part in a home-based, progressive, moderate-intensity exercise program and its effect on treatment-related side effects. We propose that providing the necessary education and continual behavioural support will empower patients and enhance their self-efficacy, a key variable in maintenance. The potential utility of home-based exercise in assisting disease management is of clinical interest and warrants further scientific investigation, highlighting a rationale for the current study.
Trial status
This feasibility trial commenced recruitment in December 2018 and is currently ongoing. At the time of submission of this protocol, seven patients have consented and entered the trial.
Availability of data and materials
Not applicable.
References
Northern Ireland Cancer Registry. Prostate cancer official statistics. 2016. Retrieved from http://www.qub.ac.uk/research-centres/nicr/CancerInformation/official-statistics/BySite/Prostate/
Hart NH, Galvão DA, Newton RU. Exercise medicine for advanced prostate cancer. Curr Opin Support Palliat Care. 2017;11:247–57.
Sountoulides P, Rountos T. Adverse effects of androgen deprivation therapy for prostate cancer: prevention and management. ISRN Urol. 2013;2013:240108.
Galvão DA, Taaffe DR, Cormie P, Spry N, Chambers SK, Peddle-McIntyre C, et al. Efficacy and safety of a modular multi-modal exercise program in prostate cancer patients with bone metastases: a randomised controlled trial. BMC Cancer. 2011;11:517.
Lee RJ, Saylor PJ, Smith MR. Treatment and prevention of bone complications from prostate cancer. Bone. 2010;48:88–95.
Resnick MJ, Penson DF. Quality of life with advanced metastatic prostate cancer. Urol Clin. 2012;39(4):505–15.
Bourke L, Smith D, Steed L, Hooper R, Carter A, Catto J, et al. Exercise for men with prostate cancer: a systematic review and meta-analysis. Eur Urol. 2016;69(4):693–703.
Newton RU, Kenfield SA, Hart NH, Chan JM, Courneya KS, Catto J, et al. Intense exercise for survival among men with metastatic castrate-resistant prostate cancer (INTERVAL-GAP4): a multicentre, randomised, controlled phase III study protocol. BMJ Open. 2018;8:e022899.
Kenfield SA, Stampfer MJ, Giovannucci E, Chan JM. Physical activity and survival after prostate cancer diagnosis in the health professionals follow-up study. J Clin Oncol. 2011;29:726–32.
Galvão DA, Nosaka K, Taaffe DR, Spry N, Kristjanson LJ, McGuigan MR, et al. Resistance training and reduction of treatment side effects in prostate cancer patients. Med Sci Sports Exerc. 2006;38:2045–52.
Galvão DA, Taaffe DR, Spry N, Joseph D, Newton RU. Combined resistance and aerobic exercise program reverses muscle loss in men undergoing androgen suppression therapy for prostate cancer without bone metastases: a randomized controlled trial. J Clin Oncol. 2010;28:340–7.
Richman EL, Kenfield SA, Stampfer MJ, Paciorek A, Carroll PR, Chan JM. Physical activity after diagnosis and risk of prostate cancer progression: data from the cancer of the prostate strategic urologic research endeavor. Cancer Res. 2011;71:3889–95.
Newton RU, Galvão DA. Exercise in prevention and management of cancer. Curr Treat Options in Oncol. 2008;9:135–46.
Koelwyn GJ, Quail DF, Zhang X, White RM, Jones LW. Exercise-dependent regulation of the tumour microenvironment. Nat Rev Cancer. 2017;17:545–632.
Thomas RJ, Kenfield SA, Jimenez A. Exercise-induced biochemical changes and their potential influence on cancer: a scientific review. Br J Sports Med. 2017;51:640–4.
Cormie P, Newton RU, Spry N, Joseph D, Taaffe DR, Galvão DA. Safety and efficacy of resistance exercise in prostate cancer patients with bone metastases. Prostate Cancer Prostatic Dis. 2013;18:196.
Cormie P, Galvão DA, Spry N, Joseph D, Taaffe DR, Newton RU. Functional benefits are sustained after a program of supervised resistance exercise in cancer patients with bone metastases: longitudinal results of a pilot study. Support Care Cancer. 2014;22:1537–48.
Friedenreich CM, Wang Q, Neilson HK, Kopciuk KA, McGregor SE, Courneya KS. Physical activity and survival after prostate cancer. Eur Urol. 2016;70:576–85.
Hayes SC, Spence RR, Galvão DA, Newton RU. Australian association for exercise and sport science position stand: optimising cancer outcomes through exercise. J Sci Med Sport. 2009;12(4):428–34.
Schmitz KH, Courneya KS, Matthews C, Demark-Wahnefried W, Galvão DA, Pinto BM, et al. American College of Sports Medicine roundtable on exercise guidelines for cancer survivors. Med Sci Sports Exerc. 2010;42(7):1409–26.
Campbell A, Stevinson C, Crank H. The BASES expert statement on exercise and cancer survivorship. J Sports Sci. 2012;30:949–52.
Buffart LM, Galvão DA, Brug J, Chinapaw MJ, Newton RU. Evidence-based physical activity guidelines for cancer survivors: current guidelines, knowledge gaps and future research directions. Cancer Treat Rev. 2014;40(2):327–40.
Clinical Oncology Society of Australia. COSA position statement on exercise in cancer care. April 2018.
Lee DH, Lee J, Lee MK, Kim N, Kim J, Kim D, et al. Effect of home-based exercise intervention on fasting insulin and adipocytokines in colorectal cancer survivors: a randomized controlled trial. Metab. 2017;76:23–31.
Banck-Petersen A, Olsen CK, Djurhuus SS, Herrstedt A, Thorsen-Streit S, Ried-Larsen M, et al. The “interval walking in colorectal cancer” (I-WALK-CRC) study: design, methods and recruitment results of a randomized controlled feasibility trial. Contemp Clin Trials Commun. 2018;9:143–50.
Hardcastle SJ, Maxwell-Smith C, Kamarova S, Lamb S, Millar L, Cohen PA. Factors influencing non-participation in an exercise program and attitudes towards physical activity amongst cancer survivors. Support Care Cancer. 2018;26:1289–95.
Eldridge SM, Lancaster GA, Campbell MJ, Thabane L, Hopewell S, Coleman CL, et al. Defining feasibility and pilot studies in preparation for randomised controlled trials: development of a conceptual framework. PLoS ONE. 2016;11(3):e0150205.
El-Kotob R, Giangregorio LM. Pilot and feasibility studies in exercise, physical activity, or rehabilitation research. Pilot Feasibility Stud. 2018;4:137.
Blatch-Jones AJ, Pek W, Kirkpatrick E, Ashton-Key M. Role of feasibility and pilot studies in randomised controlled trials: a cross-sectional study. BMJ Open. 2018;8:e022233.
Avery KNL, Williamson PR, Gamble C, Francischetto E, Metcalfe C, Davidson P, et al. Informing efficient randomised controlled trials: exploration of challenges in developing progression criteria for internal pilot studies. BMJ Open. 2017;7:e013537.
Browne RH. On the use of a pilot sample for sample size determination. Stat Med. 1995;14:1933–40.
Lancaster GA, Dodd S, Williamson PR. Design and analysis of pilot studies: recommendations for good practice. J Eval Clin Pract. 2004;10:307–12.
Billingham SAM, Whitehead AL, Julious SA. An audit of sample sizes for pilot and feasibility trials being undertaken in the United Kingdom registered in the United Kingdom clinical research network database. BMC Med Res Methodol. 2013;13:104.
Michie S, van Stralen MM, West R. The behaviour change wheel: a new method for characterising and designing behaviour change interventions. Implement Sci. 2011;6(42):11.
Cleeland CS, Ryan KM. Pain assessment: global use of the brief pain inventory. Ann Acad Med Singap. 1994;23(2):129–38.
Mystakidou K, Mendoza T, Tsilika E, Befon S, Parpa E, Bellos G, et al. Greek brief pain inventory: validation and utility in cancer pain. Oncol. 2001;60(1):35–42.
Andrés Ares J, Cruces Prado LM, Canos Verdecho MA, Penide Villanueva L, Valle Hoyos M, Herdman M, et al. Validation of the short form of the brief pain inventory (BPI-SF) in Spanish patients with non-cancer-related pain. Pain Pract. 2015;15:643–53.
Yellen SB, Cella DF, Webster K, Blendowski C, Kaplan E. Measuring fatigue and other anemia-related symptoms with the functional assessment of cancer therapy (FACT) measurement system. J Pain Symptom Manag. 1997;13(2):63–74.
Esper P, Mo F, Chodak G, Sinner M, Cella D, Pienta KJ. Measuring quality of life in men with prostate cancer using the functional assessment of cancer therapy - prostate instrument. Urol. 1997;50:920–8.
Devlin NJ, Krabbe PFM. The development of new research methods for the valuation of EQ-5D-5L. Eur J Health Econ. 2013;14:1–3.
Sellers L, Savas AN, Davda R, Ricketts K, Payne H. Patient-reported outcome measures in metastatic prostate cancer. Trends Urol Mens Health. 2016;7:28–32.
Craig CL, Marshall AL, Sjöström M, Bauman AE, Booth ML, Ainsworth BE, et al. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc. 2003;35:1381–95.
Lee PH, Macfarlane DJ, Lam TH, Stewart SM. Validity of the international physical activity questionnaire short form (IPAQ-SF): a systematic review. Int J Behav Nutr Phys Act. 2011;8:115.
Migueles JH, Cadenas-Sanchez C, Ekelund U, Nyström CD, Mora-Gonzalez J, Löf M, et al. Accelerometer data collection and processing criteria to assess physical activity and other outcomes: a systematic review and practical considerations. Sports Med. 2017;47:1821–45.
Peddle-McIntyre CJ, Cavalheri V, Boyle T, McVeigh JA, Jeffery E, Lynch BM, et al. A review of accelerometer-based activity monitoring in cancer survivorship research. Med Sci Sports Exerc. 2018;50(9):1790–801.
Braun V, Clarke V. Using thematic analysis in psychology. Qual Res Psychol. 2006;3(2):77–101.
Mays N, Pope C. Qualitative research: rigour and qualitative research. BMJ. 1995;311:109.
Gunnell AS, Joyce S, Tomlin S, Taaffe DR, Cormie P, Newton RU, et al. Physical activity and survival among long-term cancer survivor and non-cancer cohorts. Front Public Health. 2017;5:1-8.
Peisch SF, Van Blarigan EL, Chan JM, Stampfer MJ, Kenfield SA. Prostate cancer progression and mortality: a review of diet and lifestyle factors. World J Urol. 2017;35:867–74.
Culos-Reed SN, Robinson JW, Lau H, Stephenson L, Keats M, Norris S, et al. Physical activity for men receiving androgen deprivation therapy for prostate cancer: benefits from a 16-week intervention. Support Care Cancer. 2010;18:591–9.
Acknowledgements
We thank all primary care health professionals that have screened and recruited patients to the trial. We also thank the Northern Ireland Cancer Research Consumer Forum (Prostate Cancer Research Advisory Group) for input into the trial design.
Ethical approval and consent to participate
This study has been approved by the Office for Research Ethics Committees Northern Ireland (ORECNI reference number: 18/NI/0108). Participation is voluntary and all patients are required to provide signed informed consent (obtained by MB or GP), prior to undertaking any study procedures.
Funding
This research has received funding from the HSC Public Health Agency, Research and Development Division, as part of an opportunity-led scheme (HSC R&D Reference: COM/5310/16).
Author information
Authors and Affiliations
Contributions
GP, MM, JMO and SJ contributed to the conception of the study. All authors collaborated to define the study protocol and criteria. MM and LM developed the exercise intervention. All authors contributed to the design of the final study. MB, SJ and GP will co-ordinate this trial. MB, JMO, SJ and GP will contribute to the acquisition of data. HM will conduct the statistical analysis and all authors will contribute to the interpretation of data and writing of the final manuscript. MB and GP prepared this protocol paper. All authors provided feedback on drafts of this paper and have read and approved the final manuscript.
Corresponding author
Ethics declarations
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Brown, M., Murphy, M., McDermott, L. et al. Exercise for advanced prostate cancer: a multicomponent, feasibility, trial protocol for men with metastatic castrate-resistant prostate cancer (EXACT). Pilot Feasibility Stud 5, 102 (2019). https://doi.org/10.1186/s40814-019-0486-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40814-019-0486-6