Abstract
Background
The aim of this work was to study the prevalence and distribution of Porcine astrovirus (PAstV), Porcine kobuvirus (PKoV), Porcine torovirus (PToV), Mammalian orthoreovirus (MRV) and Porcine mastadenovirus (PAdV) as well as their association with widely recognized virus that cause diarrhoea in swine such as coronavirus (CoVs) and rotavirus (RVs) in diarrhoea outbreaks from Spanish swine farms. Furthermore, a selection of the viral strains was genetically characterized.
Results
PAstV, PKoV, PToV, MRV and PAdV were frequently detected. Particularly, PAstV and PKoV were detected in almost 50% and 30% of the investigated farms, respectively, with an age-dependent distribution; PAstV was mainly detected in postweaning and fattening pigs, while PKoV was more frequent in sucking piglets. Viral co-infections were detected in almost half of the outbreaks, combining CoVs, RVs and the viruses studied, with a maximum of 5 different viral species reported in three investigated farms. Using a next generation sequencing approach, we obtained a total of 24 ARN viral genomes (> 90% genome sequence), characterizing for first time the full genome of circulating strains of PAstV2, PAstV4, PAstV5 and PToV on Spanish farms. Phylogenetic analyses showed that PAstV, PKoV and PToV from Spanish swine farms clustered together with isolates of the same viral species from neighboring pig producing countries.
Conclusions
Although further studies to evaluate the role of these enteric viruses in diarrhoea outbreaks are required, their wide distribution and frequent association in co-infections cannot be disregard. Hence, their inclusion into routine diagnostic panels for diarrhoea in swine should be considered.
Similar content being viewed by others
Background
Enteric diseases caused by viruses are highly prevalent and negatively impact productivity in pig production [1]. Coronaviruses (CoVs) and rotaviruses (RVs) are the most common and well-recognized viruses responsible for diarrhoea in pigs [2,3,4]. Among CoVs, Transmissible gastroenteritis virus (TGEV) and Porcine epidemic diarrhoea virus (PEDV), two Alphacoronavirus, have swept farms all over the world since their emergence in the last century [5, 6]. More recently, a recombinant virus between TGEV and PEDV, known as Swine enteric coronavirus (SeCoV), has been identified on European pig farms [7,8,9,10]. These three CoVs target the small intestine in pigs of all ages, shortening villi and inducing an undistinguishable diarrhoeic disease [3, 11]. Other two porcine CoVs, Porcine delta coronavirus (PDCoV) [12] and Swine acute diarrhoea syndrome coronavirus (SADS-CoV) [13, 14], an Alphacoronavirus, have been also recently described in diarrhoea outbreaks, although they have never been detected on European swine farms. RVs are also a major cause of acute gastroenteritis in swine worldwide with Rotavirus A (RVA) and Rotavirus C (RVC) as the most relevant species [3]. They are mainly involved in diarrhoea outbreaks in both nursing and weaned piglets although they can be detected in all stages in porcine production [3].
In addition, several other swine enteric viruses such as Porcine astrovirus (PAstV) [15,16,17,18,19,20], Porcine kobuvirus (PKoV) [16, 21,22,23,24], Porcine torovirus (PToV) [25,26,27,28,29], Mammalian orthoreovirus (MRV) [30,31,32,33,34] or Porcine mastadenovirus (PAdV) [1, 35,36,37] have been detected in pigs with diarrhoea worldwide (Table 1), although their etiological role remains unclear. These viruses are frequently identified in co-infections with CoVs and RVs [4, 38,39,40,41,42,43,44,45], and are also commonly detected in healthy animals [39, 46,47,48,49,50]. The enteropathogenicity of PAstV, MRV and PAdV has been demonstrated on experimentally infected conventional piglets [51,52,53,54,55], but to the best of the authors’ knowledge, no experimental challenge has been reported for PKoV and PToV.
The aim of this work was to study the prevalence and distribution of PAstV, PKoV, PToV, MRV and PAdV in diarrhoea outbreaks on swine farms in Spain, as well as to identify their association with widely recognized virus that cause diarrhoea, such as CoV and RV. In addition, a selection of the viral strains was genetically characterized. Altogether, our results provide information on the aetiology of enteric disease in swine that can be used to devise and implement appropriate diagnostic and control strategies.
Results
Prevalence and distribution of individual swine enteric viruses in diarrhoea outbreaks in Spain
Globally, 160 out of 206 (77.7%) farms were positive to at least one of the enteric viruses tested. PAstV was the most frequently detected virus (48.5%; n = 100) followed by PKoV (27.2%; n = 56), PEDV (19.9%; n = 41), PAdV (14.1%; n = 29), RVA (13.6%; n = 28), PToV (11.2%; n = 23), RVC (6.3%; n = 13) and MRV (4.4%; n = 9). TGEV or SeCoV were not detected in any of the investigated farms.
The prevalence of positive diarrhoea outbreaks (a positive result for at least one of the tested viruses) was similar (p = 0.787) among nursing (75.8%, 50 out of 66), postweaning (76.6%, 49 out of 64) and fattening periods (80.3%, 61 out of 76). However, different age distribution patterns were observed for each of the investigated viruses (Fig. 1). Detection of PAstV and RVA was significantly lower in nursing diarrhoea outbreaks as compared with postweaning (p < 0.001 and p = 0.025, respectively) and fattening outbreaks (p < 0.001 and p = 0.036, respectively). In contrast, PKoV were more prevalent in nursing and postweaning than in fattening (p < 0.001), while RVC were only detected in diarrhoea outbreaks affecting lactating piglets. Finally, PToV and PAdV were more frequently detected in fattening outbreaks as compared to nursing (p = 0.016 and p = 0.005, respectively). A similar trend was observed for PEDV and MRV although without statistical significance.
Prevalence of positive diarrhoea outbreaks to each of the different investigated enteric viruses according to the age of the affected animals. Out of the 206 investigated diarrhoea outbreaks, 66 affected nursing piglets (< 21 days), 64 postweaning-growing piglets (21–70 days) and 76 fattening pigs (> 70 days)
In addition, it was evidenced that most of the investigated outbreaks (50.0%; 103 out of 206) occurred during the first trimester as compared with the rest of the year (p < 0.001). PEDV was more commonly detected during the winter (January to March) (p = 0.011) while no associated seasonality could be confirmed for any of the other investigated enteric viruses (Additional file 1).
Based on clinical criteria, the diarrhoea outbreaks included in this research were classified by the veterinarian responsible for the farm into outbreaks with or without a suspected viral aetiology. The risk of a positive result to at least one the tested viruses was higher in viral suspected outbreaks (OR = 2.52, CI 95% 1.27–5.04) as compared with those without viral suspicion (85.4% vs. 69.9%). Accordingly, the prevalence of each of the investigated viruses was higher in outbreaks with suspected viral aetiology, with the exception of PKoV, RVA and RVC (Fig. 2), although statistical differences were only demonstrated for PEDV (p < 0.001) and PAdV (p = 0.002).
Prevalence and distribution of viral co-infections in diarrhoea outbreaks in Spain
In 69 out of the 206 analysed outbreaks (33.5%) a single viral aetiological agent was detected, while 56 (27.2%) presented a co-infection of two viruses at the same time and 35 (17.0%) were positive for more than two (up to 5 different virus species simultaneously detected on three different farms). Detection of a single viral agent was more frequent in diarrhoea outbreaks in lactation as compared with postweaning (26.6%, 17 out of 64, p = 0.019) and fattening (28.9%, 22 out of 76, p = 0.031). On the contrary, outbreaks with two or more co-infecting viruses were more prevalent in postweaning (50.0%, 32 out of 64, p = 0.017) and fattening (51.3%, 39 out of 76, p = 0.009) as compared with nursing (30.3%, 20 out of 66). Besides, outbreaks presenting two or more co-infecting viruses were more frequent when viral aetiology was suspected according to the criteria of the veterinarian responsible for the farm (55.3% vs. 33.0%, p = 0.001).
As shown in Additional file 2, PAstV and PKoV were detected in almost 30% of the positive outbreaks as a single infection while PToV, MRV and PAdV were detected in over 90% of positive outbreaks co-infecting with PEDV, RVs or other enteric viruses. The most frequent viral combinations (top 8) are shown in Table 2. Once again, a clear age-dependent distribution was observed with co-infections including PAstV more prevalent in postweaning and fattening outbreaks and co-infections with PKoV recorded among the top 3 in nursing outbreaks.
The viruses most frequently associated with PEDV, RVA and RVC are shown in Fig. 3. PAstV was identified in more than 50% of the PEDV and RVA outbreaks while RVC mainly co-infects with PKoV, probably as a consequence of the age dependent distribution of these infections. Comparison of the prevalence of each of the investigated viruses between PEDV, RVA and RVC positive and negative outbreaks reveal that PToV were more commonly detected in PEDV and RVA positive outbreaks (p = 0.021 and p = 0.005, respectively) while PAstV detection was more common in RVA positive farms (p = 0.027).
Detection of each of the different investigated enteric viruses (Porcine astrovirus or PAstV, Porcine kobuvirus or PKoV, Porcine torovirus or PToV, Mammalian orthoreovirus or MRV and Porcine adenovirus or PAdV) in positive outbreaks involving well-recognized viruses that cause diarrhoea (Porcine epidemic diarrhoea virus or PEDV—n = 41-, Rotavirus A or RVA—n = 28- and Rotavirus C or RVC—n = 13-)
Sequencing and phylogenetic analysis of PAstV, PKoV and PToV
By using a next generation sequencing (NGS) in 14 pooled faecal samples, we identified 10 RNA virus species from six different genera: PAstV2 (6), PAstV3 (7), PAstV4 (10), PAstV5 (7), PkoV (8), PToV (5), PEDV (10), RV (2·RVA and 2 RVC) and MRV (1) (Additional file 3). From this array of viruses, the whole or almost whole genome was sequenced in 24, including PAstV2 (5), PAstV3 (1), PAstV4 (8), PAstV5 (2), PKoV (3), PToV (1) and PEDV (4).
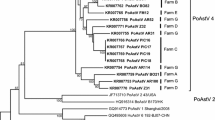
Four species of PAstV (PAstV2 to PAstV5) were detected in the investigated outbreaks on Spanish farms, being PAstV4 and PAstV2 the most represented. The nucleotide sequence identities between PAstV2 and PAstV4 strains recovered in our research were 77.2–95.2% and 87.2–93.7% respectively, while its comparison with previously described sequences provided nucleotide identities of 68.7–91.0% for PAstV2, 87.6–89.1% for PAstV3, 73.4–91.8% for PAstV4 and 83.3–94.4% for PAstV5. Most of the Spanish PAstV sequences clustered together with North American strains of the same lineage, although PAstV4 clustered with sequences from Japan and some PAstV2 with sequences from Europe and China (Fig. 4).
Neighbor-joining phylogenetic tree based on the p-distance among the nucleotide sequences of the complete genomes for PAstV. Along the branches, percentage of bootstrap values based on 10,000 replicates (only values equal or larger than 70% are shown). The filled circles identified the isolates sequenced in this research. GenBank accession number, country and year of the outbreak are also shown below the strains. The PAstV lineages referred in the text are included on the right of the tree. Scale bars indicate nucleotide substitutions per site
Regarding the phylogenetic analysis of PKoV (Fig. 5), the three sequences obtained in our study clustered together with previously reported Spanish and Hungarian isolates. The nucleotide sequence identity was 90.3–91.4% among the Spanish isolates identified in this study and 87.2–91.9% with the previously reported whole genome PKoV sequences.
Neighbor-joining phylogenetic tree based on the p-distance among the nucleotide sequences of the complete genomes for PKoV. Along the branches, percentage of bootstrap values based on 10,000 replicates (only values equal or larger than 70% are shown). The filled circles identified the isolates sequenced in this research. GenBank accession number, country and year of the outbreak are also shown below the strains. Scale bars indicate nucleotide substitutions per site
Finally, a single PToV complete genome was obtained. When compared with previously described PToV its nucleotide identity was 88.6–94.1%, but was lower when compared with torovirus sequences from other animal species (77.3–87.9%). This Spanish PToV sequence clustered together with viral sequences from Europe, particularly Germany, but also with sequences from the USA and Japan (Fig. 6).
Neighbor-joining phylogenetic tree based on the p-distance among the nucleotide sequences of the complete genomes for PToV. Along the branches, percentage of bootstrap values based on 10,000 replicates (only values equal or larger than 70% are shown). The filled circles identified the isolates sequenced in this research. GenBank accession number, country and year of the outbreak are also shown below the strains. Scale bars indicate nucleotide substitutions per site
Discussion
The enteropathogenic role of PAstV, PKoV, PToV, MRV and PAdV is still controversial and has not been evidenced in a number of case–control studies comparing detection ratios in diarrhoeic and healthy pigs [39, 46,47,48,49,50]. Taking this fact into account, we investigated the prevalence of these enteric viral species focusing on diarrhoea outbreaks and looking into their association with well-recognized viral enteropathogens as CoVs (PEDV, TGEV and SeCoV) and RVs (RVA and RVC). The design of our study does not allow to draw conclusions about the role of these viruses in the aetiology of enteric disease, but it aims to increase knowledge about their frequency, distribution and co-infections on farms with enteric problems.
Our results confirmed that PAstV and PKoV infections are highly prevalent on European farms, as previously proposed by a number of studies [19, 46, 49, 50, 56]. PAstV was the most frequent enteric virus in our research, detected in almost half of the outbreaks, followed by PKoV, detected in more than 25% of the investigated farms. In agreement with a previous report in Hungary [49] PAdV was less common (14.1% of positive outbreaks) while PToV was only identified in 11.2% of the investigated farms in spite of the broad distribution of this virus, evidenced by another Spanish study which estimated over 99% of seropositive animals among pigs older than 11 weeks of age [57]. Finally, MRV was the least frequently detected enteric virus (4.4%) among those with an unclear aetiological role in diarrhoea outbreaks. To the authors knowledge there are no previous reports on the prevalence of MRV on European swine farms. Regarding well-recognized enteropathogenic viruses, neither TGEV nor SeCoV were detected. PEDV was identified in 19.9% of the investigated diarrhoea outbreaks and the prevalence of RVA and RVC positive outbreaks was 13.6 and 6.3%, respectively.
Three age distribution patterns were observed among the investigated enteric viruses. Similarly to previous studies in China [16, 47], Canada [44] and several European countries [38, 49, 50], PKoV were more prevalent in nursing and postweaning piglets as compared with fattening stages. A similar distribution has been reported for RVC [3] and was confirmed in our research, with no positive RVC outbreak occurring after weaning. In contrast, PAstV and RVA showed a significant increase in their prevalence in weaned pigs as compared to nursing pigs. Previous studies on European swine farms have reported a similar trend for PAstV [46, 49, 50]. As described for endemic RVA on swine farms [1], the decline of specific maternal antibodies could explain the increase in prevalence of PAstV in the postweaning period. Finally and in line with previous studies [28, 49], PToV and PAdV were identified more frequently in diarrhoea outbreaks during the fattening period.
A seasonal distribution was only demonstrated for PEDV positive outbreaks which were more common during the coldest months of the year (January–March). Low temperatures allowing for CoV survival in the environment and facilitating indirect transmission may explain seasonal variation in PEDV outbreaks [58, 59]. Moreover, it has been proposed that the exposure to low ambient temperatures may enhance PEDV replication through up-regulated heat shock protein Hsp70 in the pig intestine, contributing to PEDV seasonality [60]. In contrast, no seasonality has been described for porcine RV [3].
Interestingly, veterinary clinical criteria suggesting a presumptive viral aetiology was associated with the viral detection using molecular methods. This was particularly evident for PEDV positive outbreaks but also for PAdV and to a lesser extent for PAstV, PToV or MRV. In contrast, RVA, RVC and PKoV were detected mainly in non-viral suspected outbreaks. The fact that RV infections are endemic in swine populations and frequently associated with other diarrhea-producing agents such as enterotoxigenic Escherichia coli or Clostridium perfringens type A [3, 61, 62] could complicate its recognition by practitioners.
Co-infections were relatively frequent with two or more viruses detected in almost 40% of the investigated outbreaks. This result is in line with previous studies on European farms [16, 49, 63] reporting a high frequency of co-infections involving several enteric viruses in pigs with diarrhoea. A higher proportion of viral enteric co-infections, close to 70%, has been described on swine farms from Asia [4, 40]. In agreement with previous studies conducted in Europe [49], China [4, 47] or Canada [44], the number of different virus species detected within an outbreak increased with the age of the affected pigs. Hence, single infections were more common in nursing piglets while simultaneous co-infections with up to five viruses were observed in postweaning and fattening pigs. Many factors associated with weaning such as dietary changes, placement into a new and more contaminated environment, mixing of pigs or loss of passive immunity can favor the infection by different viral species. Also, it has been proposed that the maturation of the gut associated immune system after weaning may prevent the development of enteric disease associated to single infections for those enteric viruses with a moderate enteropathogenicity [64].
The most common co-infections varied depending on the age or life stage of the affected pigs. As expected from the observed patterns of age distribution, co-infections involving PKoV were the most prevalent in lactating piglets (27.2%) while co-infections with PAstV predominated in postweaning and fattening outbreaks (87.4% and 60.6%, respectively). Although it has been recently proposed that PEDV and PKoV might play some synergetic role [65], no significant association between both viruses was observed in our research. The fact that most of the PEDV outbreaks occurred in postweaning or fattening pigs when PKoV is less prevalent may explain this result. In contrast, PToV shedding was more frequent in PEDV and RVA positive outbreaks. Although there is very little information known about PToV infections, it is worth mentioning that the first report of PToV in the United States was in a PEDV positive pig [66]. In addition, an increase in the prevalence of PAstV shedding was observed in RVA positive outbreaks. Further research is required to decipher the interactions between enteric viruses which may play a primary or secondary role in the aetiology of enteric disease in pigs.
Given the high prevalence of viral co-infections detected, the use of conventional PCR for viral detection is a major limitation of our research. Quantitative approaches based on qPCR or next generation sequencing (NGS) would have allowed for viral load estimations. As proposed by Cortey et al. [63] the determination of the relative abundance of different viral species in co-infections makes it possible to identify the predominant virus which can most likely be proposed as the primary etiological agent.
Finally, the study involved the fine tune genomic characterization of a selection of samples to obtain the complete sequence of part of these viruses. Phylogenetic analysis revealed that the majority of the PAstV strains were classified as PAstV4 (50.0%, 8/16) and PAstV2 (31.2%, 5/16), indicating that these two PAstV species are the most prevalent in swine with diarrhoea in Spain, as it has been described in several European countries [46, 50, 67], in North America [68] and in Asia [39, 69, 70]. PKoV phylogenetic analyses revealed that all available Spanish complete sequences (including the three identified in our study) were included in a single local cluster together with one Hungarian strain and well differentiated from other European isolates [38]. Finally, the single PToV whole genome sequence obtained in this research clustered together with other PToV isolates from Europe, America or Asia [25, 26].
Conclusions
Although the design of our study does not allow for the evaluation of the role of PAstV, PKoV, PToV, MRV or PAdV as causative agents of diarrhoea in pigs, we have demonstrated a high prevalence of these enteric viruses in diarrhoea outbreaks, particularly for PAstV and PKoV detected in almost 50% and 30% of the investigated farms. The fact that these enteric viruses are frequently involved in co-infections among them and also with well-recognized viral enteropathogens as CoVs or RVs make necessary further studies for surveillance and characterization as well as investigations to evaluate their pathogenic potential in both experimental and natural infections. Once these studies are available, the inclusion of these viruses into the routine diagnostic panels for diarrhoea in swine should be considered.
Methods
Sample collection and nucleic acid extraction
The study was conducted between January 2017 and October 2020 on 206 Spanish swine farms with diarrhoea outbreaks affecting nursing piglets (< 21 days) (66 farms), postweaning-growing (21–70 days) (64 farms) or fattening pigs (> 70 days) (76 farms). From each farm, two to six individual fecal samples were submitted for diagnostic purposes to the Infectious Diseases Unit of the Animal Health Department of the University of León (Spain). The veterinarian responsible for the farm classified the outbreak as viral or non-viral suspicion, according to criteria such as speed of progression of the disease or response to antimicrobials.
Individual fecal samples were mixed to prepare one pooled sample per farm that was diluted 1:2 (v/v) in sterile phosphate buffered saline (PBS), homogenized by vortex mixing and centrifuged for 10 min at 20,000 g. The nucleic acid was extracted from 140 µl of the supernatant using QIAMP Viral RNA and DNA Mini Kit (QIAGEN), following the manufacturer’s instructions, and stored at − 80 °C until use.
Molecular detection of porcine enteric viruses
Three duplex and one triplex RT-PCRs were carried out using Verso 1-Step RT-PCR ReddyMix kit (Thermo Scientific) for the detection of PEDV, TGEV, SeCoV (simultaneous detection of S gene of PEDV and N gene of TGEV), RVA, RVC, PToV, PAstV, PKoV, MRV and PAdV (Table 3). The reactions were conducted under the following conditions: 50 °C for 30 min, 95 °C for 2 min, 45 cycles at 95 °C for 20 s, 50 °C for 30 s, and 72 °C for 1 min and 30 s, followed by a final extension step at 72 °C for 10 min.
The RT-PCR products were visualized on a 1.5% agarose gel containing RedSafe Nucleic Acid Staining Solution (iNtRON Biotechnology, Inc.) and compared with expected lengths (Table 3).
Sequencing and phylogenetic analysis
A total of 14 pooled faecal samples were chosen for sequencing based on their RT-PCR results (samples with the highest number of different viruses detected). From each pooled sample, total RNA was extracted using a TRIzol LS reagent (Thermo Scientific) protocol. The total RNA extraction was directly sequenced at the Genomics Bioinformatics Service (SGB) of the Autonomous University of Barcelona (UAB), without using any primer or amplification step. NGS was carried out using an Illumina Miseq Platform. RNA virus sequences were obtained from NGS outputs applying a tailor-made, virus-specific script developed by Cortey et al. [63]. Subsequently, a database for every RNA virus species identified was constructed by downloading the available complete genome sequences from GenBank. Whole genome (> 90%) sequences were deposited in NCBI GenBank with the accession numbers ON649880, ON714996–ON715001 and ON792965–ON792977. Four whole genome sequences of PEDV also recovered in this work were already deposited with accession numbers MN692786, MN692787, MN692789 and MN692791 [8]. Finally, the sequences obtained were aligned and phylogenetically compared with the datasets constructed for every complete or near-complete genome identified. The phylogenetic relationships among sequences were analyzed using the software MEGA11 [71], by means of a Neighbor-joining (NJ) algorithm, using the model of maximum composite likelihood and 10,000 bootstrap replicates to estimate the confidence of the internal branches of the trees.
Statistical analysis
Statistical analyses of data were performed by Fisher’s exact and ANOVA tests using Epi Info™. A two-sided p value of ≤ 0.05 was considered to indicate statistical significance.
Availability of data and materials
Data are available in the GenBank database and by direct contact with the correspondence author.
Abbreviations
- CI:
-
Confidence interval
- CoV:
-
Coronavirus
- MRV:
-
Mammalian orthoreovirus
- NGS:
-
Next generation sequencing
- PAdV:
-
P orcine mastadenovirus
- PAstV:
-
Porcine astrovirus
- PBS:
-
Phosphate-buffered saline
- PEDV:
-
Porcine epidemic diarrhoea virus
- PDCoV:
-
Porcine deltacoronavirus
- PKoV:
-
Porcine kobuvirus
- PToV:
-
Porcine torovirus
- RNA:
-
Ribonucleic acid
- RT-PCR:
-
Reverse transcription-polymerase chain reaction
- RV:
-
Rotavirus
- RVA:
-
Rotavirus A
- RVC:
-
Rotavirus C
- SADS:
-
Swine acute diarrhoea syndrome coronaviruS
- SeCoV:
-
Swine enteric coronavirus
- TGEV:
-
Transmissible gastroenteritis virus
References
Thomson JR, Friendship RM. Digestive system. In: Zimmerman JJ, Karriker LA, Ramirez A, Schwartz K, Stevenson GW, Zhang J, editors. Dis swine. 11th ed. Hoboken: Wiley; 2019. p. 234–63.
Pensaert MB, Martelli P. Porcine epidemic diarrhea: a retrospect from Europe and matters of debate. Virus Res. 2016;226:1–6.
Vlasova AN, Amimo JO, Saif LJ. Porcine rotaviruses: epidemiology, immune responses and control strategies. Viruses. 2017;9:1–27.
Zhang Q, Hu R, Tang X, Wu C, He Q, Zhao Z, et al. Occurrence and investigation of enteric viral infections in pigs with diarrhea in China. Arch Virol. 2013;158:1631–6.
Wang Q, Vlasova AN, Kenney SP, Saif LJ. Emerging and re-emerging coronaviruses in pigs. Curr Opin Virol. 2019;34:39–49.
Jung K, Saif LJ, Wang Q. Porcine epidemic diarrhea virus (PEDV): an update on etiology, transmission, pathogenesis, and prevention and control. Virus Res. 2020;286:198045.
Boniotti MB, Papetti A, Lavazza A, Alborali G, Sozzi E, Chiapponi C, et al. Porcine epidemic diarrhea virus and discovery of a recombinant swine enteric coronavirus, Italy. Emerg Infect Dis. 2016;22:83–7.
de Nova PJG, Cortey M, Díaz I, Puente H, Rubio P, Martín M, et al. A retrospective study of porcine epidemic diarrhoea virus (PEDV) reveals the presence of swine enteric coronavirus (SeCoV) since 1993 and the recent introduction of a recombinant PEDV-SeCoV in Spain. Transbound Emerg Dis. 2020;67:2911–22.
Belsham GJ, Rasmussen TB, Normann P, Vaclavek P, Strandbygaard B, Bøtner A. Characterization of a novel chimeric swine enteric coronavirus from diseased pigs in Central Eastern Europe in 2016. Transbound Emerg Dis. 2016;63:595–601.
Akimkin V, Beer M, Blome S, Hanke D, Höper D, Jenckel M, et al. New chimeric porcine coronavirus in Swine Feces, Germany, 2012. Emerg Infect Dis. 2016;22:1314–5.
Puente H, Díaz I, Arguello H, Mencía-Ares Ó, Gómez-García M, Pérez-Pérez L, et al. Characterization and cross-protection of experimental infections with SeCoV and two PEDV variants. Transbound Emerg Dis. 2022;69:1–13.
Woo PCY, Lau SKP, Lam CSF, Lau CCY, Tsang AKL, Lau JHN, et al. Discovery of seven novel Mammalian and avian coronaviruses in the genus deltacoronavirus supports bat coronaviruses as the gene source of alphacoronavirus and betacoronavirus and avian coronaviruses as the gene source of gammacoronavirus and deltacoronavi. J Virol. 2012;86:3995–4008.
Gong L, Li J, Zhou Q, Xu Z, Chen L, Zhang Y, et al. A new bat-HKU2-like coronavirus in swine, China, 2017. Emerg Infect Dis. 2017;23:1607–9.
Pan Y, Tian X, Qin P, Wang B, Zhao P, Yang Y-L, et al. Discovery of a novel swine enteric alphacoronavirus (SeACoV) in southern China. Vet Microbiol. 2017;211:15–21.
Bhatta TR, Chamings A, Alexandersen S. Exploring the cause of diarrhoea and poor growth in 8–11-week-old pigs from an Australian pig herd using metagenomic sequencing. Viruses. 2021;13:1608.
Capai L, Piorkowski G, Maestrini O, Casabianca F, Masse S, de Lamballerie X, et al. Detection of porcine enteric viruses (Kobuvirus, Mamastrovirus and Sapelovirus) in domestic pigs in Corsica, France. PLoS ONE. 2022;17:1–15.
Flores C, Ariyama N, Bennett B, Mena J, Verdugo C, Mor S, et al. Case report: first report and phylogenetic analysis of porcine astroviruses in Chile. Front Vet Sci. 2021;8:764837.
Rawal G, Linhares DCL. Scoping review on the epidemiology, diagnostics and clinical significance of porcine astroviruses. Transbound Emerg Dis. 2022;69:974–85.
Stamelou E, Giantsis IA, Papageorgiou KV, Petridou E, Davidson I, Polizopοulou ZS, et al. Epidemiology of Astrovirus, Norovirus and Sapovirus in Greek pig farms indicates high prevalence of Mamastrovirus suggesting the potential need for systematic surveillance. Porcine Health. 2022;8:1–15.
Tassoni L, Zamperin G, Schiavon E, Vendramin V, Cavicchio L, Mion M, et al. First whole genome characterization of porcine astrovirus detected in swine faeces in Italy. Vet Ital. 2019;55:221–9.
Akagami M, Ito M, Niira K, Kuroda M, Masuda T, Haga K, et al. Complete genome analysis of porcine kobuviruses from the feces of pigs in Japan. Virus Genes. 2017;53:593–602.
Nantel-Fortier N, Lachapelle V, Letellier A, L’Homme Y, Brassard J. Kobuvirus shedding dynamics in a swine production system and their association with diarrhea. Vet Microbiol. 2019;235:319–26.
Milićević V, Kureljušić B, Maksimović-Zorić J, Savić B, Spalević L, Žutić J. Molecular detection and characterization of Porcine Kobuvirus in domestic pigs and wild boars in Serbia. Res Vet Sci. 2020;132:404–6.
Zhai SL, Zhang H, Lin T, Chen SN, Zhou X, Chen QL, et al. A novel porcine kobuvirus emerged in piglets with severe diarrhoea in China. Transbound Emerg Dis. 2017;64:1030–6.
Hu ZM, Le YY, Xu LD, Wang B, Qin P, Huang YW. Porcine Torovirus (PToV)-a brief review of etiology, diagnostic assays and current epidemiology. Front Vet Sci. 2019;6:1–6.
Fujii Y, Kashima Y, Sunaga F, Aoki H, Imai R, Sano K, et al. Complete genome sequencing and genetic analysis of a Japanese porcine torovirus strain detected in swine feces. Arch Virol. 2020;165:471–7.
Shin DJ, Park SI, Jeong YJ, Hosmillo M, Kim HH, Kim HJ, et al. Detection and molecular characterization of porcine toroviruses in Korea. Arch Virol. 2010;155:417–22.
Pignatelli J, Jiménez M, Grau-Roma L, Rodríguez D. Detection of porcine torovirus by real time RT-PCR in piglets from a Spanish farm. J Virol Methods. 2010;163:398–404.
Zhou L, Wei H, Zhou Y, Xu Z, Zhu L, Horne J. Molecular epidemiology of Porcine torovirus (PToV) in Sichuan Province, China: 2011–2013. Virol J. 2014;11:1–9.
Qin P, Li H, Wang J-W, Wang B, Xie R-H, Xu H, et al. Genetic and pathogenic characterization of a novel reassortant mammalian orthoreovirus 3 (MRV3) from a diarrheic piglet and seroepidemiological survey of MRV3 in diarrheic pigs from east China. Vet Microbiol. 2017;208:126–36.
Singh F, Rajukumar K, Senthilkumar D, Venkatesh G, Srivastava D, Kombiah S, et al. First report on co-isolation and whole-genomic characterisation of mammalian orthorubulavirus 5 and mammalian orthoreovirus type 3 from domestic pigs in India. Arch Virol. 2022;167:1529–45.
Day JM. The diversity of the orthoreoviruses: molecular taxonomy and phylogentic divides. Infect Genet Evol. 2009;9:390–400.
Luo Y, Fei L, Yue H, Li S, Ma H, Tang C. Prevalence and genomic characteristics of a novel reassortment mammalian orthoreovirus type 2 in diarrhea piglets in Sichuan, China. Infect Genet Evol. 2020;85:104420.
Lelli D, Beato MS, Cavicchio L, Lavazza A, Chiapponi C, Leopardi S, et al. First identification of mammalian orthoreovirus type 3 in diarrheic pigs in Europe. Virol J. 2016;13:1–5.
Kumthip K, Khamrin P, Kongkaew A, Vachirachewin R, Malasao R, Ushijima H, et al. Molecular epidemiology and characterization of porcine adenoviruses in pigs with diarrhea in Thailand. Infect Genet Evol. 2019;67:73–7.
De Motes CM, Clemente-Casares P, Hundesa A, Martín M, Girones R. Detection of bovine and porcine adenoviruses for tracing the source of fecal contamination. Appl Environ Microbiol. 2004;70:1448–54.
Buitrago D, Cano-Gómez C, Agüero M, Fernandez-Pacheco P, Gómez-Tejedor C, Jiménez-Clavero MÁ. A survey of porcine picornaviruses and adenoviruses in fecal samples in Spain. J Vet Diagn Investig. 2010;22:763–6.
Jackova A, Sliz I, Mandelik R, Salamunova S, Novotny J, Kolesarova M, et al. Porcine kobuvirus 1 in healthy and diarrheic pigs: genetic detection and characterization of virus and co-infection with rotavirus A. Infect Genet Evol. 2017;49:73–7.
Kumthip K, Khamrin P, Saikruang W, Kongkaew A, Vachirachewin R, Ushijima H, et al. Detection and genetic characterization of porcine astroviruses in piglets with and without diarrhea in Thailand. Arch Virol. 2018;163:1823–9.
Zhou Y, Chen L, Zhu L, Xu Z. Molecular detection of porcine torovirus in piglets with diarrhea in southwest China. Sci World J. 2013;2013:1–5.
Kwon HJ, Kim HH, Kim HJ, Park JG, Son KY, Jung J, et al. Detection and molecular chracterization of porcine type 3 orthoreoviruses circulating in South Korea. Vet Microbiol. 2012;157:456–63.
Gunn L, Collins PJ, Fanning S, McKillen J, Morgan J, Staines A, et al. Detection and characterisation of novel bocavirus (genus Bocaparvovirus) and gastroenteritis viruses from asymptomatic pigs in Ireland. Afr J Disabil. 2015;5:27270.
García-Hernández M-E, Trujillo-Ortega M-E, Alcaraz-Estrada S-L, Lozano-Aguirre-Beltrán L, Sandoval-Jaime C, Taboada-Ramírez BI, et al. Molecular detection and characterization of porcine epidemic diarrhea virus and porcine Aichivirus C coinfection in México. Viruses. 2021;13:738.
Nantel-Fortier N, Gauthier M, L’Homme Y, Lachapelle V, Fravalo P, Brassard J. The swine enteric virome in a commercial production system and its association with neonatal diarrhea. Vet Microbiol. 2022;266:109366.
Su M, Qi S, Yang D, Guo D, Yin B, Sun D. Coinfection and genetic characterization of porcine astrovirus in diarrheic piglets in China from 2015 to 2018. Front Vet Sci. 2020;7:3–6.
Salamunova S, Jackova A, Mandelik R, Novotny J, Vlasakova M, Vilcek S. Molecular detection of enteric viruses and the genetic characterization of porcine astroviruses and sapoviruses in domestic pigs from Slovakian farms. BMC Vet Res. 2018;14:1–9.
Werid GM, Ibrahim YM, Chen H, Fu L, Wang Y. Molecular detection and genetic characterization of potential zoonotic swine enteric viruses in Northern China. Pathogens. 2022;11:28–30.
Chuchaona W, Khamrin P, Yodmeeklin A, Kongkaew A, Vachirachewin R, Kumthip K, et al. Detection and molecular characterization of porcine kobuvirus in piglets in 2009–2013 in northern Thailand. Trop Anim Health Prod. 2017;49:1077–80.
Valkó A, Marosi A, Cságola A, Farkas R, Rónai Z, Dán Á. Frequency of diarrhoea-associated viruses in swine of various ages in Hungary. Acta Vet Hung. 2019;67:140–50.
Zhou W, Ullman K, Chowdry V, Reining M, Benyeda Z, Baule C, et al. Molecular investigations on the prevalence and viral load of enteric viruses in pigs from five European countries. Vet Microbiol. 2016;182:75–81.
Ulloa JC, Olarte-Aponte AM, Ospina JC, Rincon MA. Experimental infection of conventional newly-weaned piglets with porcine astrovirus. Acta Virol. 2019;63:96–102.
Fang Q, Wang C, Liu H, Wu Q, Liang S, Cen M, et al. Pathogenic characteristics of a porcine astrovirus strain isolated in China. Viruses. 2019;11:1–17.
Thimmasandra Narayanappa A, Sooryanarain H, Deventhiran J, Cao D, Ammayappan Venkatachalam B, Kambiranda D, et al. A novel pathogenic Mammalian orthoreovirus from diarrheic pigs and Swine blood meal in the United States. MBio. 2015;6:e00593-e615.
Ducatelle R, Coussement W, Hoorens J. Sequential pathological study of experimental porcine adenovirus enteritis. Vet Pathol. 1982;19:179–89.
McAdaragh J, Eustis S, Benfield D. Adenovirus associated with diarrhea in pigs. In: Conference of research workers in animal diseases, Chicago; 1980.
Goecke NB, Hjulsager CK, Kongsted H, Boye M, Rasmussen S, Granberg F, et al. No evidence of enteric viral involvement in the new neonatal porcine diarrhoea syndrome in Danish pigs. BMC Vet Res. 2017;13:315.
Alonso-Padilla J, Pignatelli J, Simon-Grifé M, Plazuelo S, Casal J, Rodríguez D. Seroprevalence of porcine torovirus (PToV) in Spanish farms. BMC Res Notes. 2012;5:1–6.
Carvajal A, Argüello H, Martínez-Lobo FJ, Costillas S, Miranda R, de Nova PJG, et al. Porcine epidemic diarrhoea: new insights into an old disease. Porcine Health Manag. 2015;1:1–8.
Dortmans JCFM, Li W, van der Wolf PJ, Buter GJ, Franssen PJM, van Schaik G, et al. Porcine epidemic diarrhea virus (PEDV) introduction into a naive Dutch pig population in 2014. Vet Microbiol. 2018;221:13–8.
Kong F, Xu Y, Ran W, Yin B, Feng L, Sun D. Cold exposure-induced up-regulation of HSP70 positively regulates PEDV mRNA synthesis and protein expression in vitro. Pathogens. 2020;9:246.
Vidal A, Martín-valls GE, Tello M, Mateu E, Martín M. Prevalence of enteric pathogens in diarrheic and non-diarrheic samples from pig farms with neonatal diarrhea in the North East of Spain. Vet Microbiol. 2019;237:108419.
Mesonero-Escuredo S, Strutzberg-Minder K, Casanovas C, Segalés J. Viral and bacterial investigations on the aetiology of recurrent pig neonatal diarrhoea cases in Spain. Porcine Health Manag. 2018;4:1–6.
Cortey M, Díaz I, Vidal A, Martín-Valls G, Franzo G, Gómez De Nova PJ, et al. High levels of unreported intraspecific diversity among RNA viruses in faeces of neonatal piglets with diarrhoea. BMC Vet Res. 2019;15:1–13.
Bauer E, Williams BA, Smidt H, Verstegen MWA, Mosenthin R. Influence of the gastrointestinal microbiota on development of the immune system in young animals. Curr Issues Intest Microbiol. 2006;7:35–52.
Qiu M, Li S, Xiao Y, Li J, Zhang Y, Li X, et al. Pathogenic and metagenomic evaluations reveal the correlations of porcine epidemic diarrhea virus, porcine kobuvirus and porcine astroviruses with neonatal piglet diarrhea. Microb Pathog. 2022;170:105703.
Anbalagan S, Peterson J, Wassman B, Elston J, Schwartz K. Genome sequence of torovirus identified from a pig with porcine epidemic diarrhea virus from the United States. Genome Announc. 2014;2:3–4.
Dufkova L, Scigalkova I, Moutelikova R, Malenovska H, Prodelalova J. Genetic diversity of porcine sapoviruses, kobuviruses, and astroviruses in asymptomatic pigs: an emerging new sapovirus GIII genotype. Arch Virol. 2013;158:549–58.
Mor SK, Chander Y, Marthaler D, Patnayak DP, Goyal SM. Detection and molecular characterization of Porcine astrovirus strains associated with swine diarrhea. J Vet Diagn Investig. 2012;24:1064–7.
Cai Y, Yin W, Zhou Y, Li B, Ai L, Pan M, et al. Molecular detection of Porcine astrovirus in Sichuan Province, China. Virol J. 2016;13:1–5.
Lee MH, Jeoung HY, Park HR, Lim JA, Song JY, An DJ. Phylogenetic analysis of porcine astrovirus in domestic pigs and wild boars in South Korea. Virus Genes. 2013;46:175–81.
Tamura K, Stecher G, Kumar S. MEGA11: molecular evolutionary genetics analysis version 11. Mol Biol Evol. 2021;38:3022–7.
Liu Q, Wang HY. Porcine enteric coronaviruses: an updated overview of the pathogenesis, prevalence, and diagnosis. Vet Res Commun. 2021;45:75–86.
Saif LJ, Wang Q, Vlasova AN, Jung K, Xiao S. Coronaviruses. In: Zimmerman JJ, Karriker LA, Ramirez A, Schwartz KJ, Stevenson GW, Zhang J, editors. Dis swine. 11th ed. Hoboken: Wiley; 2019. p. 488–523.
Li C, Lu H, Geng C, Yang K, Liu W, Liu Z, et al. Epidemic and evolutionary characteristics of swine enteric viruses in South-Central China from 2018 to 2021. Viruses. 2022;14:1420.
Wang L, Byrum B, Zhang Y. Detection and genetic characterization of deltacoronavirus in pigs, Ohio, USA, 2014. Emerg Infect Dis. 2014;20:1227–30.
Kong F, Wang Q, Kenney SP, Jung K, Vlasova AN, Saif LJ. Porcine deltacoronaviruses: origin, evolution, cross-species transmission and zoonotic potential. Pathogens. 2022;11:1–17.
Xu Z, Zhang Y, Gong L, Huang L, Lin Y, Qin J, et al. Isolation and characterization of a highly pathogenic strain of Porcine enteric alphacoronavirus causing watery diarrhoea and high mortality in newborn piglets. Transbound Emerg Dis. 2018;66:119–30.
Fu X, Fang B, Liu Y, Cai M, Jun J, Ma J, et al. Newly emerged porcine enteric alphacoronavirus in southern China: identification, origin and evolutionary history analysis. Infect Genet Evol. 2018;62:179–87.
Zhou P, Fan H, Lan T, Yang XL, Shi WF, Zhang W, et al. Fatal swine acute diarrhoea syndrome caused by an HKU2-related coronavirus of bat origin. Nature. 2018;556:255–9.
Marthaler D, Homwong N, Rossow K, Culhane M, Goyal S, Collins J, et al. Rapid detection and high occurrence of porcine rotavirus A, B, and C by RT-qPCR in diagnostic samples. J Virol Methods. 2014;209:30–4.
Wu FT, Liu LTC, Jiang B, Kuo TY, Wu CY, Liao MH. Prevalence and diversity of rotavirus A in pigs: evidence for a possible reservoir in human infection. Infect Genet Evol. 2022;98:105198.
Chepngeno J, Takanashi S, Diaz A, Michael H, Paim FC, Rahe MC, et al. Comparative sequence analysis of historic and current porcine rotavirus C strains and their pathogenesis in 3-day-old and 3-week-old piglets. Front Microbiol. 2020;11:1–13.
Kim O, Choi C, Kim B, Chae C. Detection and differentiation of porcine epidemic diarrhoea virus and transmissible gastroenteritis virus in clinical samples by multiplex RT-PCR. Vet Rec. 2000;146:637–43.
Kim S, Song D, Park B. Differential detection of transmissible gastroenteritis virus and porcine epidemic diarrhea virus by duplex RT-PCR. J Vet Diagn Investig. 2001;13:516–20.
Amimo JO, Vlasova AN, Saif LJ. Prevalence and genetic heterogeneity of porcine group C rotaviruses in nursing and weaned piglets in Ohio, USA and identification of a potential new VP4 genotype. Vet Microbiol. 2013;164:27–38.
Fujii Y, Shimoike T, Takagi H, Murakami K, Todaka-Takai R, Park Y, et al. Amplification of all 11 RNA segments of group A rotaviruses based on reverse transcription polymerase chain reaction. Microbiol Immunol. 2012;56:630–8.
Acknowledgements
We acknowledge the excellent technique assistance provided by Diana Molina, as well as the help and willingness of veterinary practitioners and farmers.
Funding
This work was supported by the program of the National Institute of Agricultural and Food Research and Technology (INIA project E-RTA2015-0003-C02-01 and E-RTA2015-0003-C02-02) of Spanish Government. H. Puente, O. Mencía-Ares, L. Pérez-Pérez, M. Cortey and H. Argüello were supported by Spanish Government (FPU17/00466, FPU16/03485, PRE2020-093762, RYC-2015-1715-4 and BEAGAL- 18-106, respectively) and M. Gómez-García by Junta de Castilla & León (LE131-18).
Author information
Authors and Affiliations
Contributions
Study design and direction: HP and AC. In vitro experiments: HP, LPP, MGG and OM. Analysis of the results and writing of the manuscript: HP, MC, ID, HA and AC. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1
: Percentage of positive detection to each of the different investigated enteric viruses in diarrhoea outbreaks occurring between January 2017 and December 2019.
Additional file 2
: Distribution of single infections and co-infections for Porcine astrovirus (PAstV), Porcine kobuvirus (PKoV), Porcine torovirus (PToV), Mammalian orthoreovirus (MRV) and Porcine mastadenovirus (PAdV) with well recognized pathogenic enteric viruses (Porcine epidemic diarrhoea virus or PEDV, Rotavirus A or RVA and Rotavirus C or RVC) and potential pathogenic enteric viruses (PAstV, PKoV, PToV, MRV and PAdV) in the investigated diarrhoea outbreaks (n = 206)
Additional file 3
: Summary of the results of next generation sequencing (NGS) in 14 selected faecal samples. C, consensus sequence (whole genome obtained or near complete genome, >90%); P, partial sequence (less than 90% genome covered) and T, traces (less than 10% of the genome covered). N is the number of viruses detectedper sample. An asterisk indicates PEDV genomes that were already published [8].
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Puente, H., Arguello, H., Cortey, M. et al. Detection and genetic characterization of enteric viruses in diarrhoea outbreaks from swine farms in Spain. Porc Health Manag 9, 29 (2023). https://doi.org/10.1186/s40813-023-00326-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40813-023-00326-w