Abstract
Background
As a novel and time-efficient exercise form, high-intensity interval training (HIIT) has shown great potential in improving health-related physical fitness among diverse populations. However, empirical evidence on its efficacy among the elderly has not been well summarized. This systematic review and meta-analysis aimed to determine the effect of HIIT interventions on the parameters related to physical fitness and health of older adults, including resting heart rate (HR), systolic blood pressure (SBP), diastolic blood pressure (DBP), cardiorespiratory fitness (CRF), body mass index (BMI), body fat percent (BF%), waist circumference (WC), muscular endurance (ME), muscular strength (MS), muscular power (MP), balance and flexibility, compared to non-exercise and other-exercise (e.g., moderate-intensity continuous training, resistance training) conditions.
Methods
Literature published from January 2000 to May 2023 was collected through extensive searches across eight databases and relevant review papers. Randomized controlled trials (RCTs) featuring a minimum 2-week exercise intervention for older adults (≥ 60 years) were included. The pooled effect size of Hedges’g was estimated using random-effects models in R. Meta-regression was performed for both categorical (health status, duration of training programme, and frequency) and continuous moderators (mean age, male rate, and attrition rate).
Results
Forty-four eligible RCTs with 1863 participants (52.1% female; 60.5–81.2 years) were included in the quantitative analysis. Compared to non-exercise condition, HIIT significantly improved resting HR (g = -0.36, 95%CI = [-0.67, -0.05], P = 0.032), SBP (g = -0.29, 95%CI = [-0.54, -0.03], P = 0.008), CRF (g = 0.77, 95%CI = [0.51, 1.04], P < 0.001), BF% (g = -0.26, 95%CI = [-0.41, -0.11], P = 0.006), MS (g = 0.47, 95%CI = [0.23, 0.71], P = 0.004), ME (g = 0.65, 95%CI = [0.10, 1.19], P = 0.036), and balance (e.g., timed-up-and-go) (g = -0.79, 95%CI = [-1.19, -0.40], P = 0.035). Compared to other-exercise condition, HIIT significantly improved resting HR (g = -0.11, 95%CI = [-0.21, -0.01], P = 0.029), SBP (g = -0.14, 95%CI = [-0.28, -0.01], P = 0.038), and CRF (g = 0.23, 95%CI = [0.07, 0.38], P = 0.008). No significant difference was found between HIIT and non-exercise condition for DBP, BMI and WC, as well as between HIIT and other-exercise condition for DBP, BMI, BF%, WC, ME, and balance (all P > 0.05). Meta-regression indicated that mean age moderated the HIIT effect on resting HR (b = -0.02, P = 0.014; HIIT vs. other-exercise condition) and SBP (b = 0.03, P = 0.048; HIIT vs. non-exercise), and attrition rate moderated the effect on CRF (b = 0.03, P = 0.007; HIIT vs. non-exercise).
Conclusion
This study supports the efficacy of HIIT in improving resting HR, SBP, CRF, BF%, MS, ME and balance among older adults. More empirical evidence is needed to determine the efficacy of HIIT for MP and flexibility in this population.
Trial Registration
PROSPERO CRD42022316246.
Key Points
• HIIT is an effective approach for improving older adults’ resting heart rate (HR), systolic blood pressure (SBP), cardiorespiratory fitness (CRF), body fat percent (BF%), muscular strength (MS), and balance, compared with non-exercise condition.
• HIIT outperformed the other exercise interventions in improving older adults’ resting HR, SBP, CRF, and muscular endurance (ME).
• Mean age and attrition rate were identified as potential moderators for the HIIT effects on resting HR, SBP and CRF.
Similar content being viewed by others
Background
Ageing is one of the greatest public health challenges faced by countries worldwide. The World Health Organization (WHO) has stated that the number and proportion of older adults aged ≥ 60 years is increasing rapidly. The number was 1 billion in 2019, and it is expected to increase to 1.4 billion by 2030 and 2.1 billion by 2050 [1]. This unprecedented increase in the ageing population is unavoidable and will accelerate in the coming decades, especially in developing countries [1]. It is known that a decline in physical fitness is a common health problem that accompanies ageing, which affects physical function, the risk of chronic diseases, and quality of life [2, 3]. According to the American College of Sport Medicine (ACSM), health-related physical fitness is defined as ‘a set of attributes that people have or achieve that relates to the ability to perform physical activity’, which is closely related to individuals’ physical, mental and social health [4]. The health-related components of physical fitness consist of cardiorespiratory fitness (CRF), body composition (e.g., body mass index [BMI], body fat percent [BF%], waist circumference [WC]), muscular endurance (ME), muscular strength (MS), muscular power (MP), balance and flexibility [4]. Traditionally, muscular power and balance should be considered as skill-related physical fitness components instead of health-related physical fitness components. However, the 11th ACSM’s guidelines suggest including muscular power in the assessment of muscular fitness. This is because muscular power tends to decline at a faster rate compared to muscular strength or muscular endurance with aging [5], and it may be the most significant of the muscular fitness variables for predicting maintenance of functional independence and improving quality of life [6]. Regarding balance, the ACSM position statement recommends that balance training is an effective way for fall prevention [7], which is closely related to aging health. Balance is increasingly becoming an additional component of health-related fitness [4]. Additionally, the ACSM’s guidelines emphasize that a comprehensive physical fitness assessment includes the measurement of resting heart rate (HR) and resting blood pressure (BP) [4]. Those two parameters closely relate to the health of older people as well. Therefore, the parameters related to physical fitness and health of older adults include resting HR, BP, CRF, BMI, BF%, WC, ME, MS, MP balance and flexibility in this study.
An overwhelming group of evidence has demonstrated that exercise training is a crucial part of healthy ageing and is conducive to improving health-related physical fitness of older adults [8, 9]. Moderate-intensity continuous training (MICT), as a ‘traditional’ aerobic exercise protocol, has been a leading exercise recommendation in older adults for nearly three decades [10, 11]. It refers to moderate intensity of effort (55–69% HRmax or 40–59% VO2peak) performed continuously at a steady state for a set duration [12]. MICT has been shown to be associated with a wide range of physical fitness and health indicators, including CRF, BP and body composition [13,14,15]. While traditional exercise programmes can offer numerous benefits, their implementation can be challenging for older adults, mainly because of their long duration, which often leads to diminished engagement, motivation, and compliance with the exercise prescription [16]. In this scenario, high-intensity interval training (HIIT), which is suggested as an alternative to traditional MICT, has attracted increasing interest in recent years. As a novel and time-efficient exercise form, HIIT consists of repeated bouts of high-intensity exercise that last seconds to minutes, interspersed with periods of rest [17, 18]. Similar to traditional MICT, HIIT can include diverse forms of exercise modalities such as cycling, dancing, treadmill running, jumping-based exercise etc. [19]. The main distinction is that HIIT involves alternating short bursts of vigorous exercise (lasting from 10 s to 5 min) that typically elevate one’s heart rate to at least 80% of their maximum capacity (HRmax), with recovery periods of rest or light exercise (lasting no more than 5 min) at ≤ 70% HRmax [19, 20]. A typical HIIT session lasts about half the duration of a MICT session [11, 15]. The feasibility, safety, and tolerability of HIIT programmes amongst older adults have been demonstrated by a recent scoping review [19]. In addition, previous studies have found that HIIT can improve older adults’ CRF [21, 22], body composition [23], muscle fitness [24], metabolic parameters [14], cognitive function [25, 26], and mental health [27].
Recently, several reviews have provided preliminary evidence on the effect of HIIT in improving older adults’ physical fitness [11, 28, 29]. However, most of them focused mainly on the CRF of older adults [28, 29], while evidence on the other crucial parameters of health-related physical fitness and health (e.g., resting HR, WC, MP, balance, and flexibility) has not been well summarized. Furthermore, existing reviews have shown several methodological limitations. For example, a recent meta-analysis demonstrated that HIIT has a significant medium effect on functional movement, as assessed using the 30-s chair sit-to-stand test (STS) and 8-foot (timed) up and go (TUG) test in older adults compared with the non-intervention group [30]. This review used standardized mean difference (SMD) to pool effect sizes, which may have introduced upward bias because of the inclusion of several studies with sample sizes lower than 20 [31]. Another review measured seven outcomes (i.e., 6-min walking test [6MWT], TUG test, chair test, upper limb MS, lower limb MS, MP, and citrate synthase activity) to examine the effect of HIIT [28]. Although this review showed that HIIT had significant effects on the 6MWT, TUG test, chair test, MP, and citrate synthase activities, the consistency of the results might have been influenced by two substantial shortcomings. First, four of the experimental groups included in the review used a HIIT intervention combined with other approaches, such as resistance training and a nutritional strategy. Second, some of the included studies did not satisfy the randomized controlled trials (RCTs) criteria because of the lack of a control group. Additionally, existing reviews reported conflicting findings regarding the effects of HIIT on the resting BP of older adults. Carpes et al. found that HIIT significantly decreased the systolic BP (SBP) and diastolic BP (DBP) of older adults compared with the non-intervention group, whereas another meta-analysis found no such significant effect [32]. Overall, the conflicting outcomes and variations in research methods across these studies indicate the need for further studies on this topic.
Therefore, the purpose of this systematic review and meta-analysis was to identify the evidence on and quantify the impact of HIIT interventions on a wide range of parameters related to physical fitness and health (i.e., resting HR, resting BP, body composition, CRF, ME, MS, MP, balance, and flexibility) in both clinical and non-clinical older adults compared with other-exercise (e.g., MICT, resistance training) and non-exercise control conditions. In addition, the following specific characteristics of interest were tested as moderators of the effects of HIIT: participants’ characteristics (e.g., age, male-to-female ratio, health status, and attrition rate) and intervention characteristics (e.g., frequency and duration of the training sessions).
Methods
Protocol and Registration
This study was conducted following the Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 statement [33]. The protocol was registered in the International Prospective Register of Systematic Reviews (PROSPERO) database (Prospero ID: CRD42022316246). The PROSPERO database and Cochrane Library of systematic reviews were searched for existing or pending systematic reviews and meta-analyses to avoid duplication.
Identification of Studies and Search Strategy
A systematic literature search of eight electronic databases (i.e., Medline, PsycINFO, SPORTDiscus, Scopus, Embase, CT.gov, the Cochrane Library, and PubMed) was conducted. Article titles and abstracts were searched using the key terms that were generated from a summary of previous review papers and commonly used synonyms for HIIT (see Supplementary file 1). To enable a more specific search, the following limitations were applied: (1) English language, (2) human subjects, (3) journal articles, and (4) published from 1st January 2000 to 31st May 2023 (given prior reviews indicating that HIIT has primarily been utilized in health promotion fields since the beginning of the 21st century [19, 30, 32], this systematic review commenced the literature search from the year 2000). In addition to the structured database search, literature from the bibliographies of relevant review articles was searched by hand. Two authors (XW & SC) completed the systematic search for articles and the removal of duplicates. The titles and abstracts of the remaining articles were then screened by the same two authors.
Inclusion and Exclusion Criteria
The full texts of the articles were screened for inclusion by two authors (XW & SC). Another author (WL) was consulted in cases of doubt or disagreement between the first two authors. The full-text screening was conducted based on the following PICOS criteria.
-
(1)
Participants: The mean age of participants was ≥ 60 years, and there were no restrictions regarding participants’ demographics and medical conditions.
-
(2)
Interventions: The intervention protocol included at least one group performing HIIT intervention, defined as brief, intermittent bursts of vigorous activity interspersed with periods of low-to-moderate-intensity activity or rest [17]. Exercise intensity is commonly assessed based on oxygen uptake (VO2), HR, and heart rate reserve (HRR). High intensity was categorised as ‘very hard’ effort (≥ 90% of peak HR; ≥ 85% of HRR; ≥ 80% of peak VO2) or ‘vigorous’ effort (70%-89% of peak HR; 60%-84% of HRR; 60%-79% of peak VO2) [19, 34]. To increase the generalisability of the findings, we also included the studies that used perceived exertion of at least 16 on the Borg scale to define high intensity. Additionally, the minimum duration of the intervention was set at 2 weeks to allow for the capture of training adaptations rather than just the acute effects [30]. No limitations were set for other intervention characteristics (e.g., exercise mode, frequency, and recovery mode).
-
(3)
Comparators: The comparator groups included in the studies were mainly another exercise intervention group (e.g., the MICT intervention) or a non-exercise control group.
-
(4)
Outcomes: The studies evaluated at least one of the following outcomes: resting HR, resting BP (usually SBP and DBP), CRF (usually relative VO2max or VO2peak), body composition (usually BMI, BF%, and WC), MS (usually the chair stand test and grip strength test), ME (usually the sit-to-stand test and arm curl test), MP (usually the countermovement vertical jump test), balance (usually the TUG test), and flexibility (usually the chair sit and reach test, and back scratch test).
-
(5)
Study design and article type: Individual or cluster RCTs were included. Reviews, editorial and commentaries, non-peer-reviewed papers, and non-English papers were excluded.
Risk of Bias and Certainty of Evidence
Two authors (CS & WL) independently evaluated the risk of bias for the included studies using version 2 of the Cochrane Risk of Bias Tool (RoB2) [35], based on five criteria: (1) randomisation process, (2) deviations from the intended interventions, (3) missing outcome data, (4) measurement of the outcome, and (5) selection of the reported results. For each criterion, the risk of bias was judged as low, some concerns, or high. Based on the assessment, the quality of the included studies was classified into three levels: low risk, some concern and high risk.
Additionally, the quality and certainty of the evidence was determined using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) system [36]. Specifically, the quality was downgraded in case of each of the following limitations: imprecision of results (wide 95% confidence intervals [CIs]), inconsistency of results (I2 values > 50% were considered to indicate substantial heterogeneity) [37], indirectness of evidence (indirect population, intervention, control, and outcomes), risk of bias (> 50% studies with more than one item with a high risk of bias) [37], and high probability of publication bias (Egger’s regression test results being significant). After two authors independently evaluated the quality of evidence according to the criteria, consensus was reached on the ratings and the overall quality of the summary statistics. Any disagreement between the two authors was resolved by discussion or consultation with a third investigator (XW/YD).
Data Extraction
Data extraction from the included articles was conducted by two authors (XW & SC). The extracted data included basic information about the study, study methodology, participants’ characteristics, intervention characteristics, and measurement outcomes. The specific variables were authors, publication year, participants’ health condition (i.e., healthy older or older adult patients), the country and common setting (refers to the environment or context in which the HIIT exercise was conducted), study design, sampling method, sample size, age (mean and standard deviation [SD]), sex (male rate), attrition rate, attendance rate, exercise type, recovery mode (i.e., active or passive), intensity assessment (i.e., objective or subjective), duration of the training programme, frequency, volume of HIIT, durations of HIIT intervals (i.e., work interval and rest interval), interval repetitions, warm-up time, cool-down time, and statistical data for outcome variables (i.e., n, mean, and SD). The intervention groups were categorized as the HIIT group, other-exercise group (engaged in an exercise training programme other than HIIT), and control group (not engaged in any training programme). In cases of articles missing relevant data, their authors were contacted via email for the missing data.
Summary of Measures
The primary outcomes assessed in this review were resting HR, resting BP, CRF, body composition (i.e., BMI, BF % and WC), MS, ME, MP, balance, and flexibility. The moderators were participants’ characteristics (age, male rate, health status, and attrition rate), and intervention characteristics (duration of the training programme, frequency of exercise sessions, recovery mode and exercise mode). These descriptive data were also summarized in this review (Table 1).
Meta-Analysis
For the effect size calculation and data analysis procedure, Cohen’sd (also known as the SMD) [38] values were calculated for all 229 sets of data identified in 44 studies. For studies that reported both pre-test and post-test outcomes of the experimental and control groups, change scores were used to calculate the effect sizes. The following formulas were used:
where \(\stackrel{-}{\text{x}}1\), and \(\stackrel{-}{\text{x}}2\) were obtained by subtracting the post-test mean from the pre-test mean; \(n1\) and \(n2\) are the sample sizes of the two groups, respectively; the SD of change (i.e., \(s1\) and \(s2\)) was transformed using the following the formula given below; and Corr (correlation coefficient) was assumed to be 0.5 between the baseline and follow-up measurements [35]. Notably, Cohen’s d for effect size has been found to have an upward bias when the study sample size is small, especially when n ≤ 20 [31]. Therefore, all the Cohen’s values were converted into Hedges’ g by using the following formula suggested by Hedges (1981) to correct for overestimation [31].
We examined whether there were outliers to provide evidence of the robustness of the findings. For each effect size, values that were outside the interval of \(\stackrel{-}{\text{x}}\) – 2sd and \(\stackrel{-}{\text{x}}\) +2sd were considered outliers [39], and analyses were repeated without these effects. Meta-analyses were conducted if at least three datasets provided effect sizes of HIIT for the same outcome [40]. A random-effects model was used to pool the overall effect size of HIIT because we assumed that the true effect size could vary from study to study [41]. The Tau2 and I2 statistic was used to assess the heterogeneity across studies [42, 43]. Tau2 is the estimate of between-study variance of the group. A larger Tau2 value indicates higher heterogeneity beyond what would be expected by chance alone [43]. I2 values of < 25%, 50%, and 75% indicated low, moderate, and high heterogeneity, respectively [42]. For pooling effect sizes that were significant (P < 0.05) or had higher values of heterogeneity and at least 8 datasets [44], meta-regression was used to examine the moderator for explaining the variability.
Finally, Egger’s regression test was used to identify the existence of publication bias [44]. If publication bias was found (i.e., P < 0.1 in Egger’s regression test), the selection model by Vevea and Woodds was used to obtain an overall effect size corrected by publication bias [45]. In this method, the adjusted overall effect size was measured by specifying a weight function of the probability of being published assigned to the observed effect size according to their P values. The weights for the weight function were selected based on those suggested by Vevea and Woods for moderate one-tailed selection [45]. In addition, sensitivity analysis was performed using the leave-one-out function, in which the meta-analysis is performed by removing one effect size at a time. These removed effect sizes were from studies with high risks of bias.
All of the analyses were performed in R (version 4.3.0, R Foundation for Statistical Computing). Specifically, the effect sizes were calculated using dmetar [46] and tidyverse packages [47], the main meta-analysis was conducted using the meta package [48], the meta-regression was performed using the metafor package [49], and publication bias was examined using the weightr package [50].
Results
Study Selection
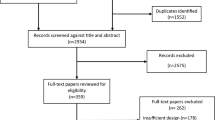
Literature searches were performed in eight databases, which resulted in a total of 29,865 articles. Following the removal of 4881 duplicates, the titles and abstracts of the remaining 24,984 articles were screened. Of these, 132 full-text articles were screened for eligibility, and 92 were excluded for several reasons as shown in Fig. 1. Additionally, 280 articles were collected from other sources (website and reference lists of relevant review articles), of which 16 were included in the eligibility check. Finally, a total of 44 studies satisfied the inclusion criteria for both qualitative and quantitative analyses (Fig. 1).
Study Characteristics
Participants’ characteristics from included studies are outlined in Table 1. A total of 1863 participants (872 male and 970 female; two studies did not provide sex information) from 15 countries were divided into HIIT groups (n = 821), other-exercise groups (i.e., MICT, moderate-intensity interval training [MIIT], resistance training [RT], combined training [CT], high-intensity continuous training [HICT]; n = 513) or non-exercise control groups (n = 529). The attrition rates ranged from 0 to 35.29% in the HIIT groups (the rate was < 20% in 37 studies), 0–35.29% in the other-exercise groups (the rate was < 20% in 26 studies), and 0–34.29% in the non-exercise control groups (the rate was < 20% in 25 studies). In addition, 20 studies reported the attendance rates of the HIIT groups (the rate was > 80% in 17 studies), and 14 studies reported the attendance rates of the other-exercise groups (the rate was > 80% in nine studies). The mean age ranged from 60.6 to 81.2 years, and the mean BMI ranged from 21.6 to 33.9 kg/m2. The studies included patients with heart failure [24, 51,52,53,54], atrial fibrillation [55], subjective cognitive decline [25], coronary artery disease [22, 56, 57], metabolic syndrome and hyper-triglyceridemic waist phenotype [58], type 2 diabetes [59], hypertension [60], and lacunar stroke [61].
HIIT protocol features from included studies are outlined in Table 2. In terms of the intervention design, the training lasted between 2 weeks to 24 weeks, with 12 weeks being the most common duration (n = 18), and training sessions were conducted one to five times per week. The HIIT training included work intervals ranging from 6 s to 4 min, rest intervals ranging from 12 s to 4 min, cool-down and warm-up times ranging from 2 to 15 min, and exercise modes consisting of treadmill (n = 12), cycling (n = 21), and others (e.g., sprints, resistance exercises, and comprehensive exercise) (n = 11). Regarding work-to-rest ratio (WRR) of HIIT, the most widely applied ratio was 1:1 (n = 15) including 15s:15s [62, 63], 30s:30s [55, 64], 1 min:1 min [22, 58, 65, 66], 2 min:2 min [67], 3 min:3 min [52, 59, 68, 69], 4 min:4 min [14, 70], followed by 4:3 (n = 11) including 4 min:3 min [25, 26, 51, 53, 54, 56, 57, 71,72,73,74]. HIIT implementation settings included the laboratory, fitness centers, healthcare facilities, university and home. The laboratory setting was the most common (n = 25), followed by the healthcare facilities setting (n = 5) and the fitness centre setting (n = 4).
The outcome measures were: resting HR assessed with the 12-lead electrocardiograph (ECG) (n = 6) and blood pressure measurement (n = 1), photoelectric pulse wave method (n = 4), resting blood pressure (SBP and DBP), cardiorespiratory fitness (VO2peak, VO2max) assessed with a treadmill test (n = 9), cycle ergometer test (n = 16) and Chester step test (n = 1), BMI, WC, BF% assessed with the dual-energy X-ray absorptiometry method (n = 10), bioelectrical impedance analysis (n = 2) and skinfold caliper (n = 1), MS assessed with the chair stand test (n = 5), handgrip strength test (n = 3) and maximal isometric knee extensor muscle strength (n = 1), ME assessed with the 6MWT (n = 5), loaded 50 m walk test (n = 1), Bruce protocol (n = 1), MP assessed with the Nottingham leg extensor power rig (n = 1) and step test (n = 1), balance assessed with the TUG (n = 6), static balance (n = 1) and standing on one leg with eyes closed (n = 1), flexibility assessed with the sit and reach test (n = 1).
Twenty-five studies reported no adverse events during HIIT, and six studies reported adverse events (one study did not provide detailed information [73]) including: (1) Achilles tendinitis, lateral epicondylitis, knee bursitis, muscle strain, and swelling in the metacarpophalangeal joint [75]; (2) low-back pain, hip soreness, hypertensive crisis, knee soreness, and muscle soreness [25]; (3) shortness of breath, dizziness and hypotension [59] (4) nausea/vomiting, knee swelling/medial collateral ligament tear, and uncontrolled HR [55]; (5) an abnormally high blood pressure response to exercise and mild vasovagal episodes [70]. Thirteen studies did not provide relevant information on adverse events.
Risk of Bias in the Included Studies
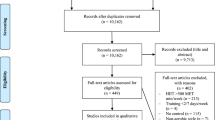
As outlined in Fig. 2, six studies were judged to be at a low risk of bias, and 36 studies were assessed as having some concerns. The main possible bias was the lack of pre-registration of intentions/methodology, which was particularly relevant for the overall results. In addition, two studies were considered to have a high risk of bias due to the possible bias in deviations from the intended interventions and missing outcome data (12 out of 34 participants discontinued the intervention).
Resting Heart Rate
HIIT Versus Non-exercise The effect of HIIT versus non-exercise groups on the resting HR outcome was investigated in six studies (n = 217) [52, 59, 60, 74,75,76]. Table 3 shows a small significant effect (g = -0.358, 95%CI = [-0.671, -0.045]) of the HIIT interventions with a moderate-GRADE quality of evidence (Table 4). No heterogeneity was evident.
HIIT Versus Other-exercise The effect of HIIT versus other-exercise groups on resting HR was investigated in 13 studies (n = 478) [14, 22, 25, 51, 52, 55,56,57, 59, 63, 66, 74, 76]. The HIIT groups showed a significant reduction in resting HR (g = -0.113, 95%CI = [-0.213, -0.014]) (Table 1) with a high-GRADE quality of evidence (Table 3). No statistically significant heterogeneity was identified.
Resting Blood Pressure
HIIT Versus Non-exercise The effect of HIIT versus non-exercise conditions on the SBP outcome was investigated in 10 studies (n = 340) [52, 59,60,61, 70, 74,75,76,77,78], in which the HIIT groups showed a significant reduction in SBP (g = -0.287, 95%CI = [-0.542, -0.032]) (Table 3). No statistically significant heterogeneity was observed across the studies, but the result was of a low GRADE quality of evidence (Table 4). Furthermore, the effect of HIIT versus non-exercise groups on the DBP outcome was investigated in 8 studies (n = 291) [52, 60, 61, 70, 74,75,76, 78]. The HIIT groups showed a small non-significant reduction in DBP (g = -0.249, 95%CI [-0.606, 0.108]) (Table 3). A small heterogeneity was evident (Tau2 = 0.09, I = 36.1%). The result was of a moderate GRADE quality of evidence (Table 4).
HIIT Versus Other-exercise The effect of HIIT versus other-exercise groups on the SBP outcome was investigated in 17 studies (n = 693) [14, 22, 23, 25, 52, 53, 55,56,57,58,59, 63, 66, 74, 76, 77, 79]. The HIIT groups also showed a significant reduction in SBP compared with the other-exercise groups (g = -0.143, 95%CI = [-0.277, -0.009]) (Table 3). No heterogeneity was evident. In addition, the effect of HIIT versus active group on the DBP outcome was investigated in 15 studies (n = 621) [14, 23, 25, 52, 53, 55,56,57,58,59, 63, 66, 74, 76, 79]. The HIIT groups showed a trivial non-significant reduction in DBP relative to the other-exercise groups (g = -0.022, 95%CI = [-0.172, 0.128]) (Table 1). No statistically significant heterogeneity was noted across the studies. These results provide high and moderate GRADE quality of evidence for the SBP and DBP outcomes, respectively (Table 4).
Cardiorespiratory Fitness
HIIT Versus Non-exercise The effect of HIIT versus non-exercise control on the CRF outcome was investigated in 14 studies (n = 413) [26, 54, 59, 68,69,70, 72, 74, 76, 78, 80,81,82], in which the HIIT groups showed a large, significant increase in CRF relative to the control groups (g = 0.774, 95%CI = [-0.506, 1.041]) (Table 1) with a moderate-GRADE quality of evidence (Table 3). Only trivial heterogeneity was observed among these studies (Tau2 = 0.07, I2 = 26.6%).
HIIT Versus Other-exercise The effect of HIIT versus other-exercise groups on the CRF outcome was investigated in 19 studies (n = 572) [22, 23, 26, 52, 53, 56, 57, 59, 63, 66,67,68,69, 74, 79, 81,82,83,84]. The HIIT groups also showed a small but significant increase in CRF relative to the other-exercise conditions (g = 0.228, 95%CI = [0.067, 0.383]) (Table 3) with a high-GRADE quality of evidence (Table 4). No heterogeneity was evident.
Body Composition
HIIT Versus Non-exercise The between-group differences in the effects of HIIT versus non-exercise control group on BMI, BF%, and WC are shown in Table 3. Eleven studies (n = 354) [59, 61, 64, 68, 69, 73, 74, 76, 78, 80, 85] evaluated BMI, seven studies (n = 241) [59, 64, 68, 69, 73, 78, 81] evaluated BF%, and three studies [59, 73, 74](n = 108) evaluated WC. Overall, the pooled effect size illustrated a significant difference between the HIIT and non-exercise groups in terms of BF% (g = -0.257, 95%CI = [-0.406, -0.108]), but non-significant differences in BMI (g = -0.127, 95%CI = [-0.266, 0.014]) and WC (g = -0.155, 95%CI = [-0.462, 0.152]). The GRADE quality of pooled effect size for BF% was high, and that for the other outcomes was moderate (Table 4).
HIIT Versus Other-exercise The between-group difference in the effects of HIIT and other-exercise interventions on BMI, BF%, and WC were evaluated in 15 studies (n = 479) [23, 53, 55, 59, 62, 63, 65, 67,68,69, 73, 74, 76, 83, 86], 11 studies (n = 410) [14, 55, 59, 65, 67,68,69, 73, 81, 83, 86], and eight studies (n = 271) [23, 55, 59, 67, 73, 74, 83, 86], respectively. According to Table 3, the pooled effect size illustrated a non-significant difference between the HIIT and other-exercise interventions in terms of BMI (g = -0.086, 95%CI = [-0.202, 0.031]), BF% (g = -0.064, 95%CI = [-0.186, 0.059]), and WC (g = 0.027, 95%CI = [-0.066, 0.120]). No further analyses were performed because the values of Tau2 and I2 for all these pooled effects were 0.
Muscle Fitness and Mobility
HIIT Versus Non-exercise The between-group differences in the effects of HIIT versus non-exercise control groups on ME, MS, and balance are shown in Table 3. Four studies (n = 115) [64, 69, 72, 80] evaluated MS, two studies tested (n = 81) [64, 80] ME, and five studies (n = 125) [64, 69, 71, 80, 85] evaluated balance. Overall, the pooled effect size demonstrated significant differences between the HIIT and non-exercise condition in terms of MS (g = 0.469, 95%CI = [0.225, 0.713]), ME (g = 0.648, 95%CI = [0.101, 1.194]) and balance (g = -0.794, 95%CI = [-1.187, -0.401]). No heterogeneity was evident for the outcomes of MS, ME and balance.
HIIT Versus Other-exercise The between-group differences in the effects of HIIT versus other-exercise interventions on MS, ME, and balance were evaluated in five studies (n = 165) [14, 23, 69, 79, 87], four studies [14, 23, 55, 71] (n = 159), and four studies (n = 104) [23, 69, 71, 87], respectively. According to Table 3, the pooled effect size demonstrated a significant difference between the HIIT and other-exercise interventions in terms of MS (g = 0.272, 95%CI = [0.028, 0.516]), but non-significant differences in ME (g = 0.158, 95%CI = [-0.074, 0.389]) and balance (g = 0.008, 95%CI = [-0.724, 0.741]). No heterogeneity was evident for the outcomes of MS and ME, and a low heterogeneity was evident for balance (Tau2 = 0.12, I2 = 41.8%). The GRADE quality of pooled effect size for MS was low, and that for the other outcomes was moderate (Table 4).
Meta-Regression Outcome
The meta-regression conducted on the significant pooled results of the meta-analysis revealed that mean age significantly moderated the effects on resting HR (HIIT vs. other-exercise condition, n = 16, b = -0.021, P = 0.014) and SBP (HIIT vs. non-exercise control, n = 11, b = 0.034, P = 0.042), and that attrition rate significantly moderated the effects on CRF (HIIT vs. non-exercise control, n = 14, b = 0.027, P = 0.012). The other moderators, including the male rate, health status, duration of the intervention programme, and frequency of exercise sessions, showed no significant moderating effects on any outcome measures (Supplementary file 2, Table S1).
Publication Bias and Sensitivity Analysis
Egger’s regression test was used to identify potential publication bias. A significant publication bias is indicated if the intercept corresponds to P < 0.10; otherwise, there is no publication bias. As outlined in Table 2, publication bias was identified for SBP in the HIIT versus non-exercise comparison (P = 0.06) and for MS in the HIIT versus other-exercise comparison (P = 0.06). The selection model of Vevea and Woods [45] indicated that the adjusted pooled effects of HIIT on SBP and MS were − 0.323 and 0.204, respectively. Therefore, the overall effects observed on SBP may be somewhat deflated, and those on MS may be somewhat inflated. Furthermore, sensitivity analyses were conducted on the results that showed heterogeneity. According to the results (see Supplementary file 3), the heterogeneity of the pooled effect size for DBP (HIIT vs. non-exercise control) decreased to 0% after one study [60] was removed, but the significance or direction of the overall effect was not changed. The sensitivity analyses showed the same pattern of results for CRF and ME (HIIT vs. non-exercise control) after two studies [71] were removed respectively. However, in the results for balance (HIIT vs. other-exercise condition), the g value for heterogeneity decreased to 0% and the direction of the overall results changed (but remained nonsignificant) after one study [87] was excluded.
Discussion
Summary of the Characteristics of Included Studies
To the best of our knowledge, this is the first study to conduct a comprehensive systematic review and meta-analysis of the evidence on the effects of HIIT interventions versus non-exercise and other-exercise conditions on the parameters of physical fitness and health of older adults. Compared with the literature on the benefits of traditional exercise for older adults, the research on HIIT interventions is limited. The current review found that there was an increasing trend of HIIT interventions for older adults over the past decade, with most of the relevant studies (32/44, 72.7%) having been conducted in the last 7 years. The current analyses included 44 studies involving a total of 1863 participants (872 male, 970 female) aged 60 years and above from 15 countries, with BMIs ranging from 21.6 to 33.9 kg/m2. Our findings showed that compared with both non-exercise control and other-exercise interventions, HIIT was an effective approach for improving parameters related to physical fitness and health, including resting HR, SBP, CRF, BF%, MS, ME, and balance among older adults. Notably, our study observed considerable variability in the characteristics of HIIT interventions (e.g., frequency, duration, and content of intervention). This diversity likely reflects the varied objectives and health requirements of the studies included in our review. Such variability emphasizes the adaptability of HIIT protocols, enabling practitioners to tailor these interventions to meet the specific needs and preferences of individuals, thereby enhancing their practical application and effectiveness.
Despite the high variability in the included studies, some trends are notable. First, a majority of the HIIT intervention studies were conducted in Western countries (41/44, 93.2%), with only three studies conducted in Asia, i.e. China (n = 2) [52, 69] and Iran (n = 1) [60]. A possible explanation for this might be that HIIT originated in the West [88] and has a relatively well-developed training model [17]. Therefore, the HIIT intervention is mostly adopted in Western countries. Another possible explanation is that several non-English reviews or dissertations were not included in this review because they did not meet the inclusion criteria.
Second, most studies (36/44, 81.8%) have used objective methods to monitor the intensity of the HIIT intervention, such as ECG belts and wrist photoplethysmogram (PPG) sensors. Objective methods are more commonly used during HIIT because the intensity of HIIT is typically high and can be difficult to judge subjectively. HIIT requires individuals to push themselves to their limits during the high-intensity intervals, and thus, subjective results vary in accuracy based on the individual’s perception of exertion [89]. In addition, some participants may report dishonest data to show they are performing well. In contrast, objective monitoring methods, such as HR monitoring, provide accurate and measurable data on the individual’s physiological responses to the exercise intensity [90]. These data can help the individual to adjust their exercise intensity to ensure that they are exercising at the appropriate intensity to derive maximum benefit and avoid overexertion or injury.
Third, the most commonly adopted methods to facilitate HIIT were cycle ergometers and treadmills (32/44, 72.7%), which is in line with a previous study [19]. These types of exercises (e.g., using these devices in the laboratory) are easy to perform for older adults who desire exercise to be part of their daily lives. This is also consistent with an earlier review that showed that cycle ergometer training is particularly suitable for older adults because of its benefits for CRF, endurance parameters, and BP and because it is safer and exerted lower pressure on the joints than other typical components of exercise programmes [91]. However, it is worth noting that implementing these types of exercise in a real-world context that targets a larger sample size of populations can be challenging. Additionally, exercises performed using machines are limited in their ability to fully develop the essential skills of balance, gait and coordination that are vital to the daily lives of older adults [92]. Therefore, developing and evaluating a HIIT protocol tailored to meet the needs of older adults in a real-world setting, incorporating a group-based, machine-free exercise format, with supervised multifaceted exercise movements and greater enjoyment levels, is warranted in the future.
Although nearly 80% of included studies applied active recovery mode during HIIT, it was difficult to conclude that active recovery mode was superior to passive recovery mode. The latest systematic review investigated the effect of active or passive recovery mode in long-term interval training on physical fitness, and demonstrated that regardless of recovery mode, long-term interval exercise training has the potential to improve health-related physical fitness in adults [93]. A similar result was found in this study. There were no significant differences between active recovery and passive recovery of effect on health-related physical fitness outcomes (e.g., CF, BF %) in older adults (more details, see Supplementary file 2). Therefore, the participants and researchers can use either active or passive recovery mode when conducting HIIT programmes. The decision should be based on various factors, such as participants’ fitness level, and exercise workload.
Additionally, HIIT appears to be safe among older adults. Although only 70% (31/44) studies reported on adverse outcomes, this figure was higher than the previous review (58%, 7/12) [94], and 81% (25/31) studies explicitly reported that there was no adverse event during HIIT intervention. For six studies with adverse outcomes [25, 55, 59, 70, 73, 75], there were no serious adverse events requiring hospitalization or medical treatment. In addition, most adverse cases were resolved within the duration of study and none lasted throughout the entire intervention period [25, 59, 70, 73, 75]. For example, after resting and rehydrating with water, participants quickly recovered from shortness of breath and dizziness during HIIT and returned to the subsequent exercise without any problem [59]. Thirty percent of the included studies did not report any information on adverse events. This omission could introduce bias into the aggregated results, potentially skewing them towards a more favourable outcome than might be warranted [94]. Consequently, our findings cannot definitively ascertain the safety of HIIT for the elderly. Recognizing this gap, we advocate for future HIIT interventions to prioritize safety considerations. This can be achieved through meticulous protocol design involving healthcare professionals, rigorous scientific monitoring of participants, and the implementation of safety and emergency measures. Such strategies are vital to ensuring the well-being of participants and to bolster the credibility and acceptability of HIIT as a safe exercise modality for older adults.
Effects on Physical Fitness and Health
As expected, this review found that HIIT significantly improved CRF in older adults, compared with other-exercise interventions (g = 0.23) and non-exercise controls (g = 0.76). This is in line with a meta-analysis by Wu et al. who reported that VO2peak increased substantially after more than 12 weeks of HIIT at 2 sessions/week compared with MICT (weighted mean difference [WMD] = 1.74 ml/kg/min) and non-exercise controls (WMD = 2.28 ml/kg/min) [28]. Bouaziz et al. also found that HIIT led to a greater improvement in the VO2peak of older adults aged ≥ 65 years compared with endurance training (mean difference [MD] = 3.76 ml/kg/min) and no-intervention control (MD = 4.61 ml/kg/min) [95]. This indicates that HIIT may be an effective way to increase aerobic capacity in older adults, as it induces greater central and peripheral adaptations than other traditional exercise modalities [95]. A possible explanation for this result might be improvement of the oxidative pathway due to increased muscle work during HIIT. Oxidative phosphorylation reduces lactate accumulation during interval periods. Training-induced reductions in blood lactate levels also lead to a slower breakdown of glycogen, which in turn favours more efficient oxidative pathways [17, 96, 97]. Notably, our meta-regression analysis showed that greater increases in VO2peak were associated with a higher attrition rate. A possible explanation for this relationship between attrition rate and the effect of HIIT on VO2peak is related duration of the HIIT programme. Based on common understanding in the field, the longer intervention periods yield better positive results [98]. However, the results of the meta-analysis did not support this. A possible explanation is the diversity of the HIIT protocol. Further empirical research is needed to identify the differential effects of intervention durations.
In addition, the current review found a significant reduction in resting HR after HIIT relative to other-exercise interventions (g = -0.11) and no-intervention controls (g = -0.36). To the best of our knowledge, this is the first systematic review and meta-analysis to assess the effect of HIIT on the resting HR of older adults. Our results are similar to those of a previous meta-analysis that identified the effect of HIIT on overweight/obese adults [99] and of school-based HIIT on children and adolescents [100]. The reduction in resting HR might be related to the increase in stroke volume and improvement of cardiac autonomic function via increased baroreflex-mediated modulation of the sinoatrial node [101, 102]. Our meta-regression analysis showed a greater reduction in resting HR in participants of older mean age. This result might be related to the baseline physical fitness level. Compared with the relatively low mean age group (mean age from 62 to 67, resting HR from 55 to 70 beats per minute (bpm); mean value of resting HR was 63.7 bpm) [22, 56, 59, 66, 74], the older mean age group had a higher resting HR at baseline (mean age from 67.2 to 80.8, resting HR from 55 to 76.9 bpm; mean value of resting HR was 69.6 bpm) [14, 25, 51, 57, 63]. To some extent, resting HR is a viable alternative measurement for monitoring physical fitness [103]. Therefore, the effect of HIIT on resting HR is more prominent in participants with poor physical fitness.
There is ongoing debate regarding the effectiveness of HIIT in reducing BP among older adults. A recent meta-analysis demonstrated that HIIT elicited a significant reduction in the BP of older adults when compared with the no-intervention group, whereas HIIT and MICT resulted in similar reductions in BP [32]. However, another study reported no correlation between HIIT and reductions in BP among older adults [28]. In this review, compared with the other-exercise intervention and no-intervention controls, HIIT showed a greater lowering effect on SBP. The characteristics of the included participants may be one of the reasons for these inconsistent results. In our study, the participants comprised both older adults with cardiovascular diseases or type diabetes and those without, whereas the HIIT group in the previous study that reported no significant effect included only healthy individuals. The possible mechanism of the BP-lowering effect of HIIT may involve an intensity-dependent increase in blood flow velocity, resulting in elevated levels of endothelial nitric oxide (NO) [104]. This increase in endothelial NO availability and bioactivity enhances NO-dependent vasodilation in the vasculature, leading to improved peripheral compliance and reduced BP [105]. Furthermore, our meta-regression analysis showed that the effect of HIIT on SBP declined with age. Age-related declines in physical fitness and exercise capacity may play a role in the observed decline in the effect of HIIT on SBP. As individuals age, they may experience declines in muscle mass, strength, and endurance, which could potentially reduce their ability to perform high-intensity exercise and experience the full benefits of HIIT [106, 107]. This explanation is somewhat speculative, as the exact mechanisms behind the effectiveness of HIIT in older populations are not fully understood. Overall, our study suggests that HIIT is a promising physical training intervention to improve SBP in older adults.
In terms of body composition, we found that HIIT significantly improved BF% compared with no-intervention controls yet led to no significant changes in BMI and WC. This finding is echoed by a recent systematic review and meta-analysis conducted by Wu et al. [28], which reported a large effect of HIIT on BF% (WMD = -0.97) but no significant change in BMI in older adults compared with the control group. Regarding WC, an umbrella review reported that HIIT caused no significant improvement in this parameter across the lifespan [108]. A systematic review and meta-analysis by Batacan et al. also suggested that HIIT is an effective approach for reducing BF% in overweight or obese populations [99]. A possible explanation for this result might be HIIT induced the increased activity of catecholamines, which contributes to enhancing fat oxidation and releasing fat from visceral fat storage [109, 110]. Another possible explanation for this is the development of an elevated fat loss state due to decreased appetite and increased lipid metabolism for exercise recovery after the HIIT intervention [111, 112]. Although an observed decline in BF% was identified, there was no noticeable change in BMI, which may be explained by the gain in muscle mass [99]. HIIT appears to be effective in reducing body fat while simultaneously increasing muscle mass, likely due to multiple factors involving a range of physiological adaptations beyond just the afterburn effect (excess post-exercise oxygen consumption or EPOC) [113, 114].
The TUG test is a widely used tool for assessing functional balance in fall risk assessments [115]. It is recommended by the American Geriatrics Society and the British Geriatric Society as an instrument for measuring fall risk [116]. The present review found that HIIT had a moderate yet significant effect on balance compared with no-intervention controls, which is in line with previous studies [28, 30]. Notably, four studies in this review measured the TUG test outcome and one study measured the one-leg stand time (OLST). Two of these studies applied sprint interval training as the HIIT protocol [80, 85], and three studies conducted HIIT with jump-based aquatic exercise [64], cycling [69], or treadmill walking [71]. The dynamic balance and lower limb muscle strength resulting from the HIIT intervention may explain the improvement of balance capacity observed among older adults [117]. Additionally, HIIT had a significant small effect on MS compared with other-exercise intervention and no-intervention controls, which is in line with previous studies [28, 30]. These improvements may be due to the high-intensity nature of the exercise, which places greater demands on the muscles and can lead to adaptations such as increased muscle fibre recruitment, improved neuromuscular function, and increased muscle hypertrophy [79]. In comparison, other-exercise interventions typically engaged in low-to-moderate intensity exercise such as walking or cycling [14, 23, 79, 87], which may not provide the same level of stimulus to the muscles as HIIT. Thus, HIIT showed better benefits for MS. Regarding ME, HIIT showed nonsignificant overall effects compared with other-exercise interventions. These results are in line with those obtained in the study by Stern et al. [30] involving older adults aged 50 years and above, which concluded that the effect of HIIT vs. MICT on the STS was nonsignificant. A potential limitation to interpreting this result is that the intensities of HIIT and MICT protocols were inconsistent. One study [23] used low-volume HIIT (i.e., six sessions of 1-min intervals at 90% HRR with 2-min active recovery at 40% HRR) in comparison with MICT at 55%HRR for 50 min; another study [14] applied formal HIIT with four sets of 4-min intervals at 85-95% HRmax with 4-min recovery at 65% HRmax compared with MICT at 55-75%HRmax for 30 min. It remains unclear whether it is necessary to equalise energy expenditure or workload in comparative studies because of the possible differences in protocol intensities and programming [53]. We did not conduct meta-analyses of evidence on MP and flexibility due to the limited number of relevant studies. Regarding MP, two studies showed small beneficial effects of HIIT on leg power [72] and stair climb power [85] compared with no-intervention controls. Regarding flexibility, HIIT showed significant improvement when compared with no-intervention controls [69], but the difference was nonsignificant when compared with MICT [23, 69]. Overall, it is possible to improve balance and MS in older adults through HIIT.
Limitations and Future Directions
Several limitations that could impact the results of this meta-analysis must be taken into consideration. (1) The GRADE quality of evidence reported in this study was generally low to moderate, and two potential factors caused these ratings to constrain the results’ certainty. First, the quality of the included studies was mixed. Most studies were at low risk of bias or had some concerns regarding quality (38/44) and had relatively small sample sizes. Therefore, the results should be interpreted with caution. Additional studies, particularly high-quality RCTs, are required to confirm out results. Second, given the generally low quality of grey literature (e.g., theses or dissertations), we did not include this type of literature, which might have caused potential publication bias [118]. In future reviews, diverse types of literature should be included to obtain more robust findings. (2) The results of meta-regression analyses of SBP and resting HR seemed to contradict common expectations. The underlying reasons for this are complex and should be further disentangled in future studies. They may relate to the quality of the included studies, including heterogeneity, and the difference in HIIT protocols. Future research can further explore the reasons for the contradiction. (3) Although we split the sample size of the shared group [78, 80] to address the unit-of-analysis problem during meta-analysis, the calculated effect sizes remained correlated [119]. Future studies should use advanced approaches (e.g. three-level meta-analysis model) to address this problem [119]. (4) Despite the inclusion of diverse indicators, some outcomes were rarely reported, namely MP, ME, and flexibility; therefore, the impact of HIIT on these outcomes remains unclear. Further high-quality studies should be implemented with a focus on the influence of HIIT on these outcomes.
The findings of our review suggest that HIIT, irrespective of sex and health status, can potentially improve physical fitness and health (e.g., resting HR, resting BP, CRF) in older adults. More specifically, our findings suggest that there are similar HIIT training-induced outcomes, irrespective of sex (male ratio), health status (clinical, non-clinical), duration (< 12 weeks, ≥ 12 weeks), frequency (< 3 times/weeks, ≥ 3 times/weeks), exercise mode (cycling, treadmill, others) and recovery mode (active, passive). Though there was still much variation in HIIT protocols, some components appeared in the literature more often, e.g. frequency is usually 2 times/week or 3 times/week; duration is usually 8 weeks or 12 weeks; exercise mode is usually cycling or treadmill mode; and WRR is usually 1:1 or 4:3. Future studies should compare these and other protocols for outcomes related to physical fitness and health as well as feasibility and tolerability.
Conclusion
The significance of health-related physical fitness for older adults stems from its potential to uphold their physical independence, reduce the risk of chronic diseases, foster mental well-being, and augment social connections. The current systematic review and meta-analysis showed that HIIT may serve as a more efficacious intervention than other-exercise interventions or no-intervention controls in enhancing older adults’ physical fitness and health-related indicators, particularly in terms of improving their resting HR, SBP, CRF, BF%, MS, ME and balance. Our meta-regression analysis suggests that age may be a significant moderator for HR and SBP when compared to a non-exercise control group. Overall, this review demonstrates the need for more high-quality empirical studies specifically focused on understanding how participant characteristics, such as age, influence the effectiveness of HIIT interventions. Further investigation in this area could help optimize HIIT protocols to achieve the greatest benefits for older adults.
Data Availability
The datasets used and/or analyses during the current study are available from the corresponding authors on reasonable request.
Abbreviations
- WHO:
-
World Health Organization
- ACSM:
-
American College of Sport Medicine
- CRF:
-
Cardiorespiratory fitness
- BMI:
-
Body mass index
- BF%:
-
Body fat percent
- WC:
-
Waist circumference
- ME:
-
Muscular endurance
- MS:
-
Muscular strength
- MP:
-
Muscular power
- HR:
-
Heart rate
- BP:
-
Blood pressure
- MICT:
-
Moderate-intensity continuous training
- HIIT:
-
High-intensity interval training
- HRmax :
-
Maximum heart rate
- STS:
-
Sit-to-stand test
- TUG:
-
Timed up and go
- SMD:
-
Standardized mean difference
- 6MWT:
-
6-min walking test
- RCTs:
-
Randomized controlled trials
- SBP:
-
Systolic blood pressure
- DBP:
-
Diastolic blood pressure
- PRISMA:
-
Preferred Reporting Items for Systematic reviews and Meta-Analyses
- VO2 :
-
Oxygen uptake
- HRR:
-
Heart rate reserve
- VO2max :
-
Maximum oxygen uptake
- VO2peak :
-
Peak oxygen uptake
- RoB2:
-
Version 2 of the Cochrane Risk of Bias Tool
- GRADE:
-
Grading of Recommendations Assessment, Development and Evaluation
- CIs:
-
Confidence intervals
- SD:
-
Standard deviation
- Corr:
-
Correlation coefficient
- RT:
-
Resistance training
- CT:
-
Combined training
- HICT:
-
High-intensity continuous training
- WMD:
-
Weighted mean difference
- MD:
-
Mean difference
- NO:
-
Nitric oxide
- EPOC:
-
Excess post-exercise oxygen consumption
- OLST:
-
One-leg stand time
- ECG:
-
Electrocardiogram
- PPG:
-
Photoplethysmogram
References
Organization WH. Ageing 2022 [cited 2023 Jan]. https://www.who.int/health-topics/ageing#tab=tab_1
Singh-Manoux A, Dugravot A, Kauffmann F, Elbaz A, Ankri J, Nabi H, et al. Association of lung function with physical, mental and cognitive function in early old age. Age. 2011;33(3):385–92. https://doi.org/10.1007/s11357-010-9189-x. Epub 2010/09/30.
Harada K, Lee S, Lee S, Bae S, Harada K, Shimada H. Changes in objectively measured outdoor time and physical, psychological, and cognitive function among older adults with cognitive impairments. Arch Gerontol Geriatr. 2018;78:190–5. https://doi.org/10.1016/j.archger.2018.06.003. Epub 2018/07/15.
Medicine ACoS. ACSM’s guidelines for exercise testing and prescription, 11th Edition. Ringgold, Inc; 2021.
Reid KF, Fielding RA. Skeletal muscle power: a critical determinant of physical functioning in older adults. Exerc Sport Sci Rev. 2012;40(1):4–12. https://doi.org/10.1097/JES.0b013e31823b5f13. PubMed PMID: 22016147; PubMed Central PMCID: PMC3245773.
Katula JA, Rejeski WJ, Marsh AP. Enhancing quality of life in older adults: a comparison of muscular strength and power training. Health Qual Life Outcomes. 2008;6:45. https://doi.org/10.1186/1477-7525-6-45. Epub 20080613.
Garber CE, Blissmer B, Deschenes MR, Franklin BA, Lamonte MJ, Lee IM, et al. American College of Sports Medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Med Sci Sports Exerc. 2011;43(7):1334–59. https://doi.org/10.1249/MSS.0b013e318213fefb. PubMed PMID: 21694556.
Kerr J, Rosenberg D, Frank L. The role of the built environment in healthy aging: community design, physical activity, and health among older adults. J Plan Lit. 2012;27(1):43–60.
Bauman A, Merom D, Bull FC, Buchner DM, Fiatarone Singh MA. Updating the evidence for physical activity: summative reviews of the epidemiological evidence, prevalence, and interventions to promote active aging. Gerontologist. 2016;56(Suppl2):S268–80.
Manini TM, Pahor M. Physical activity and maintaining physical function in older adults. Br J Sports Med. 2009;43(1):28–31. Epub 20081016. https://doi.org/10.1136/bjsm.2008.053736. PubMed PMID: 18927164; PubMed Central PMCID: PMC3104323.
Keating CJ, Parraga Montilla JA, Latorre Roman PA, Moreno Del Castillo R. Comparison of high-intensity interval training to moderate-intensity continuous training in older adults: a systematic review. J Aging Phys Act. 2020:1–10. Epub 2020/04/18. https://doi.org/10.1123/japa.2019-0111. PubMed PMID: 32303000.
Keating SE, Johnson NA, Mielke GI, Coombes JS. A systematic review and meta-analysis of interval training versus moderate-intensity continuous training on body adiposity. Obes Rev. 2017;18(8):943–64. https://doi.org/10.1111/obr.12536. Epub 20170517.
Costa EC, Hay JL, Kehler DS, Boreskie KF, Arora RC, Umpierre D et al. Effects of high-intensity interval training versus moderate-intensity continuous training on blood pressure in adults with pre- to established hypertension: a systematic review and meta-analysis of randomized trials. Sports Medicine. 2018;48(9):2127-42. Epub 2018/06/28. https://doi.org/10.1007/s40279-018-0944-y. PubMed PMID: 29949110.
Coswig VS, Barbalho M, Raiol R, Del Vecchio FB, Ramirez-Campillo R, Gentil P. Effects of high vs moderate-intensity intermittent training on functionality, resting heart rate and blood pressure of elderly women. J Transl Med. 2020;18:1–11. https://doi.org/10.1186/s12967-020-02261-8. Epub 2020/02/19.
Poon ET, Wongpipit W, Ho RS, Wong SH. Interval training versus moderate-intensity continuous training for cardiorespiratory fitness improvements in middle-aged and older adults: a systematic review and meta-analysis. J Sports Sci. 2021;39(17):1996–2005. https://doi.org/10.1080/02640414.2021.1912453. Epub 20210407.
Thum JS, Parsons G, Whittle T, Astorino TA. High-intensity interval training elicits higher enjoyment than moderate intensity continuous Exercise. PLoS ONE. 2017;12(1):e0166299. https://doi.org/10.1371/journal.pone.0166299. Epub 20170111.
Gibala MJ, Little JP, Macdonald MJ, Hawley JA. Physiological adaptations to low-volume, high-intensity interval training in health and disease. J Physiol. 2012;590(5):1077–84. https://doi.org/10.1113/jphysiol.2011.224725. Epub 2012/02/01.
Coates AM, Joyner MJ, Little JP, Jones AM, Gibala MJ. A perspective on high-intensity interval training for performance and health. Sports Med. 2023;53(Suppl 1):85–96. https://doi.org/10.1007/s40279-023-01938-6. Epub 20231007.
Marriott CFS, Petrella AFM, Marriott ECS, Boa Sorte Silva NC, Petrella RJ. High-intensity interval training in older adults: a scoping review. Sports Medicine-Open. 2021;7(1):49. https://doi.org/10.1186/s40798-021-00344-4. Epub 2021/07/20.
Martland R, Korman N, Firth J, Vancampfort D, Thompson T, Stubbs B. Can high-intensity interval training improve mental health outcomes in the general population and those with physical illnesses? A systematic review and meta-analysis. Br J Sports Med. 2022;56(5):279–91. https://doi.org/10.1136/bjsports-2021-103984. Epub 2021/09/18.
Briggs BC, Ryan AS, Sorkin JD, Oursler KK. Feasibility and effects of high-intensity interval training in older adults living with HIV. J Sports Sci. 2021;39(3):304–11. PubMed PMID: 32962523; PubMed Central PMCID: PMC8212165.
Currie KD, Dubberley JB, McKelvie RS, MacDonald MJ. Low-volume, high-intensity interval training in patients with CAD. Med Sci Sports Exerc. 2013;45(8):1436–42. https://doi.org/10.1249/MSS.0b013e31828bbbd4. Epub 2013/03/09.
Boukabous I, Marcotte-Chenard A, Amamou T, Boulay P, Brochu M, Tessier D, et al. Low-volume high-intensity interval training (HIIT) versus moderate-intensity continuous training on body composition, cardiometabolic profile and physical capacity in older women. J Aging Phys Act. 2019;27(4):879–89. https://doi.org/10.1123/japa.2018-0309. Epub 2019/04/30.
Nilsson BB, Westheim A, Risberg MA. Long-term effects of a group-based high-intensity aerobic interval-training program in patients with chronic heart failure. Am J Cardiol. 2008;102(9):1220–4. https://doi.org/10.1016/j.amjcard.2008.06.046. Epub 2008/10/23.
Boa Sorte Silva NC, Petrella AFM, Christopher N, Marriott CFS, Gill DP, Owen AM, et al. The benefits of high-intensity interval training on cognition and blood pressure in older adults with hypertension and subjective cognitive decline: results from the heart & mind study. Front Aging Neurosci. 2021;13:643809. https://doi.org/10.3389/fnagi.2021.643809. Epub 2021/05/04.
Kovacevic A, Fenesi B, Paolucci E, Heisz JJ, Fenesi B, Paolucci E, et al. The effects of aerobic exercise intensity on memory in older adults. Appl Physiol Nutr Metab. 2020;45(6):591–600.
Jimenez-Garcia JD, Hita-Contreras F, de la Torre-Cruz MJ, Aibar-Almazan A, Achalandabaso-Ochoa A, Fabrega-Cuadros R, et al. Effects of HIIT and MIIT suspension training programs on sleep quality and fatigue in older adults: randomized controlled clinical trial. Int J Environ Res Public Health. 2021;18(3). https://doi.org/10.3390/ijerph18031211. PubMed PMID: 33572909; PubMed Central PMCID: PMC7908512. Epub 2021/02/13.
Wu ZJ, Wang ZY, Gao HE, Zhou XF, Li FH. Impact of high-intensity interval training on cardiorespiratory fitness, body composition, physical fitness, and metabolic parameters in older adults: a meta-analysis of randomized controlled trials. Exp Gerontol. 2021;150:111345. https://doi.org/10.1016/j.exger.2021.111345. Epub 2021/04/10.
McLeod KA, Jones MD, Thom JM, Parmenter BJ. Resistance training and high-intensity interval training improve cardiometabolic health in high risk older adults: a systematic review and meta-anaylsis. Int J Sports Med. 2022;43(3):206–18. https://doi.org/10.1055/a-1560-6183. Epub 2021/07/29.
Stern G, Psycharakis SG, Phillips SM. Effect of High-Intensity Interval Training on Functional Movement in older adults: a systematic review and Meta-analysis. Sports Medicine-Open. 2023;9(1):5. https://doi.org/10.1186/s40798-023-00551-1. Epub 2023/01/16.
Hedges LV. Distribution theory for Glass’s estimator of effect size and related estimators. J Educ Stat. 1981;6(2):107–28.
Carpes L, Costa R, Schaarschmidt B, Reichert T, Ferrari R. High-intensity interval training reduces blood pressure in older adults: a systematic review and meta-analysis. Exp Gerontol. 2022;158:111657. https://doi.org/10.1016/j.exger.2021.111657. Epub 2021/12/19.
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;88:105906. https://doi.org/10.1016/j.ijsu.2021.105906. Epub 20210329.
Norton K, Norton L, Sadgrove D. Position statement on physical activity and exercise intensity terminology. J Sci Med Sport. 2010;13(5):496–502. https://doi.org/10.1016/j.jsams.2009.09.008. Epub 20091210.
Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al. Cochrane handbook for systematic reviews of interventions. Hoboken: Wiley; 2019.
Group GW. Grading quality of evidence and strength of recommendations. BMJ. 2004;328(7454):1490.
King A, Helms E, Zinn C, Jukic I. The ergogenic effects of acute carbohydrate feeding on resistance exercise performance: a systematic review and meta-analysis. Sports Med. 2022;52(11):2691–712. https://doi.org/10.1007/s40279-022-01716-w. Epub 20220709.
Cohen J. Statistical power analysis for the behavioral sciences. Routledge; 2013.
Acuna E, Rodriguez C. A meta analysis study of outlier detection methods in classification. Technical paper, Department of Mathematics, University of Puerto Rico at Mayaguez. 2004;1:25.
Williams G, Hamm MP, Shulhan J, Vandermeer B, Hartling L. Social media interventions for diet and exercise behaviours: a systematic review and meta-analysis of randomised controlled trials. BMJ Open. 2014;4(2):e003926. https://doi.org/10.1136/bmjopen-2013-003926. Epub 2014/02/15.
Hedges LV, Vevea JL. Fixed-and random-effects models in meta-analysis. Psychol Methods. 1998;3(4):486.
Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–58.
Borenstein M, Higgins JP, Hedges LV, Rothstein HR. Basics of meta-analysis: I(2) is not an absolute measure of heterogeneity. Res Synth Methods. 2017;8(1):5–18. https://doi.org/10.1002/jrsm.1230. Epub 20170106.
Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ Open. 1997;315(7109):629–34.
Vevea JL, Woods CM. Publication bias in research synthesis: sensitivity analysis using a priori weight functions. Psychol Methods. 2005;10(4):428.
Harrer M, Cuijpers P, Furukawa T, Ebert DDJR. dmetar: Companion R package for the guide’Doing meta-analysis in R’. 2019;9000.
Wickham H, Averick M, Bryan J, Chang W, McGowan LDA, François R, et al. Welcome to the Tidyverse. J Open Source Softw. 2019;4(43):1686.
Schwarzer G. meta: an R package for meta-analysis. R news. 2007;7(3):40–5.
Viechtbauer W. Conducting meta-analyses in R with the metafor package. J Stat Softw. 2010;36(3):1–48.
Coburn KM, Vevea JL, Coburn MKM. Package ‘weightr’. Estimating weight-function models for publication bias; 2019.
Wisløff U, Stoylen A, Loennechen JP, Bruvold M, Rognmo O, Haram PM, et al. Superior cardiovascular effect of aerobic interval training versus moderate continuous training in heart failure patients: a randomized study. Circulation. 2007;115(24):3086–94. https://doi.org/10.1161/CIRCULATIONAHA.106.675041. Epub 2007/06/06.
Fu TC, Wang CH, Lin PS, Hsu CC, Cherng WJ, Huang SC, et al. Aerobic interval training improves oxygen uptake efficiency by enhancing cerebral and muscular hemodynamics in patients with heart failure. Int J Cardiol. 2013;167(1):41–50. PubMed PMID: 22197120.
Angadi SS, Mookadam F, Lee CD, Tucker WJ, Haykowsky MJ, Gaesser GA. High-intensity interval training vs. moderate-intensity continuous exercise training in heart failure with preserved ejection fraction: a pilot study. J Appl Physiol. 2015;119(6):753–8. https://doi.org/10.1152/japplphysiol.00518.2014. Epub 2014/09/06.
Spee RF, Niemeijer VM, Schoots T, Tuinenburg A, Houthuizen P, Wijn PF, et al. High intensity interval training after cardiac resynchronization therapy: an explorative randomized controlled trial. Int J Cardiol. 2020;299:169–74. https://doi.org/10.1016/j.ijcard.2019.07.023. Epub 2019/07/30.
Reed JL, Terada T, Vidal-Almela S, Tulloch HE, Mistura M, Birnie DH, et al. Effect of high-intensity interval training in patients with atrial fibrillation: a randomized clinical trial. JAMA Netw Open. 2022;5(10):e2239380. https://doi.org/10.1001/jamanetworkopen.2022.39380. Epub 2022/11/01.
Rognmo O, Hetland E, Helgerud J, Hoff J, Slordahl SA. High intensity aerobic interval exercise is superior to moderate intensity exercise for increasing aerobic capacity in patients with coronary artery disease. Eur J Prev Cardiol. 2004;11(3):216 – 22. Epub 2004/06/05. https://doi.org/10.1097/01.hjr.0000131677.96762.0c. PubMed PMID: 15179103.
Iellamo F, Caminiti G, Sposato B, Vitale C, Massaro M, Rosano G, et al. Effect of high-intensity interval training versus moderate continuous training on 24-h blood pressure profile and insulin resistance in patients with chronic heart failure. Intern Emerg Med. 2014;9(5):547–52. https://doi.org/10.1007/s11739-013-0980-4. Epub 2013/07/17.
de Matos DG, de Almeida-Neto PF, Moreira OC, de Souza RF, Tinoco Cabral BGA, Chilibeck P, et al. Two weekly sessions of high-intensity interval training improve metabolic syndrome and hypertriglyceridemic waist phenotype in older adults: a randomized controlled trial. Metab Syndr Relat Disord. 2021;19(6):332–9. https://doi.org/10.1089/met.2020.0136. Epub 2021/03/25.
Hwang CL, Lim J, Yoo JK, Kim HK, Hwang MH, Handberg EM, et al. Effect of all-extremity high-intensity interval training vs. moderate-intensity continuous training on aerobic fitness in middle-aged and older adults with type 2 diabetes: a randomized controlled trial. Exp Gerontol. 2019;116:46–53. PubMed PMID: 30576716; PubMed Central PMCID: PMC6404965.
Izadi MR, Ghardashi Afousi A, Asvadi Fard M, Babaee Bigi MA. High-intensity interval training lowers blood pressure and improves apelin and NOx plasma levels in older treated hypertensive individuals. J Physiol Biochem. 2018;74(1):47–55. https://doi.org/10.1007/s13105-017-0602-0. Epub 2017/12/08.
Krawcyk RS, Vinther A, Petersen NC, Faber J, Iversen HK, Christensen T, et al. Effect of home-based high-intensity interval training in patients with lacunar stroke: a randomized controlled trial. Front Neurol. 2019;10:664. PubMed PMID: 31316451; PubMed Central PMCID: PMC6611174.
Mekari S, Neyedli HF, Fraser S, O’Brien MW, Martins R, Evans K, et al. High-intensity interval training improves cognitive flexibility in older adults. Brain Sci. 2020;10(11). https://doi.org/10.3390/brainsci10110796. PubMed PMID: 33137993; PubMed Central PMCID: PMC7693870. Epub 2020/11/04.
O’Brien MW, Johns JA, Robinson SA, Bungay A, Mekary S, Kimmerly DS. Impact of high-intensity interval training, moderate-intensity continuous training, and resistance training on endothelial function in older adults. Med Sci in Sports Exerc. 2020;52(5):1057–67. Epub 2019/12/27. https://doi.org/10.1249/MSS.0000000000002226. PubMed PMID: 31876667.
Aboarrage Junior AM, Teixeira CVS, Dos Santos RN, Machado AF, Evangelista AL, Rica RL, et al. A high-intensity jump-based aquatic exercise program improves bone mineral density and functional fitness in postmenopausal women. Rejuvenation Res. 2018;21(6):535–40. https://doi.org/10.1089/rej.2018.2069. Epub 2018/06/12.
Nunes PRP, Martins FM, Souza AP, Carneiro MAS, Orsatti CL, Michelin MA, et al. Effect of high-intensity interval training on body composition and inflammatory markers in obese postmenopausal women: a randomized controlled trial. Menopause. 2019;26(3):256–64. 10.1097. Epub 2018/10/03.
Klonizakis M, Moss J, Gilbert S, Broom D, Foster J, Tew GA. Low-volume high-intensity interval training rapidly improves cardiopulmonary function in postmenopausal women. Menopause. 2014;21(10):1099–105.
Bruseghini P, Tam E, Calabria E, Milanese C, Capelli C, Galvani C. High intensity interval training does not have compensatory effects on physical activity levels in older adults. Int J Environ Res Public Health. 2020;17(3). https://doi.org/10.3390/ijerph17031083. PubMed PMID: 32046311; PubMed Central PMCID: PMC7037169. Epub 2020/02/13.
Hwang CL, Yoo JK, Kim HK, Hwang MH, Handberg EM, Petersen JW, et al. Novel all-extremity high-intensity interval training improves aerobic fitness, cardiac function and insulin resistance in healthy older adults. Exp Gerontol. 2016;82:112–9. https://doi.org/10.1016/j.exger.2016.06.009. Epub 2016/06/28.
Li X, Han T, Zou X, Zhang H, Feng W, Wang H, et al. Long-term high-intensity interval training increases serum neurotrophic factors in elderly overweight and obese Chinese adults. Eur J Appl Physiol. 2021;121(10):2773–85. Epub 2021/06/21. https://doi.org/10.1007/s00421-021-04746-w. PubMed PMID: 34148146.
Wyckelsma VL, Levinger I, Murphy RM, Petersen AC, Perry BD, Hedges CP, et al. Intense interval training in healthy older adults increases skeletal muscle [(3)H]ouabain-binding site content and elevates na(+),K(+)-ATPase alpha2 isoform abundance in type II fibers. Physiol Rep. 2017;5(7). https://doi.org/10.14814/phy2.13219. PubMed PMID: 28373411; PubMed Central PMCID: PMC5392511. Epub 2017/04/05.
Coetsee C, Terblanche E. The effect of three different exercise training modalities on cognitive and physical function in a healthy older population. Eur Rev Aging Phys Act. 2017;14:1–10. https://doi.org/10.1186/s11556-017-0183-5. Epub 2017/08/16.
Hurst C, Weston KL, Weston M. The effect of 12 weeks of combined upper- and lower-body high-intensity interval training on muscular and cardiorespiratory fitness in older adults. Aging Clin Exp Res. 2019;31(5):661–71. https://doi.org/10.1007/s40520-018-1015-9. Epub 2018/07/28.
Jimenez-Garcia JD, Martinez-Amat A, De la Torre-Cruz MJ, Fabrega-Cuadros R, Cruz-Diaz D, Aibar-Almazan A, et al. Suspension training HIIT improves gait speed, strength and quality of life in older adults. Int J Sports Med. 2019;40(2):116–24. https://doi.org/10.1055/a-0787-1548. Epub 2019/01/04.
Kim HK, Hwang CL, Yoo JK, Hwang MH, Handberg EM, Petersen JW, et al. All-extremity exercise training improves arterial stiffness in older adults. Med Sci Sports Exerc. 2017;49(7):1404–11. https://doi.org/10.1249/MSS.0000000000001229. Epub 2017/02/07.
Ballin M, Lundberg E, Sorlen N, Nordstrom P, Hult A, Nordstrom A. Effects of interval training on quality of life and cardiometabolic risk markers in older adults: a randomized controlled trial. Clin Interv Aging. 2019;14:1589–99. PubMed PMID: 31564841; PubMed Central PMCID: PMC6732517.
Ballesta-Garcia I, Martinez-Gonzalez-Moro I, Ramos-Campo DJ, Carrasco-Poyatos M. High-intensity interval circuit training versus moderate-intensity continuous training on cardiorespiratory fitness in middle-aged and older women: a randomized controlled trial. Int J Environ Res Public Health. 2020;17(5). Epub 2020/03/14. https://doi.org/10.3390/ijerph17051805. PubMed PMID: 32164314; PubMed Central PMCID: PMC7084372.
Herrod PJJ, Lund JN, Phillips BE. Time-efficient physical activity interventions to reduce blood pressure in older adults: a randomised controlled trial. Age Ageing. 2021;50(3):980–4. https://doi.org/10.1093/ageing/afaa211. Epub 2020/10/18.
Sian TS, Inns TB, Gates A, Doleman B, Bass JJ, Atherton PJ, et al. Equipment-free, unsupervised high intensity interval training elicits significant improvements in the physiological resilience of older adults. BMC Geriatr. 2022;22(1):1–11. https://doi.org/10.1186/s12877-022-03208-y. Epub 2022/06/28.
Simonsson E, Levik Sandstrom S, Hedlund M, Holmberg H, Johansson B, Lindelof N et al. Effects of controlled supramaximal high-intensity interval training on cardiorespiratory fitness and global cognitive function in older adults: the Umea HIT study-a randomized controlled trial. J Gerontol A. 2023. Epub 2023/03/28. https://doi.org/10.1093/gerona/glad070. PubMed PMID: 36972981.
Adamson S, Kavaliauskas M, Lorimer R, Babraj J. The impact of Sprint interval training frequency on blood glucose control and physical function of older adults. Int J Environ Res Public Health. 2020;17(2):454. https://doi.org/10.3390/ijerph17020454. Epub 2020/01/16.
Brown BM, Frost N, Rainey-Smith SR, Doecke J, Markovic S, Gordon N, et al. High-intensity exercise and cognitive function in cognitively normal older adults: a pilot randomised clinical trial. Alzheimers Res Ther. 2021;13(1):33. https://doi.org/10.1186/s13195-021-00774-y. Epub 2021/02/02.
Northey JM, Pumpa KL, Quinlan C, Ikin A, Toohey K, Smee DJ, et al. Cognition in breast cancer survivors: a pilot study of interval and continuous exercise. J Sci Med Sport. 2019;22(5):580–5. PubMed PMID: 30554923.
Terada T, Friesen A, Chahal BS, Bell GJ, McCargar LJ, Boule NG. Feasibility and preliminary efficacy of high intensity interval training in type 2 diabetes. Diabetes Res Clin Pract. 2013;99(2):120–9. https://doi.org/10.1016/j.diabres.2012.10.019. Epub 2012/11/28.
Varga J, Porszasz J, Boda K, Casaburi R, Somfay A. Supervised high intensity continuous and interval training vs. self-paced training in COPD. Respir Med. 2007;101(11):2297–304. https://doi.org/10.1016/j.rmed.2007.06.017. Epub 2007/08/11.
Adamson S, Kavaliauskas M, Yamagishi T, Phillips S, Lorimer R, Babraj J. Extremely short duration sprint interval training improves vascular health in older adults. Sport Sci Health. 2019;15(1):123–31. https://doi.org/10.1007/s11332-018-0498-2.
Maillard F, Rousset S, Pereira B, Traore A, de Pradel Del Amaze P, Boirie Y, et al. High-intensity interval training reduces abdominal fat mass in postmenopausal women with type 2 diabetes. Diabetes & Metabolism. 2016;42(6):433–41. https://doi.org/10.1016/j.diabet.2016.07.031. Epub 2016/08/28.
Santos GOR, Wolf R, Silva MM, Rodacki ALF, Pereira G. Does exercise intensity increment in exergame promote changes in strength, functional capacity and perceptual parameters in pre-frail older women? A randomized controlled trial. Exp Gerontol. 2019;116:25–30. https://doi.org/10.1016/j.exger.2018.12.009. Epub 2018/12/16.
Atakan MM, Li Y, Kosar SN, Turnagol HH, Yan X. Evidence-based effects of high-intensity interval training on exercise capacity and health: a review with historical perspective. Int J Environ Res Public Health. 2021;18(13). https://doi.org/10.3390/ijerph18137201. PubMed PMID: 34281138; PubMed Central PMCID: PMC8294064. Epub 2021/07/21.
Borresen J, Lambert MI. Quantifying training load: a comparison of subjective and objective methods. Int J Sports Physiol Perform. 2008;3(1):16–30.
Fisher KM, Fuller L, Chandler JP. A review of the relationship between heart rate monitoring, training load, and injury in field-based team sport athletes. Int J Sport Exerc Health Res. 2022;6:43–54.
Bouaziz W, Schmitt E, Kaltenbach G, Geny B, Vogel T. Health benefits of cycle ergometer training for older adults over 70: a review. Eur Rev Aging Phys Act. 2015;12:8. https://doi.org/10.1186/s11556-015-0152-9. Epub 2016/02/13.
Granacher U, Muehlbauer T, Gruber M. A qualitative review of balance and strength performance in healthy older adults: impact for testing and training. J Aging Res. 2012;2012. Epub 20120123. https://doi.org/10.1155/2012/708905. PubMed PMID: 22315687; PubMed Central PMCID: PMC3270412.
Zouhal H, Abderrahman AB, Jayavel A, Hackney AC, Laher I, Saeidi A, et al. Effects of passive or active recovery regimes applied during long-term interval training on physical fitness in healthy trained and untrained individuals: a systematic review. Sports Med Open. 2024;10(1):21. https://doi.org/10.1186/s40798-024-00673-0. Epub 20240305.
Martland R, Mondelli V, Gaughran F, Stubbs B. Can high intensity interval training improve health outcomes among people with mental illness? A systematic review and preliminary meta-analysis of intervention studies across a range of mental illnesses. J Affect Disord. 2020;263:629–60. PubMed PMID: 31780128.
Bouaziz W, Malgoyre A, Schmitt E, Lang PO, Vogel T, Kanagaratnam L. Effect of high-intensity interval training and continuous endurance training on peak oxygen uptake among seniors aged 65 or older: a meta-analysis of randomized controlled trials. Int J Clin Pract Suppl. 2020;74(6):e13490. https://doi.org/10.1111/ijcp.13490. Epub 2020/02/23.
Daussin FN, Ponsot E, Dufour SP, Lonsdorfer-Wolf E, Doutreleau S, Geny B, et al. Improvement of by cardiac output and oxygen extraction adaptation during intermittent versus continuous endurance training. Eur J Appl Physiol. 2007;101(3):377–83. https://doi.org/10.1007/s00421-007-0499-3. Epub 2007/07/31.
Phillips SM, Green HJ, Tarnopolsky MA, Grant SM. Increased clearance of lactate after short-term training in men. J Appl Physiol. 1995;79(6):1862–9. https://doi.org/10.1152/jappl.1995.79. Epub 1995/12/01.
Yue T, Wang Y, Liu H, Kong Z, Qi F. Effects of high-intensity interval vs. moderate-intensity continuous training on cardiac rehabilitation in patients with cardiovascular disease: a systematic review and meta-analysis. Front Cardiovasc Med. 2022;9:845225. https://doi.org/10.3389/fcvm.2022.845225. Epub 20220223.
Batacan RB Jr., Duncan MJ, Dalbo VJ, Tucker PS, Fenning AS. Effects of high-intensity interval training on cardiometabolic health: a systematic review and meta-analysis of intervention studies. Br J Sports Med. 2017;51(6):494–503. https://doi.org/10.1136/bjsports-2015-095841. Epub 2016/11/01.
Duncombe SL, Barker AR, Bond B, Earle R, Varley-Campbell J, Vlachopoulos D, et al. School-based high-intensity interval training programs in children and adolescents: a systematic review and meta-analysis. PLoS ONE. 2022;17(5):e0266427. https://doi.org/10.1371/journal.pone.0266427. Epub 2022/05/05.
Helgerud J, Hoydal K, Wang E, Karlsen T, Berg P, Bjerkaas M, et al. Aerobic high-intensity intervals improve VO2max more than moderate training. Med Sci Sports Exerc. 2007;39(4):665–71. https://doi.org/10.1249/mss.0b013e3180304570. Epub 2007/04/07.
Kiviniemi AM, Tulppo MP, Eskelinen JJ, Savolainen AM, Kapanen J, Heinonen IH, et al. Cardiac autonomic function and high-intensity interval training in middle-age men. Med Sci Sports Exerc. 2014;46(10):1960–7. Epub 2014/02/25.
Silva DAS, de Lima TR, Tremblay MS. Association between resting heart rate and health-related physical fitness in Brazilian adolescents. BioMed Res Int. 2018;2018. Epub 20180628. https://doi.org/10.1155/2018/3812197. PubMed PMID: 30050928; PubMed Central PMCID: PMC6046174.
Pal S, Radavelli-Bagatini S, Ho S. Potential benefits of exercise on blood pressure and vascular function. J Am Soc Hypertens. 2013;7(6):494–506. PubMed PMID: 23992766.
Green DJ, Maiorana A, O’Driscoll G, Taylor R. Effect of exercise training on endothelium-derived nitric oxide function in humans. J Physiol. 2004;561(Pt 1):1–25. https://doi.org/10.1113/jphysiol.2004.068197. Epub 2004/09/18.
Marzuca-Nassr GN, Artigas-Arias M, Olea MA, SanMartin-Calisto Y, Huard N, Duran-Vejar F, et al. High-intensity interval training on body composition, functional capacity and biochemical markers in healthy young versus older people. Exp Gerontol. 2020;141:111096. https://doi.org/10.1016/j.exger.2020.111096. Epub 20200922.
Sculthorpe NF, Herbert P, Grace F. One session of high-intensity interval training (HIIT) every 5 days, improves muscle power but not static balance in lifelong sedentary ageing men: a randomized controlled trial. Medicine. 2017;96(6):e6040. PubMed PMID: 28178145; PubMed Central PMCID: PMC5313002.
Martland R, Mondelli V, Gaughran F, Stubbs B. Can high-intensity interval training improve physical and mental health outcomes? A meta-review of 33 systematic reviews across the lifespan. J Sports Sci. 2020;38(4):430–69. PubMed PMID: 31889469.
Boutcher SH. High-intensity intermittent exercise and fat loss. J Obes. 2011;2011(1). https://doi.org/10.1155/2011/868305. Epub 2010/11/30. PubMed PMID: 21113312; PubMed Central PMCID: PMC2991639.
LaForgia J, Withers RT, Gore CJ. Effects of exercise intensity and duration on the excess post-exercise oxygen consumption. J Sports Sci. 2006;24(12):1247–64. Epub 2006/11/15. https://doi.org/10.1080/02640410600552064. PubMed PMID: 17101527.
Moniz SC, Islam H, Hazell TJ. Mechanistic and methodological perspectives on the impact of intense interval training on post-exercise metabolism. Scand J Med Sci Sports. 2020;30(4):638–51. Epub 20200103. https://doi.org/10.1111/sms.13610. PubMed PMID: 31830334.
Vanderheyden LW, McKie GL, Howe GJ, Hazell TJ. Greater lactate accumulation following an acute bout of high-intensity exercise in males suppresses acylated ghrelin and appetite postexercise. J Appl Physiol. 2020;128(5):1321–8.
Moniz SC, Islam H, Hazell TJ. Mechanistic and methodological perspectives on the impact of intense interval training on post-exercise metabolism. Scand J Med Sci Sports. 2020;30(4):638–51.
Ahlert M, Matzenbacher F, Albarello JCS, Halmenschlager GH. Comparison of epoc and recovery energy expenditure between Hiit and continuous aerobic exercise training. Revista Brasileira De Medicina do Esporte. 2019;25(1):20–3. https://doi.org/10.1590/1517-869220192501181346.
Elboim-Gabyzon M, Buxbaum R, Klein R. The effects of high-intensity interval training (HIIT) on fall risk factors in healthy older adults: a systematic review. Int J Environ Res Public Health. 2021;18(22):11809. https://doi.org/10.3390/ijerph182211809. Epub 20211111.
Diao Y, Lou N, Liang S, Zhang Y, Ning Y, Li G, et al. A novel environment-adaptive timed up and go test system for fall risk assessment with wearable inertial sensors. IEEE Sens J. 2021;21(16):18287–97. https://doi.org/10.1109/jsen.2021.3082982.
Chen T, Chou LS. Effects of muscle strength and balance control on sit-to-walk and turn durations in the timed up and go test. Arch Phys Med Rehabil. 2017;98(12):2471–6. https://doi.org/10.1016/j.apmr.2017.04.003. Epub 2017/05/04.
Bellefontaine SP, Lee CM. Between black and white: examining grey literature in meta-analyses of psychological research. J Child Fam Stud. 2013;23(8):1378–88. https://doi.org/10.1007/s10826-013-9795-1.
Harrer M, Cuijpers P, Furukawa TA, Ebert DD. Doing meta-analysis with R: a hands-on guide. Chapman and Hall/CRC; 2021.
Acknowledgements
Not applicable.
Funding
This research was funded by the Humanities and Social Science Fund of Ministry of Education of China (23YJCZH121), the Humanities and Social Sciences Revitalization Grant of Shenzhen University (WKZX0312), as well as the Jockey Club Mus-Fit for Health Project (Ref no.: 2021 − 0401) of Hong Kong Jockey Club Charities Trust. The funding organization had no role in the study design, study implementation, manuscript preparation, or publication decision. This work is the responsibility of the authors.
Author information
Authors and Affiliations
Contributions
Conception and design of study: WL, XW, YD; Retrieve articles and acquisition of data: XW, SSC, WL; Analysis and/or interpretation of data: XW, WL; Drafting the manuscript: WL, XW; Revising the manuscript critically for important intellectual content: WL, WX, JJ, XGZ, YD. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics Approval and Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Competing Interests
All authors declare that they have no conflicts of interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Liang, W., Wang, X., Cheng, S. et al. Effects of High-Intensity Interval Training on the Parameters Related to Physical Fitness and Health of Older Adults: A Systematic Review and Meta-Analysis. Sports Med - Open 10, 98 (2024). https://doi.org/10.1186/s40798-024-00767-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40798-024-00767-9