Abstract
Background
Iron deficiency (ID) and anaemia of inflammation (AI) coexist where infections and nutritional deficiencies are common. The aim of this study was to determine burden of ID, anaemia, inflammation and AI in children in malaria endemic Limbe, Mount Cameroon as well as decipher the contribution of some inflammatory cytokines on the concentration of haemoglobin and ferritin.
Methods
A total of 520 children aged ≤ 15 years old from the Limbe Health District (LHD) were randomly selected and examined in a cross-sectional study for iron deficiency, anaemia, inflammation and inflammation anaemia. Collected blood samples were used for full blood count and inflammatory marker analyses with the aid of a haemoanalyzer and ELISA machine, respectively. Spearman’s rank correlation analysis was used to determine the correlation between cytokines and haemoglobin while multiple linear regression analysis was used to evaluate the effects of inflammatory cytokines on haemoglobin and ferritin concentrations.
Results
The overall prevalence of anaemia, ID, IDA, inflammation and AI were respectively, 67.5%, 34.6%, 12.9%, 63.1% and 30.2%. Children aged 12‒15 years (P = 0.001), enrolled from the community (P < 0.001), whose parents are civil servants (P < 0.001), living in a home with 6‒10 occupants (P = 0.016), afebrile (P < 0.001) and malaria negative (P = 0.007) had the highest prevalence of ID while, children ≤ 5 years old (P = 0.001), with a family size of 1‒5 occupants (P = 0.033) had the highest prevalence of AI. Haemoglobin concentration positively correlated with concentrations of IFN-γ (P < 0.001), TNF-α (0.045) and ferritin (P < 0.001) while a negative correlation was observed with IL-10 (P = 0.003). In the multiple linear regression analysis only IL-6 significantly (P = 0.030) influenced haemoglobin concentration.
Conclusions
While IL-6 is of significance in the pathology of anaemia, iron deficiency and anaemia of inflammation are of moderate public health concerns in the Mount Cameroon area. Hence, appropriate intervention against anaemia, ID and AI should be directed at children ≤ 5 years and counterparts > 10 years old that bear the highest burden.
Similar content being viewed by others
Background
Iron deficiency (ID) is the primary cause of anaemia especially in low-income countries [1]. Over a billion people in all age groups suffer from it [2]. ID can be caused by inadequate intake of bioavailable iron or poor absorption of iron resulting from high intake of iron-inhibitory food or dietary fibres [3] or deficiency in other such micronutrients as folate and/vitamin B12, vitamin A [4], or genetic disorders. ID can be described as marginal when the production of iron-dependent proteins is compromised but haemoglobin levels are normal; mild when iron stores are depleted, and iron deficiency anaemia (IDA) when haemoglobin synthesis is reduced [5].
Anaemia resulting from iron deficiency is common among children and women especially in the developing countries [6]. Their deleterious effect on children both on the short-term and long terms have been well documented [7]. ID often goes unnoticed especially in patients who are not anaemic and/or do not complain of fatigue [8], thus, routine laboratory checks which requires measuring the ferritin concentration in blood is necessary [9]. Notwithstanding, ferritin is an acute phase protein (APP) highly influenced by inflammation and other infections [10], and its upshot on ID remains to be investigated in children resident in this malaria area of Mount Cameroon.
Inflammation is a protective innate immune response defence mechanism of the body whereby the immune system recognizes damaged cells, irritants, and pathogens [11]. Inflammation may not be detected if the infection is minor or if the immune system is apt in removing it (acute inflammation) thereby preventing disease. However, the changes that constitute inflammation are metabolically demanding and potentially destructive leading to conditions like cancer and diabetes (chronic inflammation) which in turn may lead to anaemia [12].
Anaemia ensuing from inflammation formerly called anaemia of chronic disease (ACD) is acute or chronic anaemia associated with conditions that cause inflammation including infections, cancer, autoimmune diseases, chronic kidney disease (CKD), and inflammatory bowel diseases (IBD) [10, 13, 14]. Although anaemia of inflammation (AI) is like ID in low serum iron (< 15 µg/L) [15], it is basically a disorder in iron distribution since macrophages of the liver, spleen and bone marrow responsible for recycling old red cells still retain their iron stores [16]. A person with AI thus will feel fatigued and intolerant to exercise [17] and consequently among children, it may affect their ability to study. AI coexists with ID in areas where nutritional deficiency and infections are common [18] and is considered the second cause of anaemia after IDA [19]. Up to 40% worldwide anaemia can be considered caused by AI or combined anaemia with AI contributing significantly [1, 20] and vary according to sex, age, geography and other disease prevalence [18]. The co-existence of ID and AI coupled with iron deficiency from malnutrition makes it difficult to get a specific diagnosis for ID [17] and therefore may lead to underestimation of the burden in a population.
In the Mount Cameroon area where diseases such as malaria and other infections abound, anaemia is almost inevitable. Previous studies conducted in this area have reported the prevalence and associated risk factors most often in relation to malaria [21,22,23,24], helminths [25, 26], HIV/AIDS [27, 28], infant feeding practices [29], nutritional status [30, 31] environmental factors [32] and urbanisation [33]. Yet, the contributions of iron deficiency and inflammation-linked anaemia- reported to be the major causes of anaemia to the overall burden and severity of anaemia have been infrequently studied. Therefore, the objective of this study was to determine the prevalence of iron deficiency, inflammation, and anaemia of inflammation as well as decipher the influence of some inflammatory markers on the concentration of haemoglobin and ferritin in children ≤ 15 years living in Limbe, in the Mount Cameroon area. Findings from the study will elucidate further the factors contributing to the pathogenesis of iron deficiency and anaemia in this at-risk group to facilitate proper management and control.
Methods
Study participants and site
This study was conducted in the Limbe Health District (LHD) in the Fako division of the Mount Cameroon area. The description of the town Limbe has been established in previous literature [21, 22]. The study participants included children ≤ 15 years old living in the area, whose parents consented to their participation in the study and who had not had blood transfused two months prior to the commencement of the study.
Study design, sampling technique and unit
This was a cross sectional study conducted between December 2018 and August 2019. Upon receipt of administrative and ethical authorizations, participants were enrolled from their respective communities and hospitals within the LHD. Informed consent/assent forms were given to parents explaining the purpose, benefits, and risks of the study. Clinical evaluation was done after which structured questionnaires were administered to obtain socio-demographic data and clinical history. Blood samples were collected to determine the presence of malaria parasites, for full blood count (FBC) and biochemical analyses.
A convenience multistage method of sampling was used for data collection. For representativeness of each council, a health area was randomly selected from each of the 3 council areas that make up the LHD. This was followed by a random selection of representative health facilities and neighbourhoods in the selected health area. Following education by the community relay agents, enrolment into the study was consecutive in hospitals while in the community it was on planned visits. The formula n = z2pq/d2 [34] was used to calculate the sample size where n = required sample size; z = 1.96, the standard normal deviate for a 95% confidence interval (CI); p = 62%, the anaemia prevalence in the region [22]; q = (1-p) and d = 0.05, the acceptable error margin. The optimum sample size was 362. To allow for losses due to incomplete data entry the sample size was increased to 520 individuals.
Data collection
Clinical examination for each child was done by a trained physician. Symptoms such as diarrhoea, headaches, and joint or muscle pains were recorded. The parent/guardian of the child was given a self-administered pre-tested questionnaire [S1 Questionnaire] developed for this study to be filled with the aid of an interviewer. This questionnaire included data on socio-demography and clinical symptoms. Axillary temperature was measured using a clinical thermometer and fever was defined as temperature ≥ 37.5 °C. Weight was measured using a Terraillon weighing scale to the nearest 0.1 kg while height was measured using a measuring tape to the nearest 0.1 cm. The anthropometric measurements were used to calculate nutritional indices: height-for-age (HA) for stunting, weight-for-age (WA) for underweight and weight-for-height (WH) for wasting, based on WHO growth reference curves [35]. A child was considered undernourished if he/she scored < -2 SD in one of the above indices [36].
Laboratory methods
Determining malaria parasites
About 3 ml venous blood was collected using sterile techniques into labelled EDTA and dry tubes. The tubes were transported on ice to the Malaria Research Laboratory of the University of Buea for further analysis. Thin and thick blood films were prepared on the spot immediately after dispensing blood into tubes. The thin films were fixed with absolute methanol and with the thick films stained with 10% Giemsa stain for 15 min. They were examined for malaria parasites by microscopy according to standard procedures [37].
Haematology
Following the manufacturer’s instructions, the Nihon Kohden Celltac α (Tokyo, Japan) haemoanalyzer was used to run a full blood count analysis. Values for white blood cell (WBC) counts and haemoglobin (Hb) concentration were obtained. The classification of anaemia based on WHO [38] standard was as follows: Hb < 11.0 g/L for children 1–5 years, Hb < 11.5 g/dL for children 6 ‒ 11 years and Hb < 12.0 g/dL for children 12 ‒ 15 years old. Anaemia severity was categorised as mild anaemia = Hb 10.0 g/dL ‒ 10.9 g/dL for children 1 ‒ 5 years, Hb 11.0 g/dL ‒ 11.4 g/dL for children 6 ‒ 11 years and 11.0 ‒ 11.9 g/dL for 12 ‒ 15 years; moderate = 7.0 g/dL ‒ 9.9 g/dL for 1 ‒ 5 years, 7 ‒ 10.9 g/dL for 6 ‒ 15 years, and severe = Hb < 7.0 g/dL for all children.
Quantifying inflammatory cytokines and ferritin using ELISA
Blood in dry tubes were centrifuged at 3000 rpm for 5 min and the aliquots stored at – 20 °C until use. The inflammatory markers C-reactive protein (CRP), interleukin-1 beta (IL-1β), interleukin-6 (IL-6), interleukin-10 (IL-10), interferon-gamma (IFN-γ), tumour necrosis factor-alpha (TNF-α), and ferritin were measured using the sandwich ELISA technique with the ThermoFisher Scientific™ Multiskan™ Go Microplate Spectrophotometer (Waltham, Massachusetts, USA) ELISA machine following the manufacturer’s instructions.
The following cut-off values were used: CRP > 5 mg/L [39] confirmed the presence of any inflammatory process; IL-1β > 12 pg/mL [40], IL-6 > 50 pg/mL [41], IL-10 > 20 pg/mL [40], IFN-γ > 50 pg/mL [42] and TNF-α > 10 pg/mL [43]. Ferritin values < 12 µg/L for children 1 ‒ 5 years old and < 15 µg/L for children over five years old were used to define ID and IDA was defined as concurrent anaemia and low ferritin level [44] in the absence of inflammation. Anaemia of inflammation (AI) was defined as concurrent anaemia and inflammation without ID [45].
Data analyses
Data was entered into Microsoft Excel 2016 and then exported to IBM-Statistical Package for Social Sciences version 21 (SPSS, Inc., Chicago, IL, USA) for statistical analysis. Graphs were plotted with R version 4.0.5 (Boston, Massachusetts, USA). Means and standard deviations were used to summarise continuous variables while categorical data was summarised as frequencies and percentages. Parasite density was log-transformed before analysis. Spearman’s rank correlation analysis was used to determine the correlation between cytokines and haemoglobin as well as ferritin. Multiple linear regression analysis was used to evaluate the effects of inflammatory cytokines on haemoglobin and ferritin concentrations. Significance was set at P-value < 0.05 at 95% confidence interval (CI).
Ethical consideration
Clearance for the study with number 2018/811-05/UB/SG/IRB/FHS was obtained from the Ethical Review Board hosted by the Faculty of Health Sciences, University of Buea after obtaining administrative authorisation from the South West Regional Delegation of Public Health (R11/MINSANTE/SWR/RDPH/PS/430/940). Additionally, authorisations for community and hospital studies were obtained from the community chiefs and hospital director, respectively. Only participants who gave written consent documented by the investigator took part in the study. Children whose parents consented to the study were enrolled only after the purpose, risks and benefits of the study were clearly explained to them. It was emphasized that participation was fully voluntary and that a parent could at any time disallow his child to continue. All samples were coded to ensure confidentiality.
Results
Characteristic of the participants
The study included 520 participants, 46.5% (242) males and 53.5% (278) females. The mean (standard deviation: SD) age was 5.9 (4.2) years of which majority (56.3%, 293) were ≤ 5 years old. As shown in Table 1, most of the participants were enrolled from the hospitals (65.8%) and their parents had secondary level of education (42.9%). The prevalence of fever, malaria parasite, undernutrition, stunting, underweight and wasting in the study population were 36.9%, 37.9%, 18.2%, 16.0%, 4.7% and 6.8%, respectively. The geometric mean parasite density (GMPD)/µL of blood and prevalence of fever were significantly highest in children 1 ‒ 5 years old (928, 44%, respectively) when compared with their equivalents. Undernutrition prevalence varied significantly with age with children 11 ‒ 15 years old having the highest prevalence (22.2%) as well as in the prevalence of stunting (22.2%) as shown in Table 1.
Prevalence and severity of anaemia
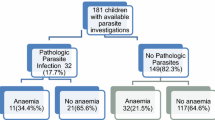
The overall anaemia prevalence in the study population was 67.5% (351). Of the 351 anaemic cases 49.0% was mild, 45.0% moderate and 6.0% severe. A significantly higher prevalence of anaemia was found in participants enrolled from the community (81.5%, P < 0.001), children whose parent had no formal/primary education (73.9%, P = 0.018), were fishermen (81.7%, P = 0.012), who lived in a home with ≤ 5 persons (71.8%, P = 0.012) and were iron deficient (76.7%, P < 0.001) when compared with their respective counterparts. The prevalence was comparable with sex (P = 0.289), age (P = 0.443), fever (P = 0.095) and malaria status (P = 0.566). Although not significant, inflammation was higher in anaemic (69.5%) than non-anaemic (64.1%) children as indicated in Table 2.
The severity of anaemia was comparable with sex (P = 0.998), education level of parent (P = 0.699), family size (P = 0.551) and the presence of inflammation (P = 0.222). Anaemia severity however, varied significantly with age (P = 0.011), enrolment site (P = 0.012), parent’s occupation (P = 0.022), febrile state (P = 0.017), malaria parasite status (P = 0.001) and ID status (P < 0.001) as revealed in Table 2. The prevalence of mild anaemia was highest in the ≤ 5 years old (53.3%) while moderate anaemia prevalence was highest (66.7%) in their 12 ‒ 15 years old counterparts. While moderate anaemia was common (53.8%) in those within the community, mild and severe anaemia occurred commonly in children presenting to hospital (53.4%, 7.8%), respectively. Of significance, children who had fever and were malaria parasite positive had higher prevalence of mild (49.6%, 50.8%) and severe anaemia (10.7%, 11.5%), respectively, as opposed to those without. Children whose parents were jobless had the highest prevalence of mild anaemia (61.6%) while those whose parents were civil servants had the highest prevalence of moderate (55.0%) and severe anaemia (20.0%). A higher prevalence of moderate anaemia was observed in iron deficient children (57.2%) while those iron replete had a higher prevalence of severe anaemia (8.5%) as shown in Table 2.
Inflammation, ID, IDA and AI prevalence
The overall prevalence of inflammation (CRP > 5 mg/L), ID (age-related ferritin concentration < 12 µg/L and < 15 µg/L), IDA (concurrent ID and anaemia) and AI (concurrent anaemia and inflammation in the absence of ID) were respectively, 63.1%, 34.6%, 12.9% and 30.2%. Commonly, inflammation was highest in females (64.7%), children ≤ 5 years (65.5%), malaria parasite negatives (63.8%), those without fever (64.3%) and were not undernourished (63.3%) than their compeers. Of statistical significance, the prevalence of ID was highest in children 12 ‒ 15 years (56.2%, P < 0.001), those enrolled in the community (57.9%, P < 0.001), whose parents were civil servants (48.6%, P < 0.001), who lived in homes with 6‒10 occupants (41.2%, P = 0.016), had no fever (41.8%, P < 0.001) and were malaria parasite negative (39.0%, P = 0.007) when compared with their respective counterparts as revealed in Table 3.
Statistically significant differences in the prevalence of IDA were observed with age (P < 0.001), enrolment site (P < 0.001), parent’s occupation (P = 0.005), febrile status (P = 0.004). The 12–15 years old (23.3%), children within the community (22.5%), whose parents were fishermen (21.7%), and who were afebrile (16.2%) had the highest prevalence of IDA. In relation to AI, children 1 ‒ 5 years old and who lived in homes with 1 ‒ 5 occupants had the highest prevalence (36.9% and 35.3%) than their peers. This difference was significant at P = 0.001 and P = 0.033, respectively. Contrarily, children with fever (31.8%), malaria parasite positives (30.5%) and were undernourished (31.2%) had a higher preponderance of AI than counterparts that was none significant (Table 3).
Haemoglobin and ferritin concentrations and inflammatory markers
Correlations between haemoglobin and some inflammatory markers revealed a significant negative correlation between haemoglobin and IL-10 (P = 0.003) as shown in Fig. 1(c). Although not significant, a negative trend was also observed with haemoglobin concentration and IL-6 (P = 0.082) as revealed in Fig. 1(b). Meanwhile a significant positive correlation was observed between haemoglobin and IFN-γ (P < 0.001), TNF-α (P = 0.045) and ferritin concentration (P < 0.001) as shown in Fig. 1(d), (e) and (g), respectively. No significance however, was observed in the correlation between haemoglobin and IL-1β (Fig. 1(a) and CRP (Fig. 1(f) concentrations respectively.
On the other hand correlations between ferritin and some cytokines showed a significant positive correlation between ferritin and IL-1 (P < 0.001), IFN-γ (P < 0.001), TNF-α (P < 0.001) and CRP (P < 0.001) as shown in Fig. 2 (a), (d), (e) and (f), respectively while, a significant negative trend was observed between ferritin concentration and concentration of IL-10 (P < 0.001) (Fig. 2(c)). No significance was observed between ferritin concentration and IL-6 concentration as shown in Fig. 2(b).
Influence of independent variables on haemoglobin and ferritin concentrations
Overall, the mean (SD) Hb was 10.5 (1.7) g/dL and mean (SD) ferritin was 15.28 (3.80) µg/L. Using a multiple linear regression model, the influence of socio-demographic and clinical factors as well as cytokine concentration on haemoglobin and ferritin concentrations were examined. Among the socio-demographic factors, the age (P < 0.001), enrolment site (P < 0.001) and family size (P = 0.007) had a significant positive influence on haemoglobin concentration as shown in Table 4. On the other hand, ferritin concentration was significantly influenced by sex (P = 0.011) and enrolment site (P < 0.001) and showed a positive correlation. Moreover, haemoglobin concentration showed no significant correlation with any of the clinical factors whereas ferritin concentrations was significantly influenced by febrile status (P = 0.013), malaria status (P = 0.040) and inflammation (P = 0.007). While ferritin correlated negatively with febrile and malaria status, respectively, it correlated positively with inflammation as shown in Table 4. Regarding the influence by inflammatory markers, haemoglobin negatively correlated (P = 0.026) with the concentration of IL-6. Meanwhile ferritin was significantly influenced by the concentration of IL-1β (P = 0.016), IL-6 (P = 0.044), IFN-γ (P < 0.001), TNF-α (P < 0.001) and CRP (P = 0.001) with all the associations being positive except for IL-6 which showed a negative trend (Table 4).
Discussion
Inflammation and iron deficiency are two major causes of anaemia in disease-endemic setting. A deficiency in iron supply leads to an imbalance in the homeostatic environment of the body spurring a series of acute phase responses and leading to inflammation. Inflammation leads to more iron retention [17], and then iron deficiency which further causes inflammation and subsequent anaemia. This vicious cycle of events leads to more iron retention and anaemia unless the root cause is diagnosed and treated. The study determined the prevalence of anaemia, iron deficiency and anaemia of inflammation in children in malaria endemic Limbe - Cameroon and how different social and clinical factors as well as inflammatory cytokines influence the concentration of haemoglobin and ferritin.
The overall anaemia prevalence of 67.5% is higher than the national prevalence of 62.5% recorded in children under five years [46], lower than the 77.7% obtained by Asoba et al. [29]. in the under-fives in some parts of the Mount Cameroon area, and comparable to 66.7% obtained in children in the Western Region of Cameroon [47]. This again confirms anaemia as a severe public health problem in the Mount Cameroon area hence, there is a dire need to re-strategize the current control methods being employed.
The overall iron deficiency prevalence (34.6%) is comparable to those obtained in Kenyan (36.9%) and Ugandan (36.5%) children [48], higher than 18.2% reported by Nazari [49] in Iranian children, and lower than 76.1% and 51.1% reported in children in India [50] and in a rural area in Cameroon [47], respectively. This high prevalence observed in Cameroonian children may be a result of poor feeding practices despite having a vast diversity of food as recently reported in a study in the same area [51]. In that study inadequate weekly consumption of meat and plantain (which are iron-rich) and fruits, (which facilitate iron absorption) were reported risk factors for anaemia, and iron-deficiency has been established as the main cause of anaemia [1]. Meanwhile iron deficiency anaemia prevalence (12.9%) was lower than the recorded 33.5% in children in the Gaza Strip [52]. This difference may be attributed to the different age strata used in the study. Whilst our study population included adolescents in both urban and semi-urban setting, those in Gaza were under-fives and lived in a setting already compromised by being marginalized.
Observation from the study revealed anaemia of inflammation prevalence (30.2%) is comparable to the ID prevalence (34.6%). This is in line with studies that say ID and AI often co-exist and together cause most of the anaemia encountered in disease-prone areas [1]. A plausible explanation is that during AI, iron absorption from the intestines is restricted leading to iron retention in the recticulo-endothelial system as ferritin thus causing ID [16]. Hence, the intensity of inflammation may be directly proportional to the amount of iron sequestered as observations from the study demonstrated a positive correlation between ferritin and the pro-inflammatory markers.
Sex-wise, findings from the study showed males had a higher prevalence of anaemia than females. Correspondingly, ID and IDA prevalence were higher in males than females. The high proportion of IDA in males than females is in line with Nazari et al. [49]. and Ewusie et al. [53]. in Ghana. This may be explained by the fact that the iron requirement for growth is higher in males than in females [54] and they gain more weight during their first years of life [55]. This may imply that ID is among the major contributors of the anaemia observed in this group. Conversely, AI was more prevalent in females, which is to be expected as they had a higher proportion of inflammation and therefore probably accounted for the observed anaemia than did ID. However, as a limitation, it is uncertain if the inflammatory process was acute or chronic since alpha 1-acid glycoprotein (AGP) which is more reliable in distinguishing past from present infection than CRP [56] was not assayed.
Findings from the study demonstrated children 12–15 years old had a higher occurrence of anaemia, ID and IDA. This is probably because there is a peak in iron requirements at adolescence resulting from expansion of red blood cell mass and growing muscle tissues [57]. Also, there is a possibility that adolescents tend to frequently snack and take carbonated drinks rather than eat proper meals or consume vegetables and fruits; these snacks are usually overly processed and may not contain the much-needed iron to meet their bodies’ demand.
In many African countries iron deficiency usually goes unnoticed except when diagnosed in the hospital in the cause of finding causes for another ailment [8]. This may account for the high prevalence within the community when compared with those enrolled at presentation in the hospital. Further observation demonstrated ID was more prevalent in children whose parents work in the civil service whereas anaemia and IDA was more common in children whose parents’ main occupation is fishing. Whilst being a civil servant may ensure a steady source of income, it is no guarantee that the quality of food consume by the children in these household is iron-rich or promote iron-absorption. Furthermore, in this conflict-hit area, food security is a challenge in many of such households offering hospitality to family members and other internally displaced individuals who have fled the violence in search for food, peace and security. This may have led to low dietary diversity as quantity will be preferred over quality. Moreover, the anaemia and IDA observed in children whose parent/caregiver were fishermen may be attributed to the deficiency in iron observed or more likely the result of blood loss due to some other infection than malaria as the negative trend observed between haemoglobin and malaria status in the regression model was not significant.
Relating to family size, children in homes with 6–10 members had a higher prevalence of ID than in homes with less than 5 members. This finding is in conformity with Psirropoulou et al. [58] in Greek children 1‒2 years old. A reasonable explanation may be that in large homes, iron intake may be reduced after reduction in food portions as food is spread more widely. Furthermore, these children may be more exposed to infections and other parasites. As a limitation in the study, the effects of other infections such as bacterial or helminths were not investigated, which would have divulged to what extent they contribute to the burden of ID.
ID and IDA observed in afebrile children may have resulted from inflammatory response to an infection which the immune system was trying to fight off as observations from this study revealed higher ID prevalence in malnourished and malaria negative children. In areas of high malaria transmission people develop some degree of immunity so, harbouring parasites without overt fever or malaria-linked symptoms is common [59]. Moreover, afebrile children may have been harbouring other blood-sucking parasites such as intestinal parasites which cause iron deficiency by direct blood loss resulting from intestinal bleeding, or appetite loss leading to reduced food intake or may prevent nutrient from being absorbed [60]. However, we did not assess intestinal parasites thus their role in iron deficiency in this study cannot be ascertained.
Congruent with previous studies [48, 61, 62,] malaria negative children had a higher prevalence of ID. The sequestration of iron in macrophages and liver cells in times of deficiency starves the malaria parasite [63] of iron thus serving as a protection against the disease in African children. It may also be that the production of nitric oxide, which has been shown to be detrimental against the malaria parasite, increases in ID states [64]. Hence, a probable increase in nitric oxide may be the cause of the high IDA burden seen in non-malarious children.
Both ID and IDA were common in undernourished than well fed children in accordance with Hagan et al. [65]. even though no significant difference was observed with ID. It is expected that undernourished children will be iron deficient and anaemic, as the association between iron status and malnutrition has been previously established [66]. The vicious cycle between malnutrition, iron deficiency and anaemia may lead to inflammation which will further exacerbate anaemia.
The significant negative correlation observed between IL-6 and haemoglobin concentration is in line with studies elsewhere [67]. IL-6 stimulates the production of hepcidin which is the main iron-regulatory hormone preventing absorption of iron from the intestines and release from macrophages leading to low iron levels which may result to anaemia. On another hand, being a pro-inflammatory cytokine, produced in an early response to TNF-α [68], IL-6 will lead to iron sequestration in the presence of inflammation or iron overload [67] by stimulating hepcidin to degrade ferroprotein, thereby reducing bone marrow supply of iron and thus decreasing serum iron concentration [69] leading to anaemia.
In line with Choucair et al. [70]., haemoglobin concentration correlated negatively with IL-10 concentration. IL-10 is an anti-inflammatory cytokine and acts to reduce inflammation by reducing the production of pro-inflammatory cytokines and free radicals such as nitric oxide [71]. A reduction in pro-inflammatory cytokines will lead to a decrease in inflammation and may lead to an increase in haemoglobin. IFN-γ is another pro-inflammatory cytokine which together with TNF-α, is produced in response to malaria infection [72]. Its production suppresses ferritin [73] starving the parasites of iron but also fostering ID and IDA [74] hence the positive correlation observed between haemoglobin and IFN-y levels.
Ferritin has been reported to correlate with inflammatory markers being on one hand a promoter and on the other a regulator of inflammation. The positive link between IL-1β, IL-6, IFN-y, TNF-α, CRP and ferritin shows that inflammatory markers can induce the expression of ferritin [75]. TNF-α and IL-1β have been shown to work in synergy with IL-6 to increase the production of more TNF-α and IL-1β, thus more CRP which in turn causes a corresponding increase in ferritin concentration [76]. This cascade of events usually results in iron retention in the liver and macrophages hence anaemia. Also, the presence of ferritin may stimulate the production of more of these cytokines including the anti-inflammatory IL-10 and multifunctional IL-6 which act to reduce the inflammatory response. This is observed in the negative trend between ferritin and IL-10.
Although the study had as limitations not assessing other haemoglobinopathies, nutritional deficiencies and behaviours that may have the potentials of influencing markers of iron deficiency and anaemia, nevertheless, the variables evaluated are critical in portraying the burden of anaemia, ID and the contributions of inflammatory cytokines to markers of anaemia, a severe public health burden in the region. There is a dire need for regular monitoring of these burdens to provide accurate data for the development of sustainable control strategies to alleviate the poor health of these children.
Conclusions
With iron deficiency, iron deficiency anaemia and anaemia of inflammation as moderate public health concerns in children in the Limbe Health District of the Mount Cameroon area, along with its contribution to the overall burden of anaemia it is imperative to review the anaemia-control strategies in place. Children 12‒15 years had the highest prevalence of iron deficiency while those aged 1‒5 years old had the highest inflammation-anaemia prevalence hence, appropriate intervention should be directed at the groups with unreasonable burden. While iron deficiency contributes to about half the total anaemia prevalence, inflammation, mediated by cytokines and acute phase proteins, contributes just as much to the overall burden of anaemia. Therefore, the influence cytokines have on haemoglobin concentration should be considered in the management of anaemia.
Data Availability
All datasets on which the conclusions of the research rely are presented in this paper. However, data is available from the corresponding author on reasonable request.
References
Kassebaum NJ, Jasrasaria R, Naghavi M, Wulf SK, Johns N, Lozano R, et al. A systematic analysis of global anaemia burden from 1990 to 2010. Blood. 2014;123(5):615–24. https://doi.org/10.1182/blood-2013-06-508325.
Shill KB, Karmaker P, Kibria MG, Das A, Rahman MA, Hossain MS, et al. Prevalence of iron-deficiency anaemia among university students in Noakhali region, Bangladesh. J Health Popul Nutri. 2014;32(1):103–10. PMID: 24847599; PMCID: PMC4089078.
Mananga MJ, Kana-Sop MM, Nolla NP, Tetanye-Ekoe, Gouado I. Feeding Practices, food and nutrition insecurity of infants and their mothers in Bangang Rural Community, Cameroon. J Nutr Food Sci. 2014;4:264. https://doi.org/10.4172/2155-9600.1000164.
Azhar S, Murtaza G, Noreen S, Khan SA, Khan A, Nasir B, et al. An evaluation of pakistani pharmacy students’ knowledge of herbal medicines in Pakistan. Afr J Pharm Pharmacol. 2012;6(3):221–4. https://doi.org/10.5897/AJPP11.860.
Coad J, Pedley K. Iron deficiency anaemia in women. Scand J Clin Lab Invest Suppl. 2014;244:82–9. https://doi.org/10.3109/00365513.2014.936694.
Miller JL. Iron deficiency anaemia: a common and curable disease. Cold Spring Harb Perspect Med. 2013;3(7):a011866. https://doi.org/10.1101/cshperspect.a011866.
Allali S, Brousse V, Sacri AS, Chalumeau M, Montalembert M. Anaemia in children: prevalence, causes, diagnostic workup, and long-term consequences. Expert Rev Haematol. 2017;10(11):1023–8. https://doi.org/10.1080/17474086.2017.1354696.
Ekwochi U, Odetunde O, Maduka I, Azubuiko J, Obi I. Iron deficiency among non-anaemic under-five children in Enugu, South-East, Nigeria. Ann Med Health Sci Res. 2013;3(3):402–6. https://doi.org/10.4103/2141-9248.117943.
Kelly AU, McSorley ST, Patel P, Talwar D. Interpreting iron studies. BMJ. 2017;357:j2513. https://doi.org/10.1136/bmj.j2513.
Nemeth E, Ganz T. Anaemia of inflammation. Hematol Oncol Clin North Am. 2014;28(4):671–81. https://doi.org/10.1016/j.hoc.2014.04.005. vi.
Ferrero-Miliani L, Nielsen OH, Andersen PS, Girardin SE. Chronic inflammation: importance of NOD2 and NALP3 in interleukin-1β generation. Clin Exp Immunol. 2007;147(2):227–35. PMCID: PMC1810472. PMID: 17223962.
Thurnham DIMG. Influence of infection and inflammation on biomarkers of nutritional status with an emphasis on vitamin A and iron. In: World Health Organization Report: Priorities in the assessment of vitamin A and iron status in populations, Panama City, Panama, 15 2012.
Metzgeroth G, Hastka J. Eisenmangelanämie und Anämie der chronischen Erkrankungen [Iron deficiency anemia and anemia of chronic disorders]. Internist (Berl). 2015 Sep; 56(9):978 – 88. German. doi: https://doi.org/10.1007/s00108-015-3711-2. PMI: 2622837.
Weiss G, Ganz T, Goodnough LT. Anaemia of inflammation. Blood. 2019;133(1):40–50. https://doi.org/10.1182/blood-2018-06-856500.
Ferraro S, Mozzi R, Panteghini M. Revaluating serum ferritin as a marker of body iron stores in the traceability era. Clin Chem Lab Med. 2012;50(11):1911–6. https://doi.org/10.1515/cclm-2012-0129.
Cartwright GE. The anaemia of chronic disorders. Semin Hematol. 1966;3:351–75. https://doi.org/10.1111/j.1365-11365-2141.197.tb03424.x.
Ganz T. Anaemia of inflammation. N Engl J Med. 2019;381:1148–57. https://doi.org/10.1056/NEJMra1804281.
Shaw JG, Friedman JF. Iron deficiency anaemia: focus on infectious diseases in lesser developed countries. Anaemia. 2011;2011:260380. https://doi.org/10.1155/2011/260380.
Nayak L, Gardner LB, Little JA et al. Chapter 37—anaemia of chronic diseases: In Hematology 7th ed. Hoffman R, Benz EJ, Silberstein LE, Eds.: 491–496.Elsevier. 2018 doi: https://doi.org/10.1016/b978-0-323-35762-3.0037-8.
Steinbicker AU, Muckenthaler MU. Out of balance-systemic iron homeostasis in iron-related disorders. Nutrients. 2013; Aug 2;5(8):3034–3061. doi: https://doi.org/10.3390/nu5083034.
Kimbi HK, Sumbele IUN, Nweboh M, Anchang-Kimbi JK, Lum E, Nana Y, et al. Malaria and haematologic parameters of pupils at different altitudes along the slope of Mount Cameroon: a cross-sectional. Malar J. 2013;12(1):193. https://doi.org/10.1186/1475-2875-12-193.
Sumbele IUN, Sama SO, Kimbi HK, Taiwe GS. Malaria, moderate to severe anaemia, and malarial anaemia in children at presentation to hospital in the Mount Cameroon Area: a cross-sectional study. Anaemia. 2016; Article ID 5725634.
Ngole SIU, Theresa NA, Moses S, Thomas N, Ngwa Elsy Manka NE, Titanji VPK. Haematological changes and recovery associated with treated and untreated Plasmodium falciparum infection in children in the Mount Cameroon Region. JCMR. 2010;2(9):143–51.
Sumbele IUN, Samje M, Nkuo-Akenji T. A longitudinal study on anaemia in children with Plasmodium falciparum infection in the Mount Cameroon region: prevalence, risk factors and perceptions by caregivers. BMC Infect Dis. 2013;13, 123(2013). doi: https://doi.org/10.1186/1471-2334-13-123.
Nkuo-Akenji TK, Chi PC, Cho JF, Ndamukong KKJ, Sumbele I. Malaria and helminth co-infection in children living in a malaria endemic setting of mount Cameroon and predictors of anemia. J Parasitol. 2006 Dec;92(6):1191–5. doi: 101645/GE-895R.1.
Sumbele IUN, Nkemnji GB, Kimbi HK. Soil-transmitted helminths and Plasmodium falciparum malaria among individuals living in different agroecosystems in two rural communities in the mount Cameroon area: a cross-sectional study. Infect Dis Poverty. 2017;6:67. https://doi.org/10.1186/S40249-017-02266-5.
Bate A, Kimbi HK, Lum E, Lehman LG, Onyoh EF, Ndip LM, et al. Malaria infection and anaemia in HIV-infected children in Mutengene, Southwest Cameroon: a cross sectional study. BMC Infect Dis. 2016;16:523. https://doi.org/10.1186/12879-016-1853-z.
Sandie MS, Sumbele IUN, Tasah MM, Kimbi HK. Malaria and intestinal parasite co-infection and its association with anaemia among people living with HIV in Buea, Southwest Cameroon: a community-based retrospective cohort study. PLoS ONE 202;16(1): e0245743. doi:https://doi.org/10.1371/journal.pone.0245743.
Asoba GN, Sumbele IUN, Anchang-Kimbi JK, Metuge S, Teh RN. Influence of infant feeding practices on the occurrence of malnutrition, malaria and anaemia in children ≤ 5 years in the Mount Cameroon area: a cross sectional study. PLoS ONE. 2019;14(7):e0219386. https://doi.org/10.1371/journal.pone.0219386.
Sumbele IUN, Bopda OSM, Kimbi HK, Ning TR, Nkuo-Akenji T. Nutritional status of children in a malaria meso endemic area: cross sectional study on prevalence, intensity, predictors, influence on malaria parasitaemia and anaemia severity. BMC Public Health. 2015;15:1099. https://doi.org/10.1186/S12889-015-2462-2.
Tabi ESB, Cumber SN, Juma KO, Ngoh EA, Akum EA, Eyong EM. A cross-sectional survey on the prevalence of anaemia and malnutrition in primary school children in the Tiko Health District, Cameroon. Pan Afr Med J. 2019;32:111. https://doi.org/10.11604/pamj.2019.32.111.15728.
Ndamukong-Nyanga J, Kimbi HK, Sumbele IUN, Emmaculate L, Nweboh M, Nana Y, et al. Socio-demographic and environmental factors influencing asymptomatic malaria and anaemia incidence among school children in Fako Division, South West Cameroon. JAMMR. 2014;4(20):3814–27. https://doi.org/10.9734/BMJMMR/2014/9712.
Sumbele IUN, Kimbi HK, Ndamukong-Nyanga JL, Nweboh M, Anchang-Kimbi JK, Lum E, et al. Malarial anaemia and anaemia severity in apparently healthy primary school children in urban and rural settings in the Mount Cameroon area: cross sectional survey. PLoS ONE. 2015;10(4):e0123549. https://doi.org/10.1371/journal.pone.013549.
Bryan FJ. The design and analysis of research studies. UK: University of Otago, Cambridge University Press,; 1992.
de Onis M, Oyango AW, Borghi E, Siyam A, Nishida C, Siekmann J. Development of a WHO growth reference for school-aged children and adolescents. Bull World Health Organisation. 2007;85(9):660–7. https://doi.org/10.2471/bit.07.043497.
Kateera F, Ingabire CM, Hakizimana E, Kalinda P, Mens PF, Grobusch MP, et al. Malaria, anaemia and under-nutrition: three frequently coexisting conditions among preschool children in rural Rwanda. Malar J. 2015;14:440. https://doi.org/10.1186/s12936-015-0973-z.
Cheesbrough M. District Laboratory Practice in Tropical Countries. 2nd ed. Edinburg Building, UK: Cambridge University Press; 2019. Part 2.
WHO. Haemoglobin concentrations for the diagnosis of anaemia and assessment of severity. Vitamin and Mineral Nutrition Information System. Geneva, World, Health Organisation., 2011. https://apps.who.int/iris/handle/10665/85839.
Shinoda N, Sullivan KM, Tripp K, Erhardt JG, Haynes BM, Temple VJ, et al. Relationship between markers of inflammation and anaemia in children of Papua New Guinea. Public Health Nutr. 2013;16(2):289–95. https://doi.org/10.1017/S1368980012001267.
Lyke KE, Burges R, Cissoko Y, Sangare L, Dao M, Diarra I, et al. Serum levels of the pro-inflammatory cytokines interleukin-1 beta (IL-1β), IL-6, IL-8, IL-10, tumour necrosis factor alpha, and IL-12 (p70) in malian children with severe Plasmodium falciparum malaria and matched uncomplicated malaria or healthy controls. Infect Immun. 2004;72(10):5630–7. https://doi.org/10.1128/IAI.72.10.5630-5637.2004.
Buck C, Bundschu J, Gallati H, Bartmann P, Pohlandt F. Interleukin-6: a sensitive parameter for the early diagnosis of neonatal bacterial infection. Pediatrics. 1994;93(1):54–8. PMID 8265324.
Monastero RN, Pentyala S. Cytokines as biomarkers and their respective clinical cutoff levels. Int J Inflam. 2017;7:4309485. https://doi.org/10.1155/2017/4309485.
Achidi EA, Apinjoh TO, Yafi CN, Besingi R, Anchang JK, Awah NW, et al. Plasma levels of tumour necrosis factor-alpha, interleukin-10, interleukin-12, macrophage inhibition factor and transforming growth factor-beta in children with severe and uncomplicated falciparum malaria. J Trop Dis. 2013;1(1):103.
Chandyo RK, Henjum S, Ulak M, Thorne- Lyman AL, Ulvik RJ, Shrestha PS, et al. The prevalence of anaemia and iron deficiency is more common in breastfed infants than their mothers in Bhaktapur, Nepal. Eur J Clin Nutr. 2016;70:456–62. 10.1038.ejcn.2015.199.
Finch CA, Bellotti V, Stray S, Lipschitz DA, Cook JD, Pippard MJ, et al. Plasma ferritin determination as a diagnostic tool. West J Med. 1986;145(5):657–63. PMID 3541387.
World Bank. 2016. Prevalence of anaemia among children (% of children under five) |data. https://data.worldbank.org/indicator/SH.ANM.CHLD.ZS. Accessed 13 Nov 2020.
Kana SMM, Amvam ZPH, Ndifor F. Iron bioavailability in Cameroon weaning foods and influence on the diet composition. Afr J Food Agric Nutr Dev. 2015;4(1):1–11. https://doi.org/10.4314/ajfand.v4i1.19152.
Muriuki JM, Mentzer AJ, Kimita W, Ndungu FM, Macharia AW, Webb EL, et al. Iron status and associated malaria risk among african children. Clinl Infect Dis. 2019;68(11):1807–14. https://doi.org/10.1093/cid/ciy791.
Nazari M, Mohammadnejad E, Dalvand S, Gheshlagh RG. Prevalence of iron deficiency anaemia in iranian children under 6 years of age: a systematic review and meta-analysis. J Blood Med. 2019;10:111–7. https://doi.org/10.2147/JBM.2196102.
Onyeneho NG, Ozumba BG, Subramanian SV. Determinants of childhood anaemia in India. Sci Rep. 2019;9:16540. https://doi.org/10.1038/s41598-019-52793-3.
Sama SO, Chiamo SN, Taiwe GS, Njume GE, Sumbele IUN. Microcytic and malarial anaemia prevalence in urban children ≤ 15 years in the Mount Cameroon Area: a cross-sectional study on risk factors. Anaemia 2021; Article ID 5712309, 12 pages doi: https://doi.org/10.1155/2021/5712309.
Sirdah MM, Yaghi A, Yaghi AR. Iron deficiency anaemia among kindergarten children living in the marginalized areas of Gaza Strip. Palestine Rev Bras de Hematol Hemoter. 2014;36(2):132–8. https://doi.org/10.5581/1516-8484.20140030.
Ewusie JE, Ahiadeke C, Beyene J, Hamid JS. Prevalence of anaemia among under-5 children in the ghanaian population: estimates from the Ghana demographic and health survey. BMC Public Health. 2014;14(1):626. https://doi.org/10.1186/1471-2458-14-626.
de Castro TG, Silva-Nunes M, Conde WL, Muniz PT, Cardoso MA. Anaemia and iron deficiency among schoolchildren in the western brazilian Amazon: prevalence and associated factors. Cad Saude Publica. 2011;27(1):131–42. https://doi.org/10.1590/s0102-311x2011000100014.
Lozoff B, Kaciroti N, Walter T. Iron deficiency in infancy: applying a physiologic framework for prediction. Am J Clin Nutr. 2006;84(6):1412–21. https://doi.org/10.1093/ajcn/84.6.1412.
Hsiao SY, Lai YR, Kung CT, Tsai NW, Su CM, Huang CC. α-1-acid glycoprotein concentration as an outcome predictor in adult patients with sepsis. Biomed Res Int. 2019. https://doi.org/10.1155/2019/374896. article ID 3174896.
Vasanthi G, Fawashe AB, Susie H, Sujatha T, Raman L. Iron and nutritional status of adolescent girls from rural area and urban slum. Indian Paediatr. 1994;31(2):127–32.
Psirropoulou E, Vagenas C, Dafni O, Matala A, Skopouli F. Environmental risk factors for iron deficiency anaemia in children 12–24 months old in the area of Thessalia in Greece. Hippokratia. 2008;12(4):240–50.
Northrop CA. Interpreting indicators of iron status during an acute phase response – lessons from malaria and human immunodeficiency virus. Ann Clin Biochem. 2008;45:18–32. https://doi.org/10.1258/acb.2007.007167.
Gujo AB, Kare AP. Prevalence of intestinal parasite infection and its association with anaemia among children aged 6 to 59 months in Sidama National Regional State, Southern Ethiopia. Clin Med Insights: Paediatrics. 2021. https://doi.org/10.1177/11795565211029259.
Jonker FAM, Calis JCJ, van Hensbroek MB, Phiri K, Geskus RB, Brabin BJ, et al. Iron status predicts malaria risk in malawian preschool children. PLoS ONE. 2012;7(8):e42670. https://doi.org/10.1371/journal.pone.0042670.
Barffoue MA, Schulze KJ, Coles CL, Chileshe J, Kalungwana N, Arguello M, et al. High iron stores in the low malaria season increase malaria risk in the high transmission season in a prospective cohort of rural zambian children. J Nutr. 2017;147(8):1531–6. https://doi.org/10.3945/jn.117.250381.
Portugal S, Carret C, Recker M, Armitage AE, Gonçalves LA, Epiphanio S, et al. Host-mediated regulation of superinfection in malaria. Nat Med. 2011;17(6):732–7. https://doi.org/10.1038/nm.2368.
Fritsche G, Larcher C, Schennach H, Weiss G. Regulatory interactions between iron and nitric oxide metabolism for immune defense against Plasmodium falciparum infection. J Infect Dis. 2001;183(9):1388–94. https://doi.org/10.1086/319860.
Hagan JF, Shaw JS, Duncan PM. Bright Futures Guidelines for Health Supervision of infants, children and adolescents. 3rd ed. Elk Grove Village, IL: American Academy of Paediatrics; 2008.
Cepeda-Lopez AC, Osendarp SJ, Melse-Boonstra A, Aeberli I, Gonzalez-Salazar F, Feskens E, et al. Sharply higher rates of iron deficiency in obese mexican women and children are predicted by obesity-related inflammation rather than by differences in dietary iron intake. Am J Clin Nutri. 2011;93(5):975–83. https://doi.org/10.3945/ajcn.110.005439.
Nakagawa H, Tamura T, Mitsuda Y, Goto Y, Kamiya Y, Kondo T, et al. Inverse correlation between serum interleukin-6 and iron levels among japanese adults: a cross-sectional study. B C Haematology. 2014;14:6. https://doi.org/10.1186/2052-1839-14-6.
Katz CS, Bamboat ZM, Pillarisetty VG, DeMatteo RP. Chapter 10- liver immunology in Blumgart’s surgery of the liver, biliary tract and pancreas, 2-Volume set. (Sixth Edition). 2017. https://doi.org/10.1016/B978-0-323-34062-5-00010-8.
Vardhan-Raj S, Zhou X, Bueso-Ramos CE, Patel S, Benjamin RS, Ngyuen M. Interleukin 6, hepcidin, and other biomarkers in anaemia of chronic disease and chemotherapy induced anaemia (CIA): potential therapeutic targets. Blood. 2012;120(21):2086. https://doi.org/10.1182/blood.V120.21.2086.2086.
Choucair K, Kelso JD, Duff JR, Cassidy CS, Albrethsen MT, Ashraf M, et al. Interleukin 10-mediated response and correlated anaemia in a patient with advanced non-small cell lung carcinoma. Case Rep Oncol. 2019;12:297–303. https://doi.org/10.1159/000499704.
Moore KW, O’GArra A, de Waal Malefyt R, Vieira P, Mosmann TR. Interleukin 10. Annu Rev Immunol. 1993;11:165–90. https://doi.org/10.1146/annurev.iy.11.040193.001121.
McCall MB, Sauerwein RW. Interferon-γ-central mediator of protective immune responses against pre-ertyhrocytic and blood stage of malaria. J Leukoc Biol. 2010;88(6):1131–43. https://doi.org/10.1189/jib.0310137.
Tanyong DI, Panichob P, Kheansaard W, Fucharoen S. Effect of tumour necrosis factor alpha on erythropoietin and erythropoietin receptor-induced erythroid progenitor cell proliferation in β-thalassaemia/haemoglobin E patients. Turk J Haematol. 2015;32(4):304–10. https://doi.org/10.4274/tjh.2014.0079.
Raballah E, Kempaiah P, Karim Z, Orinda GO, Otieno MF, Perkins DJ, et al. CD4 T-cell expression of IFN-γ and IL-17 in paediatric malarial anaemia. PLoS ONE. 2017;12(4):e0175864. https://doi.org/10.1371/journal.pone.0175864.
Rosário C, Zandman-Goddard G, Meyron-Holtz EG, et al. The hyperferritinemic syndrome: macrophage activation syndrome, still’s disease, septic shock and catastrophic antiphospholipid syndrome. BMC Med. 2013;11:185. https://doi.org/10.1186/1741-7015-11-185.
Thurnham DI, McCabe LD, Haldar S, Wieringa FT, Northrop-Clewes CA, McCabe GP. Adjusting plasma ferritin concentrations to remove the effects of subclinical inflammation in the assessment of iron deficiency: a meta-analysis. Am J Clin Nutr. 2010;92(3):546–55. https://doi.org/10.3945/ajcn.2010.29284.
Acknowledgements
The authors wish to thank the parents and children for their participation in this study.
Funding
This work was supported by the special fund for research and modernization given to the authors by the Government of Cameroon. The funding body only provided part of the financial means to allow the authors to carry out the study. The funding body played no role in the design of the study and collection, analysis, interpretation of data, and in writing the manuscript.
Author information
Authors and Affiliations
Contributions
SOS participated in the data collection, laboratory analysis, analysed and interpreted the data and wrote the manuscript. GST conceived, designed and supervised the study; participated in data analysis and interpretation; and was a major contributor to the write-up of the manuscript. RNT participated in the data collection and laboratory analysis, analysed and interpreted the data and also participated in the revision of the manuscript. GEN and SNC participated in the data collection and laboratory analysis. IUNS participated in the study design, supervision and revision of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
The methodology of this research conformed to the ethical principles of the Helsinki Declaration. The study was approved by the Institutional Review Board hosted by the Faculty of Health Sciences, University of Buea (2018/811-05/UB/SG/IRB/FHS) following administrative clearance from the South West Regional Delegation of Public Health, Cameroon. Written informed consent/assent forms were obtained from parent of the children at presentation. The purpose and benefits of the study as well as the protocol and methods were clearly stated in the information sheet and consent/assent forms, respectively. The participants were also informed that participation in the study was voluntary and they were at liberty to stop the interview if they were uncomfortable. Also, it was made clear to them that the data they were providing would be kept on password protected computer and only use for the purpose of this research project.
Consent for publication
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Sama, S.O., Taiwe, G.S., Teh, R.N. et al. Anaemia, iron deficiency and inflammation prevalence in children in the Mount Cameroon area and the contribution of inflammatory cytokines on haemoglobin and ferritin concentrations: a cross sectional study. BMC Nutr 9, 94 (2023). https://doi.org/10.1186/s40795-023-00748-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40795-023-00748-3