Abstract
Background
Bacillus subtilis is well known for promoting plant growth and reducing abiotic and biotic stresses. Mutant gene-defective models can be created to understand important traits associated with rhizosphere fitness. This study aimed to analyze the role of exopolymeric genes in modulating tomato rhizosphere microbiome assembly under a gradient of soil microbiome diversities using the B. subtilis wild-type strain UD1022 and its corresponding mutant strain UD1022eps−TasA, which is defective in exopolysaccharide (EPS) and TasA protein production.
Results
qPCR revealed that the B. subtilis UD1022eps−TasA− strain has a diminished capacity to colonize tomato roots in soils with diluted microbial diversity. The analysis of bacterial β-diversity revealed significant differences in bacterial and fungal community structures following inoculation with either the wild-type or mutant B. subtilis strains. The Verrucomicrobiota, Patescibacteria, and Nitrospirota phyla were more enriched with the wild-type strain inoculation than with the mutant inoculation. Co-occurrence analysis revealed that when the mutant was inoculated in tomato, the rhizosphere microbial community exhibited a lower level of modularity, fewer nodes, and fewer communities compared to communities inoculated with wild-type B. subtilis.
Conclusion
This study advances our understanding of the EPS and TasA genes, which are not only important for root colonization but also play a significant role in shaping rhizosphere microbiome assembly. Future research should concentrate on specific microbiome genetic traits and their implications for rhizosphere colonization, coupled with rhizosphere microbiome modulation. These efforts will be crucial for optimizing PGPR-based approaches in agriculture.
Similar content being viewed by others
Background
The rhizosphere microbiome is intricately linked with the host plant [1,2,3] and is primarily modulated according to the host genotype [4,5,6] and, consequently, by the plant exudate profile [7,8,9]. In turn, the rhizosphere microbial community provides readily available nutrients for plant absorption, along with other molecules such as phytohormones and secondary metabolites, which enhance host development and health [10, 11].
The symbiotic relationships between the rhizosphere microbiome and plants can also lead to intricate connections within microbial communities, ultimately benefiting the host plant [12]. For instance, the Bacillus subtilis strain UD1022 can colonize Arabidopsis thaliana roots, establishing mutualistic interactions [13]. As the host plant secretes fixed carbon through root exudates to nourish the bacteria, B. subtilis in turn facilitates rhizobacterium colonization, providing the plant with growth-promoting traits [13]. B. subtilis is one of the most studied gram-positive plant growth-promoting rhizobacteria (PGPR) [14, 15], and it has great agricultural and ecological importance [16,17,18,19]. Their ability to induce plant development and protect against pathogens and abiotic stresses has been widely explored [20,21,22,23,24,25]. B. subtilis promoted plant growth in tomato [25], cucumber [26], and wheat [27] and conferred resistance against the soil-borne pathogen Rhizoctonia solani in cotton [28] and Pseudomonas syringae pv. tomato in Arabidopsis [29]. Martins et al. [20] and Allard-Massicotte et al. [13] showed that B. subtilis and B. amyloliquefaciens can also induce plant drought tolerance by forming biofilms in bean and Arabidopsis roots through bacterial exopolysaccharide (EPS) secretion. Most importantly, various B. subtilis strains are considered generalists for multiple crops [25,26,27,28,29]. The application of plant growth-promoting rhizobacteria (PGPR) in agricultural settings not only impacts plant performance but also affects the resident soil microbiome. For instance, the use of bacillus-based products in crops such as tobacco [30], lettuce [31], and strawberry [32] can increase bacterial diversity in rhizosphere soils.
EPS constitute an enclosed matrix produced by microbial multicellular aggregates and serve as the primary component of biofilms [33,34,35]. Along with EPS, TasA is a major proteinaceous component of B. subtilis biofilms [36,37,38,39]. In addition to affecting motility and chemotaxis, TasA plays a role in B. subtilis rhizosphere colonization [13, 35, 39], which provides the host plant with an extra barrier against potential soil-borne pathogens and drought tolerance [40,41,42]. Knocking out the ability of B. subtilis to form biofilms by constructing mutant strains is an efficient way to understand its role in plant health and development [35, 43,44,45,46]. For instance, Bacillus spp. mutated models defective in YtnP (lactonase-homolog protein-encoding gene) [44], EPS, and TasA protein-encoding genes (tapA– sipW–tasA operon and bslA gene) [45, 46] have been employed in previous studies to elucidate their role in biofilm formation, interactions with the soil microbiome, and their efficacy in antagonizing pathogens.
Studies have reported the effects of mutant B. subtilis on plant growth promotion and protection, including sporulation [44], surfactin [45], and flagellar [13, 47] mutants, but the effects of these mutants on rhizosphere microbiome assembly have not yet been properly considered. In this study, EPS and TasA double mutants of B. subtilis were used to investigate the impact of exopolymeric genes on the modulation of rhizosphere microbiome assembly. The use of a mutant strain was combined with the dilution-to-extinction approach to assess the assembly of bacterial and fungal communities in the tomato rhizosphere under a gradient of soil microbial diversity.
Materials and methods
Soil microbial diversity dilution
Soil samples were collected from the "UD Fresh to You" farm (39°40′04.2″N 75°45′03.5″W) at the University of Delaware. The specific soil type used was Delanco silt loam, which had previously been cultivated with organic tomatoes and was identified through the Web Soil Survey [48]. The dilution-to-extinction method [49] was employed to obtain soils with the microbial diversity gradient used in the bioassays. Initially, 30 kg of sieved (< 2 mm sieve) and dried soil was divided into three bags, each containing 10 kg of soil. The soil in the bags was autoclaved four times at 120 °C and 1 atm pressure for 60 min. The serial dilution process involved suspending 450 g of natural soil (dry weight) in 900 mL of autoclaved deionized water, resulting in a concentration of 0.5 g mL−1 (10–1 soil dilution). Subsequently, 100 mL of the 10–1 dilution was transferred to 900 mL of autoclaved deionized water to obtain 10–2 soil dilutions. This serial dilution process was repeated until a soil dilution of 10–6 was reached, following the methods described by Wertz et al. [50] and Souza et al. [51]. Three dilutions were selected for use in the experiment: 10–1, 10–3, and 10–6, in addition to the natural and autoclaved soils. To obtain the microbial diversity gradient across treatments, pots with 200 g of soil received 40 mL of each soil suspension, 10–1, 10–3, or 10–6, and the natural and autoclaved soils received 40 mL of sterilized ultrapure water. Pots were placed in a climatized chamber set at 25 °C, with a photoperiod of 12 h light and 12 h dark. The pots were incubated under these conditions for six weeks, allowing the establishment of the microbiome before the experiment [52].
Bacillus subtilis strains and inoculum preparation
The B. subtilis strain UD1022eps−TasA−, which is defective for the EPS and TasA genes, was obtained in a previous study [53]. Wild-type B. subtilis was cultivated on Luria broth (LB) agar plates, and its respective mutant was cultivated on LB supplemented with 5 µg mL−1 tetracycline and 1 µg mL−1 erythromycin. The streaked plates were then incubated at 37 °C for 24 h. Subsequently, individual bacterial colonies were transferred to LB liquid media supplemented with antibiotics, as was the case for the mutant strain UD1022eps−TasA−, and incubated in a shaker at 150 rpm for 6 h at 37 °C. After the incubation period, the B. subtilis cultures were washed and resuspended in autoclaved distilled water. Bacterial cultures were grown until they reached a concentration of 108 cells mL−1. Seeds were disinfected by immersion in a 3% sodium hypochlorite solution and shaken for 30 s, followed by thorough rinsing with ultrapure water. Subsequently, the seeds were briefly soaked in 70% ethanol and shaken for 1 min, followed by another extensive rinse with ultrapure water. After disinfection, 1 g of tomato seeds was mixed with the bacterial suspension (108 cells g−1) and kept for 1 h in a shaker at 150 rpm before planting. A boost dose of 1 mL (108 cells mL−1) per plant was used on the 16th day of the experiment. In the control treatment, the seeds or plants were treated with autoclaved distilled water.
Tomato bioassay and experimental design
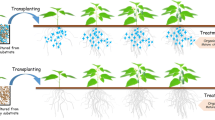
The plant bioassay used the tomato cultivar “Amish Paste” with four different treatments: (i) plants inoculated with UD1022, (ii) plants inoculated with UD1022eps−TasA−, (iii) non-inoculated plants (control), and (iv) pots without plants (bulk soil) (Fig. 1A). Each treatment was performed using five different levels of soil microbial diversity: natural soil, 10–1 dilution, 10–3 dilution, 10–6 dilution, and autoclaved soil. Thus, considering four treatments, soils with five levels of microbial diversity, and five replicates, 100 pots were used in the experiment. Each pot (8 × 6.7 cm) contained 200 g of soil (dry mass), and the plants received at least 10 tomato seeds, which were thinned after five days to leave just one plant per pot. The experiment was conducted using a randomized complete block design. Thirty days after germination, the entire root system was harvested by carefully removing the plants from the pots and gently shaking them to remove excess soil from the root system. The root-adhered soil (i.e., rhizospheric soil) was collected, transferred to 1.5 mL microtubes, and stored at -20 °C before downstream analyses. Various plant growth parameters, including plant height, root fresh and dry masses, and shoot fresh and dry masses, were measured and collected for further data analyses (Fig. 1B).
Plant bioassay experimental design and timeline. A Each treatment, including plants inoculated with UD1022, plants inoculated with UD1022eps−TasA− and non-inoculated plants (control), was cultivated in soils with five different levels of microbial diversity: natural soil, 10–1 dilution, 10–3 dilution, 10–6 dilution, and autoclaved soil. Pots without plants (bulk soil) were also used as a control. B Bioassay timeline showing bacterial inoculation, sampling, and analyses
Soil microbiome genomic DNA extraction and metataxonomic analysis
Rhizosphere and bulk soil samples were subjected to DNA extraction using the DNeasy PowerSoil® Kit (QIAGEN) according to the manufacturer’s instructions. The quality and concentration of the extracted DNA samples were evaluated using a NanoDrop spectrophotometer. In addition, to verify the integrity of the DNA, electrophoresis was performed on a 1.5% agarose gel at 80 V/400 mA for 45 min. To ensure sample DNA concentrations, quantification was performed using a QUBIT® fluorometer.
DNA samples from all five replicates per treatment (a total of 100 samples) were subjected to amplicon sequencing using the Illumina MiSeq platform at the Delaware Biotechnology Institute in Newark, Delaware, USA. Sequencing of the V4 region of the bacterial 16S rRNA gene was performed using the following primer pairs: 515F (5′-GTGYCAGCMGCCGCGGTAA-3′) [54] and 806R (5′- GGACTACNVGGGTWTCTAAT-3′) [55], and sequencing of the ITS1 region of the fungal ITS gene was performed using the following primer pairs: ITS1f (5′-CTTGGTCATTTAGAGGAAGTAA-3′) and ITS2 (5′-GCTGCGTTCTTCATCGATGC-3′) [56].
Rhizosphere and bulk soil Bacillus quantification using quantitative polymerase chain reaction (qPCR)
Initially, B. subtilis UD1022 genomic DNA was used to prepare a qPCR standard curve. Total DNA was extracted from B. subtilis strain UD1022 liquid cultures using the DNeasy® UltraClean® Microbial Kit (QIAGEN) according to the manufacturer’s instructions. The quality of the total extracted DNA was assessed using a NanoDrop® ND-2000 Spectrophotometer (Thermo Fisher Scientific, Wilmington, DE, USA). DNA was quantified using a QUBIT® 2.0 fluorometry system (Thermo Fisher Scientific, Wilmington, DE, USA). DNA was stored at -20 °C for subsequent analyses.
To prepare the standard curve, serial dilutions of B. subtilis UD1022 genomic DNA were prepared at a 1:10 ratio. The starting concentration of genomic DNA used for the dilutions was standardized at 10 ng μL−1. The target DNA was amplified using primers designed for the gyrB gene, which encodes DNA gyrase subunit B. The primers gyrB_5234_F (5′-CGGTCGTAAACGCACTATC-3′) and gyrB-5391_R (5′-AGGGTCCGGGACAAAATGTGTCG-3′) were adapted from Xie et al. [57]. Each qPCR reaction had a final volume of 10 μL and included the following components: 5 μL of PerfeCTa SYBR® Green SuperMix (Quantabio, Baverly, MA, USA), 0.2 μL of each primer (10 mM), 1 μL of template DNA, and 3.6 μL of ultrapure water. The reaction conditions were adapted from Xie et al. [54] and involved an initial denaturation step at 95 °C for 10 min, followed by 40 cycles of denaturation at 95 °C for 30 s, annealing at 61 °C for 35 s, and extension at 72 °C for 40 s. Melting curve collection was performed at the end of the cycling program. Distilled water was used as the non-template control. The qPCR assay was performed in triplicate for each dilution to ensure the accuracy and reproducibility of the results. The standard curve served as a reference to quantify the abundance of Bacillus in soil samples by interpolating their Ct values onto the curve and then converting to the number of gyrB per gram of soil. For Bacillus subtilis quantification in the rhizosphere and bulk soil samples, qPCR was performed with standard curves under the same conditions described above.
Data processing and statistical analyses
Bioassay data, including plant height, shoot and root dry mass, and qPCR (number of copies of the gyrB gene), were compared using the Scott–Knott test (P < 0.05). To generate amplicon sequence variants (ASVs) from both genes, 16S rRNA and ITS, the raw data were processed using Dada2 version 1.21.0 [58]. The primers were removed using Cutadapt version 3.4. [59] Quality control was performed and reads with low quality (Q20 or lower) were discarded, followed by taxonomic assignment using the Silva (v. 138.1) [60, 61] and UNITE (v. 9.0) databases [62,63,64]. To assess α-diversity, the Chao1 and Shannon indices were calculated. β-diversity was assessed using the Bray‒Curtis distance. Principal coordinate analysis (PCoA) was employed to visualize the similarity matrix among various soil diversity dilutions and treatments. In both analyses, rarefied and normalized data were used. The significance and effect size β-diversity were determined using the vegan package (v. 2.6-4) through permutation-based analysis (MANOVA) with the "adonis()" function [65]. To identify differentially abundant taxa among the treatment groups, ANOVA-like differential expression analysis (ALDEx2) [66] was performed using the "run_aldex()" function from the microbiomeMarker package (v. 1.28.1) [67]. In addition, a co-occurrence network analysis was performed using the PhyloSmith package (v. 1.0.6) [68] based on Spearman's pairwise correlation. To mitigate the influence of rare ASVs, ASVs occurring fewer than 20 times in each treatment with a relative abundance greater than 30% were excluded. Significant interactions were identified using Spearman pairwise correlations, with p-values less than 0.001 indicating robust associations. The nature of the co-occurrence relationships, whether negative or positive, was determined based on the strength of the correlation. In the network, each ASV represents a node, and the edges represent the correlations among the ASVs. The network layout was generated using the Fruchterman-Reingold algorithm in Gephi software (v. 0.10) [69].
Results
Quantification of Bacillus subtilis in the tomato rhizosphere
Quantitative real-time PCR (qPCR) was performed to quantify the abundance of B. subtilis gyrB gene copies in the rhizosphere and bulk soils. In natural soil, a greater amount of B. subtilis gyrB was detected in the treatment group inoculated with the UD1022eps−TasA− strain (Fig. 2A). At 10–1, 10–3, and autoclaved soil dilutions, B. subtilis UD1022 was more abundant in the wild-type soil than in the other treatments (Fig. 2BCE).
Boxplot of the gyrB gene (DNA gyrase subunit B coding gene) and qPCR quantification of B. subtilis. A Natural soil. B Soil dilution 10–1. C Soil dilution 10–3. D Soil dilution 10–6. E Autoclaved soil. Bulk soil = non-inoculated soil; Control = non-inoculated plants; UD1022 = plants inoculated with wild-type B. subtilis; and UD1022eps−TasA− = plants inoculated with mutant B. subtilis. The Scott–Knott test for pairwise comparisons of means was performed considering a 95% familywise confidence level (P < 0.05). * Indicates significant differences between treatments
Inoculation of Bacillus subtilis strains and plant performance
Significant differences in plant growth were observed for root dry mass with Bacillus inoculation (Fig. 3). According to pairwise comparisons, compared with non-inoculated plants (controls), tomato plants inoculated with the mutant strain UD1022eps−TasA− showed a reduction in root dry mass when grown in natural soil (Fig. 3A) or at a soil dilution of 10–3 (Fig. 3C). At a soil dilution of 10–3, plants inoculated with the wild-type strain UD1022 showed significantly increased root growth compared with plants inoculated with the mutant strain (Fig. 3C). No differences were observed across treatments for plant height (Additional file 1: Figure S1A to E) or shoot dry mass (Additional file 1: Figure S1F to G). Within each treatment, plants generally grew better in soils with diluted microbial diversity (Additional file 1: Figure S2).
Boxplot of the root dry mass of tomato plants 30 days after germination. A Natural soil; B Soil dilution 10–1; C Soil dilution 10–3; D Soil dilution 10–6; and E Autoclaved soil. Control = non-inoculated plants; UD1022 = plants inoculated with wild-type B. subtilis; and UD1022eps−TasA− = plants inoculated with mutant B. subtilis. The Scott–Knott test for pairwise comparisons of means was performed considering a 95% familywise confidence level (P < 0.05). Asterisks (*) indicate significant differences between treatments
Impact of the Bacillus subtilis strain UD1022 on rhizosphere microbiome assembly
The bacterial community in natural soils was dominated by Bacillus and Pseudarthrobacter in non-inoculated or inoculated soils, respectively, with strain UD1022 (Fig. 4A). These two bacterial taxa decreased in relative abundance in soils with diluted microbial diversity (Fig. 4A). Bacillus inoculation changed the relative abundance of specific bacterial and fungal groups in the tomato rhizosphere. For example, inoculation with strain UD1022 increased the relative abundance of Pseudarthrobacter in natural soil and Chthoniobacter in autoclaved soil (Fig. 4A). Twelve bacterial taxa were exclusively found in the rhizosphere of plants inoculated with the UD1022 strain (Fig. 4B), including Mucilaginibacter spp. ASV-0058, Curtobacterium spp. ASV-0097, Kaistia spp. ASV-0098, Sumerlaea spp. ASV-0135, Nocardioides spp. ASV-0181, and Methylorosula spp. ASV-0216 (Fig. 4B).
Composition of bacterial and fungal communities in the tomato rhizosphere. A Relative abundance of bacteria and fungal genera across soils with a microbial diversity gradient. NS = natural soil, D1 = soil dilution 10–1, D3 = soil dilution 10–3, D6 = soil dilution 10–6, and AS = autoclaved soil. B Venn diagram of bacterial and fungal taxa. Control = non-inoculated plant; UD1022 = plants inoculated with wild-type B. subtilis
The fungal community was dominated by Clonostachys in natural soils inoculated with or without strain UD1022 (Fig. 4A). In soils with diluted microbial diversity, the communities were dominated by Trichoderma and Fusarium (Fig. 4A). Moreover, inoculation with the UD1022 strain significantly increased the relative abundance of Talaromyces at a dilution of 10–3 (Fig. 4A). Compared with that in the control treatment, the abundance of Talaromyces increased at a dilution of 10–3 when UD1022 was inoculated (Fig. 4A). Trichoderma ghanense ASV-0001, Trichoderma spp. ASV-0003, and Cryptococcus laurentii ASV-0025 were exclusively detected in the rhizospheres of plants inoculated with strain UD1022 (Fig. 4B).
The α-diversity analysis using the Shannon index and HSD test (P < 0.05) revealed significant differences in the bacterial community across all the soil dilutions, except between the 10–6 soil dilution and autoclaved soil (Additional file 1: Figure S3A). As expected, the natural soil exhibited the highest bacterial diversity, followed by the 10–1, 10–3, 10–6 dilutions, and autoclaved soil. The same pattern was observed in the fungal community (Additional file 1: Figure S3B).
Soil microbial diversity dilution significantly affected the assembly of the rhizosphere microbiome in plants inoculated with or without strain UD1022. The relative abundance of the Bacillota phylum (Additional file 1: Figure S4), particularly the Bacillus genus (Additional file 1: Figure S4), decreased as the soil diversity decreased. The abundances of the phyla Acidobacteria and Crenarchaeota exhibited the same pattern; they decreased with soil microbial dilution and were not detected in the most diluted or autoclaved soil (Additional file 1: Figure S4). Conversely, the relative abundances of the phyla Bacteroidota, Planctomycetota, and Pseudomonadota increased with decreasing soil microbial diversity (Additional file 1: Figure S4).
For the fungal community, the relative abundance of the phyla Mortierellomycota and Basidiomycota decreased as the soil diversity decreased (Additional file 1: Figure S5). In the control treatment and in plants inoculated with the mutant UD1022eps−TasA−, they were not detected in soil diluted 10–6 or autoclaved soil (Additional file 1: Figure S5). The phylum Chytridiomycota was detected only in the 10–1 dilution soil, and Rozellomycota was exclusively found in plants growing in natural soil inoculated or not inoculated with Bacillus strains (Additional file 1: Figure S5).
To better understand how inoculation influenced the assembly of bacterial and fungal communities in the plant rhizosphere under low microbial diversity, β-diversity measurements were separately conducted using samples from each dilution (Additional file 1: Figures S6 and S7). Significant changes in the composition of the bacterial and fungal communities were observed when Bacillus strains were inoculated into autoclaved soil (Fig. 5A, B). The control and UD1022 treatments in natural soil and at dilutions of 10–1, 10–3, and 10–6 did not significantly change the bacterial or fungal β-diversity (Figures S6 and S7).
PCoA analysis of the rhizosphere microbiome of tomato plants cultivated in autoclaved soil. Statistical pairwise comparisons were performed using the Adonis method (P < 0.05, permutation = 999). A Comparison of bacterial communities between the control group and the UD1022 treatment group (P = 0.008). B Comparison of fungal communities in the control group versus the UD1022 treatment group (P = 0.025). C Comparison of the bacterial communities of the control group and the UD1022eps−TasA− treatment group (P = 0.011). D Comparison of fungal communities in the control group versus the UD1022eps−TasA− treatment group (P = 0.012). E Comparison of the bacterial communities of the UD1022eps−TasA− and UD1022-treated groups (P = 0.009). F Comparison of fungal communities in the UD1022eps−TasA− versus UD1022 treatment groups (P = 0.011). Control = non-inoculated plants; UD1022 = plants inoculated with the wild-type strain of B. subtilis; and UD1022eps−TasA− = plants inoculated with the mutant strain of B. subtilis
Role of EPS and TasA in rhizosphere microbiome assembly
Inoculation with the mutant strain UD1022eps−TasA decreased the abundance of the genus Rhizobium in the rhizosphere microbiome of natural soil compared with that in non-inoculated plants (Additional file 1: Figure S8A). Inoculation with the mutant strain resulted in a reduced abundance of Abrothallus and Clonostachys in the natural soil (Additional file 1: Figure S8B). Several bacterial and fungal taxa were detected only when the wild-type strain UD1022 was inoculated (Additional file 1: Figure S9), including Pedobacter spp. ASV-0024 and ASV-0126, Gemmatimonas spp. ASV-0066, Planococcaceae spp. ASV-0078, Flavisolibacter spp. ASV-0089, Curtobacterium spp. ASV-0097, Mesorhizobium spp. ASV-0110, Nocardioides spp. ASV-0181, and Methylorosula spp. ASV-0216. However, some microbial taxa were exclusively detected when the mutant strain UD1022eps−TasA− was inoculated, including Streptomyces spp. ASV-0076, Chthoniobacter spp. ASV-0079, Frankia spp. ASV-0083, Bryobacter spp. ASV-0100, Adhanibacter spp. ASV-0129, Parasegetibacter spp. ASV-0142, Methylorosula spp. ASV-0196, Flavisolibacter spp. ASV-0222, and Micropepsaceae ASV-0091 (Additional file 1: Figure S9).
Analysis of bacterial β-diversity revealed significant differences in the structures of bacterial and fungal communities due to Bacillus inoculation. These differences in β-diversity were observed not only in the comparison between non-inoculated and inoculated plants but also between plants inoculated with the wild-type strain UD1022 or the mutant UD1022eps−TasA− (Fig. 5 and Figures S10-S13). These differences were more remarkable in soils with lower microbial diversity (i.e., autoclaved soil) (Fig. 5).
Differential abundance analysis using ALDEx2 was employed to identify microbial taxa that were differentially enriched in plants inoculated with the wild-type strain or the defective mutant lacking EPS and TasA. Compared with those of the mutants, the abundances of the phyla Verrucomicrobiota (P < 0.01), Patescibacteria (P < 0.05), Nitrospirota (P < 0.02), Bdellovibrinota (P < 0.01), Armatimonadota (P < 0.01), and Actinomycetota (P < 0.01) in the wild-type B. subtilis strains were greater than those in the wild-type strain UD1022 (Fig. 6A). Compared with inoculation with the mutant strain, inoculation with the wild-type strain also increased the abundances of specific members of the fungal community, including Tomentella (P < 0.05), Pseudogymnoascus (P < 0.02), Preussia (P < 0.03), Motirella (P < 0.01), Lectera (P < 0.02), Humicola (P < 0.01), Fusarium (P < 0.01), Exophiala (P < 0.01), and Cystobasidium (P < 0.05) (Fig. 6B).
Heatmaps showing variations in the relative abundances of bacteria (A) and fungi (B) across treatments. ALDEx2 analysis was performed using Monte Carlo distances from the Dirichlet distribution with a P cutoff of 0.05. The color spectrum on the heatmap, ranging from dark purple to orange, represents statistically significant disparities in relative abundance (p < 0.05 to 0.01). A value of 0.01 indicates a more pronounced level of differential enrichment. NS = natural soil, D1 = soil dilution 10–1, D3 = soil dilution 10–3, D6 = soil dilution 10–6, and AS = autoclaved soil. Control = non-inoculated plants; UD1022 = plants inoculated with wild-type B. subtilis; and UD1022eps−TasA− = plants inoculated with mutant B. subtilis
Co-occurrence network analysis of the rhizosphere microbiome
The construction of the bacterial networks revealed that inoculation with Bacillus strains affected the complexity of the network (Fig. 7 and Additional file 1: Table S1). The number of edges in the network of plants inoculated with the wild-type strain UD1022 and the mutant UD1022eps−TasA− was reduced compared with that in the network of non-inoculated plants. A decrease in the number of nodes was also observed in inoculated plants, especially when the mutant UD1022eps−TasA− was inoculated (Fig. 7A). Moreover, a greater number of nodes, modularity, and number of communities were observed when UD1022 was inoculated than when UD1022eps−TasA was inoculated (Fig. 7 and Additional file 1: Table S1). In contrast, compared with wild-type inoculation, mutant inoculation resulted in a greater total number of edges, including negative and positive edges (Additional file 1: Table S1). Thus, compared with the control, UD1022eps−TasA inoculation led to a decrease in the number of positive connections and an increase in the number of negative edges, whereas wild-type UD1022 inoculation resulted in a decrease in the number of positive and negative edges (Fig. 7 and Additional file 1: Table S1).
Co-occurrence network of ASVs according to the Fruchterman Reingold distribution. ASVs were filtered considering occurrence > 20 times and > 30% abundance. A Bacterial community networks. B Fungal community networks. Non-inoculated plant = Control; plant inoculated with wild-type B. subtilis = UD1022; and plant inoculated with mutant B. subtilis = UD1022eps−TasA−
In terms of the fungal community, plants in the control treatment exhibited greater modularity than did those in the rhizosphere of plants inoculated with the wild-type strain UD1022 or the mutant strain UD1022eps−TasA− (Additional file 1: Table S1). Notably, in contrast to the bacterial networks, inoculation with the mutant strain UD1022eps−TasA− improved fungal connections (Fig. 7B). This was evident by an increase in the number of nodes, edges, and average clustering coefficient parameters, surpassing those observed in the control and UD1022 treatments (Additional file 1: Table S1). In addition, compared with the control treatment, UD1022eps−TasA inoculation increased the number of positive and negative connections, whereas UD1022 increased the number of positive edges and decreased the number of negative edges (Additional file 1: Table S1).
Discussion
Previous studies have shown that the Bacillus subtilis strain UD1022 exerts beneficial effects on plant growth and offers protection against plant pathogens [13, 23, 45, 46, 53]. Most experiments involving plant growth-promoting rhizobacteria (PGPR) are conducted using artificial soil or under controlled laboratory conditions [70]. Under such conditions, numerous bacterial isolates exhibit promising traits for plant growth promotion, including siderophore production, phosphate solubilization, and phytohormone synthesis [71]. However, when these microorganisms are applied in more complex systems, such as agricultural soil or under on-farm conditions, many of these traits may be subdued or even remain unexpressed owing to factors such as niche competition, nutrient limitation, antagonistic interactions, and environmental conditions [1, 72, 73].
Therefore, the inoculation of PGPR can encounter various challenges when interacting with the resident soil microbiome, primarily because of the diverse microbial community and the complexity of the relationships present around the roots [73,74,75]. As plants shape the rhizosphere microbiome according to their needs at each life stage [76, 77], soil diversity is considered an important microbial reservoir for plant root recruitment through exudation [3, 78].
Inoculation with the wild-type strain UD1022 increased ~ 100 to 200-fold the number of B. subtilis gyrB genes detected in the rhizosphere of plants growing in soils with diluted microbial diversity compared with that in non-inoculated plants or those inoculated with the mutant strain UD1022eps−TasA. This observation suggests that root colonization by Bacillus is enhanced in soils with lower microbial diversity because less diverse soils can generate more open environments and less nutrient and niche competition, favoring the establishment of inoculants [79,80,81,82,83]. The inverse relationship between soil microbial diversity and the survival of an invading bacterial pathogen was previously demonstrated using the dilution-to-extinction approach [79].
As expected, in soils with lower microbial diversity, the wild-type strain UD1022 was more efficient at colonizing the rhizosphere than the mutant strain UD1022 eps−TasA−. This observation confirms the importance of the EPS and TasA genes in root establishment. Surprisingly, in natural soil, a higher number of gyrB genes were detected when plants were inoculated with the mutant strain. This difference may be attributed to the naturally higher abundance of Bacillus sp. in natural soil, suggesting that inoculation with mutated B. subtilis UD1022eps−TasA stimulated the enrichment of native Bacillus sp. in the rhizosphere. The effects of B. subtilis mutation on biofilm production in soil are not well understood. Moreover, Zhu et al. [84] recently demonstrated a growth-survival fitness trade-off in B. subtilis mutants lacking the master regulator sporulation gene (Spo0A-) in vitro. Their findings showed that Spo0A-null strains exhibited increased growth capacity, both in terms of rate and yield, compared with wild-type B. subtilis because of resource reallocation [84]. However, considering that the primer pair employed to detect B. subtilis was not strain specific, we were not able to discriminate between soil-resident B. subtilis and the inoculated strain UD1022.
Inoculation with the mutant strain UD1022eps−TasA− at a soil dilution of 10–3 resulted in plants with reduced root dry mass compared with that of plants inoculated with the wild-type strain. This result implies that the inability of strain UD1022eps−TasA− to form biofilms prevents effective colonization of the plant rhizosphere, resulting in less effective plant growth promotion compared with that of the UD1022 wild-type. The genetic traits of the inoculant, such as the presence of chemoreceptors and mobility in soil, are crucial for establishing the inoculant in the plant rhizosphere [13, 85, 86]. However, in this study, the resident microbial diversity found in natural soil may have played an important role in promoting plant growth [85].
In this context, the dilution-to-extinction method has been applied to better understand the impacts of microbial invasion on the rhizosphere microbiome [49,50,51,52, 87]. Such research has shed light on changes in soil microbial communities under biotic disturbances [87]. For example, Ferrarezi et al. [88] demonstrated that the PGPR Azospirillum brasilense had a more pronounced beneficial impact on plants growing in soils with lower microbial diversity, as determined by applying the dilution-to-extinction method. It is important to emphasize that the autoclaved soil did not receive any microbial inoculum, and the microbial communities found in this treatment originated naturally from the soil. After autoclaving, the soil was pre-incubated to reach a microbial biomass similar to that of the other treatments but with reduced species richness [52]. Natural ecosystems show variable resistance to invasion by alien species, and this resistance can be related to species diversity in the system [78]. Mawarda et al. [89] reported that the response of the soil microbial community to an introduced organism is also contingent on the nature and extent of the invasion disturbance. This is closely linked to the ecological strategies and functional traits of each invader.
Concurrently, beneficial bacteria and fungi were also enriched when the wild-type strain UD1022 was inoculated. For example, the bacterial genera Chthoniobacter and Pseudarthrobacter are known to contain endophytic/PGPR strains and species that play a role in the transformation of organic carbon compounds in soil [90,91,92,93]. In general, Pseudogymnoascus, Preussia, Humicola, Fusarium, Exophiala, and Cystobasidium were the most enriched fungal genera when UD1022 was inoculated. Interestingly, some of these groups, including Preussia [94, 95], Humicola [96, 97], and Exophiala [98, 99], are known growth promoters in rice. On the other hand, the relative abundance of Actinomycetota, which is a phylum harboring well-known beneficial bacteria [99,100,101,102], decreased in plants inoculated with UD1022eps−TasA− compared to that in plants inoculated with UD1022 (Additional file 1: Figure S4). This observation may imply the importance of the EPS and TasA genes in synergistic interactions among soil resident microbiome communities. Sun and collaborators [103] demonstrated the importance of Bacillus biofilm formation in syntrophic cooperation in soil. Inoculation with the wild-type B. velezensis strain SQR9 positively induced plant-beneficial indigenous Pseudomonas stutzeri in the cucumber rhizosphere by branched-chain amino acid (BCAA) production compared with a mutant defective in EPS and TasA [103]. In the present study, the same pattern was observed in soil dilutions 10–3 and 10–6, where Pseudomonadota, a phylum of the Pseudomonas genus, was significantly enriched when the UD1022 strain was inoculated compared with the UD1022eps−TasA− strain. The impact of UD1022 inoculation on the bacterial community exhibited a stronger effect on the β-diversity of autoclaved soil than on that of natural soil. This observation suggested that the absence of competition and reduced niche occupancy in autoclaved soil may have allowed the inoculant to significantly alter the composition of the bacterial communities [104]. Mallon et al. [81] demonstrated a similar pattern for foreign microbial invaders when the soil microbial composition was compromised, fitting the paradigm of diversity-invasion effects [80,81,82, 105, 106], where less diverse communities have limited abilities to use available resources, and consequently, their ability to mitigate external microbial invasion decreases.
Previous studies have highlighted the important effect of the B. subtilis EPS and TasA genes on social interactions in rhizosphere soil using double-mutated bacteria and soil resident communities [34, 45, 107]. These studies emphasized the effect of the extracellular matrix on the bacterial consortium between two bacterial species and its importance in salt stress tolerance. This study extends this understanding by showing how EPS and TasA gene knockout in B. subtilis affects rhizosphere microbiome assembly.
Biofilm formation is essential for successful rhizosphere colonization, with the TasA gene playing a crucial role in stabilizing biofilm membrane dynamics and enabling cellular adaptation, mainly in plant interactions [34, 107, 108]. In this context, compared with UD1022 inoculation, co-occurrence network analysis revealed that inoculation with the mutant strain UD1022eps−TasA− decreased the number of nodes and increased the number of negative interactions in the bacterial network. On the other hand, plants inoculated with UD1022 exhibited denser connections within subcommunities than within the entire network, which could be one of the stages of biofilm production, which includes microcolony formation [108]. The fungal community network was also altered by inoculation with the mutant strain UD1022eps−TasA−. While the bacterial network decreased the number of nodes in the presence of the mutant strain, the fungal network showed more nodes than did the network in the presence of the wild-type strain UD1022. Therefore, as bacteria engage with eukaryotes, highlighting the significance of social interactions in the coevolution of fungi and bacteria, this dynamic process fosters specific interactions and the potential generation of metabolites that influence network outcomes [109]. Notably, both the core components of the matrix, EPS and TasA, significantly contribute to establishing robust interactions with other microorganisms [110].
Taken together, the results of this study underscore the critical role of the EPS and TasA genes in B. subtilis strain UD1022 for effective plant growth promotion and modulation of soil microbial communities. The presence of these genes significantly influenced microbial β-diversity, especially in less diverse soils, demonstrating their importance in shaping the rhizosphere microbiome. The absence of these genes, as observed in plants inoculated with UD1022eps−TasA−, altered the bacterial and fungal communities, demonstrating their role in social interactions and community dynamics. In addition, co-occurrence network analysis revealed that the absence of the EPS and TasA genes impacted the structure and dynamics of the bacterial networks in the rhizosphere. This study emphasizes that understanding genetic traits such as EPS and TasA is vital for comprehending how PGPRs interact with the rhizosphere microbiome and, consequently, influence plant health and growth. Further research on specific microbiome genetic traits and their implications for rhizosphere colonization will significantly contribute to the optimization of PGPR-based approaches in agriculture.
Availability of data and materials
The raw amplicon sequencing data are available at the NCBI project number PRJNA1054195. The soil sample access activity is registered at SISGen A5EB05F.
References
Berendsen RL, Pieterse CMJ, Bakker PAHM. The rhizosphere microbiome and plant health. Trends Plant Sci. 2012;17(8):478–86. https://doi.org/10.1016/j.tplants.2012.04.001.
Pérez-Jaramillo JE, Mendes R, Raaijmakers JM. Impact of plant domestication on rhizosphere microbiome assembly and functions. Plant Mol Biol. 2016;90:635–44. https://doi.org/10.1007/s11103-015-0337-7.
Mendes R, Garbeva P, Raaijmakers JM. The rhizosphere microbiome: significance of plant-beneficial, plant-pathogenic and human-pathogenic microorganisms. FEMS Microbiol Rev. 2013;37(5):634–63. https://doi.org/10.1111/1574-6976.12028.
Andreote FD, Silva MCP. Microbial communities associated with plants: learning from nature to apply it in agriculture. Curr Opin Microbiol. 2017;37:29–34. https://doi.org/10.1016/j.mib.2017.03.011.
Escudero-Martinez C, Coulter M, Terrazas RA, Foito A, Kapadia R, Pietrangelo L, Maver M, Sharma R, Aprile A, Morris J, Hedley PE, Maurer A, Pillen K, Naclerio G, Mimmo T, Barton GJ, Waugh R, Abbott J, Bulgarelli D. Identifying plant genes shaping microbiota composition in the barley rhizosphere. Nat Commun. 2022;13(3443):1–14. https://doi.org/10.1038/s41467-022-31022-y.
Oyserman BO, Flores SS, Griffioen T, Pan X, van der Wijk E, Pronk L, Lokhorst W, Nurfikari A, Paulson JN, Movassagh M, Stopnisek N, Kupczok A, Cordovez V, Carrión VJ, Ligterink W, Snoek BL, Medema MH, Raaijmakers JM. Disentangling the genetic basis of rhizosphere microbiome assembly in tomato. Nat Commun. 2022;13:3228. https://doi.org/10.1038/s41467-022-30849-9.
Bais HP, Weir TL, Perry LG, Gilroy S, Vivanco JM. The role of root exudates in rhizosphere interactions with plants and other organisms. Ann Rev Plant Biol. 2006;57:233–66. https://doi.org/10.1146/annurev.arplant.57.032905.105159.
Bakker GM, Schlatter DC, Otto-Hanson L, Kinkel LL. Diffuse symbioses: roles of plant-plant, plant-microbe, and microbe-microbe interactions in structuring the soil microbiome. Mol Ecol. 2014;23:1571–83. https://doi.org/10.1111/mec.12571.
Korenblum E, Dong Y, Szymanski J, Panda S, Jozwiak A, Massalha H, Meir S, Rogachev I, Aharoni A. Rhizosphere microbiome mediates systemic root metabolite exudation by root-to-root signaling. PNAS. 2020;117:7. https://doi.org/10.1073/pnas.1912130117.
Philippot L, Raaijmakers JM, Lemanceau P, van der Putten WH. Going back to the roots: the microbial ecology of the rhizosphere. Nat Rev Microbiol. 2013;11:789–99. https://doi.org/10.1038/nrmicro3109.
Sasse J, Martinoia E, Northen T. Feed your friends: do plant exudates shape the root microbiome? Trends Plant Sci. 2018;23(1):25–41. https://doi.org/10.1016/j.tplants.2017.09.003.
Fadiji AE, Barmukh R, Varshney RK, Singh BK. Exploring the connectivity between rhizosphere microbiomes and the plant genes: A way forward for sustainable increase in primary productivity. J Sustain Agric Environ. 2023;1:20. https://doi.org/10.1002/sae2.12081.
Allard-Massicotte R, Tessier L, Lécuyer F, Lakshmanan V, Lucier JF, Garneau D, Caudwell L, Vlamakis H, Bais HP, Beauregard PB. Bacillus subtilis early colonization of Arabidopsis thaliana roots involves multiple chemotaxis receptors. AMS Journals. 2016;7:6. https://doi.org/10.1128/mBio.01664-16.
Babalola OO. Beneficial bacteria of agricultural importance. Biotech Lett. 2010;32(1559):1570. https://doi.org/10.1007/s10529-010-0347-0.
Liu Y, Xu Z, Chen L, Xun W, Shu X, Chen Y, Sun X, Wang Z, Ren Y, Shen Q, Zhang R. Root colonization by beneficial rhizobacteria. FEMS Microbiol Rev. 2024;48:1–20. https://doi.org/10.1093/femsre/fuad066.
Beauregard PB, Chai Y, Vlamakis H, Losick R, Kolter R. Bacillus subtilis biofilm induction by plant polysaccharides. PNAS. 2013. https://doi.org/10.1073/pnas.1218984110.
Bishnoi U, Polson SW, Sherrier DJ, Bais HP. Draft genome sequence of a natural root isolate, Bacillus subtilis UD1022, a potential plant growth-promoting biocontrol agent. Genome Announ. 2015;3:4. https://doi.org/10.1128/genomeA.00696-15.
Liu S, Qin T, Dong B, Shi X, Lv Z, Zhang G. The influence of climate, soil properties and vegetation on soil nitrogen in sloping farmland. Sustainability. 2021;13:1480. https://doi.org/10.3390/su13031480.
Tiwari S, Prasad V, Lata C. Chapter 3–-bacillus: Plant growth promoting bacteria for sustainable agriculture and environment. In: Singh JS, Singh DP, editors. New and Future Developments in Microbial Biotechnology and Bioengineering. Hoboken: Elsevier; 2019. p. 43–55. https://doi.org/10.1016/B978-0-444-64191-5.00003-1.
Kumar AS, Lakshmanan V, Caplan JL, Powell D, Czymmek KJ, Levia DF, Bais HP. Rhizobacteria Bacillus subtilis restricts foliar pathogen entry through stomata. Plant J. 2012;72(4):694–706. https://doi.org/10.1111/j.1365-313X.2012.05116.x.
Martins SJ, Medeiros FHV, Lakshmanan V, Bais HP. Impact of seed exudates on growth and biofilm formation of Bacillus amyloliquefaciens ALB629 in common bean. Front Microbiol. 2018;8:2631. https://doi.org/10.3389/fmicb.2017.02631.
Lakshmanan V, Castaneda R, Rudrappa T, Bais HP. Root transcriptome analysis of Arabidopsis thaliana exposed to beneficial Bacillus subtilis FB17 rhizobacteria revealed genes for bacterial recruitment and plant defense independent of malate efflux. Planta. 2013;238(4):657–68. https://doi.org/10.1007/s00425-013-1920-2.
Zheng W, Zeng S, Bais HP, LaManna JM, Hussey DS, Jacobson DL, Jin Y. Plant growth-promoting rhizobacteria (PGPR) reduce evaporation and increase soil water retention. Water Resour Res. 2018;54:3673–87. https://doi.org/10.1029/2018WR022656.
Poudel M, Mendes R, Costa LAA, Bueno G, Meng Y, Folimonova SY, Garrett KA, Martins SJ. The role of plant-associated bacteria, fungi, and viruses in drought stress mitigation. Front Microbiol. 2021;12: 743512. https://doi.org/10.3389/fmicb.2021.743512.
Tahir H, Gu Q, Wu H, Raza W, Hanif A, Wu L, Colman MV, Gao X. Plant growth promotion by volatile organic compounds produced by Bacillus subtilis SYST2. Front Microbiol. 2017;8:171. https://doi.org/10.3389/fmicb.2017.00171.
Samaras A, Kamou N, Tzelepis G, Karamanoli K, Menkissoglu-Spiroudi U, Karaoglanidis GS. Root transcriptional and metabolic dynamics induced by the plant growth promoting rhizobacterium (PGPR) bacillus subtilis mbi600 on cucumber plants. Plants. 2022;11:1218. https://doi.org/10.3390/plants11091218.
Shahid I, Han J, Hanooq S, Malik KA, Borchers CH, Mehnaz S. Profiling of metabolites of Bacillus spp. and their application in sustainable plant growth promotion and biocontrol. Front Sustain Food Syst. 2021;5:605195. https://doi.org/10.3389/fsufs.2021.605195.
Medeiros FHV, Souza RM, Medeiros FCL, Zhang H, Wheeler T, Payton P, Ferro HM, Paré PW. Transcriptional profiling in cotton associated with Bacillus subtilis (UFLA285) induced biotic-stress tolerance. Plant Soil. 2011;347:327–37. https://doi.org/10.1007/s11104-011-0852-5.
Kumar AS, Lakshmanan V, Caplan JL, Powell D, Czymmek KJ, Levia DF, Bais HP. Rhizobacteria Bacillus subtilis restricts foliar pathogen entry through stomata. Plant J. 2012;72:694–706. https://doi.org/10.1111/j.1365-313X.2012.05116.x.
You C, Zhang C, Kong F, Feng C, Wang J. Comparison of the effects of biocontrol agent Bacillus subtilis and fungicide metalaxyl–mancozeb on bacterial communities in tobacco rhizospheric soil. Ecol Eng. 2016;91:119–25. https://doi.org/10.1016/j.ecoleng.2016.02.011.
Cucu MA, Gilard G, Pugliese M, Matic S, Gisi U, Gullino ML, Garibaldi A. Influence of different biological control agents and compost on total and nitrification-driven microbial communities at rhizosphere and soil level in a lettuce–-Fusarium oxysporum f. sp. lactucae pathosystem. J Appl Microbiol. 2018;126:905–18. https://doi.org/10.1111/jam.14153.
Etesami H, Jeong BR, Glick BR. Potential use of Bacillus spp as an effective biostimulant against abiotic stresses in crops—a review. Curr Res Biotechnol. 2023;5:100128. https://doi.org/10.1016/j.crbiot.2023.100128.
Beauregard PB, Chai Y, Vlamakis H, Losick R, Kolter R. Bacillus subtilis biofilm induction by plant polysaccharides. PNAS. 2013;110:17. https://doi.org/10.1073/pnas.1218984110.
Arnaouteli S, Bamford NC, Stanley-Wall NR, Kovács ÁT. Bacillus subtilis biofilm formation and social interactions. Nat Rev. 2021;19:600–14. https://doi.org/10.1038/s41579-021-00540-9.
Kobayashi K, Iwano M. BslA(YuaB) forms a hydrophobic layer on the surface of Bacillus subtilis biofilms. Mol Microbiol. 2012;85(1):51–66. https://doi.org/10.1111/j.1365-2958.2012.08094.x.
Hobley L, Ostrowski A, Rao FV, Bromley KM, Porter M, Prescott AR, MacPhee CE, van Aalten DMF, Stanley-Wall NR. BslA is a self-assembling bacterial hydrophobin that coats the Bacillus subtilis biofilm. PNAS. 2013;110(33):13600–5. https://doi.org/10.1073/pnas.1306390110.
Cámara-Almirón J, Navarro Y, Díaz-Martínez L, Magno-Pérez-Bryan MC, Molina-Santiago C, Pearson JR, de Vicente A, Pérez-García A, Romero D. Dual functionality of the amyloid protein TasA in Bacillus physiology and fitness on the phylloplane. Nat Commun. 1859;2020:11. https://doi.org/10.1038/s41467-020-15758-z.
Pandit S, Fazilati M, Gaska K, Derouiche A, Nypelo T, Mijakovic I, Kádár R. The exo-polysaccharide component of extracellular matrix is essential for the viscoelastic properties of Bacillus subtilis biofilms. Int J Mol Sci. 2020;21:6755. https://doi.org/10.3390/ijms21186755.
Costa OYA, Raaijmakers JM, Kuramae EE. Microbial extracellular polymeric substances: ecological function and impact on soil aggregation. Front Microbiol. 2018. https://doi.org/10.3389/fmicb.2018.01636.
Chen Y, Yan F, Chai Y, Liu H, Kolter R, Losick R, Guo J. Biocontrol of tomato wilt disease by Bacillus subtilis isolates from natural environments depends on conserved genes mediating biofilm formation. Environ Microbiol. 2013;15(3):848–64. https://doi.org/10.1111/j.1462-2920.2012.02860.x.
Ansari FA, Ahmad I, Pichtel J. Synergistic effects of biofilm-producing PGPR strains on wheat plant colonization, growth and soil resilience under drought stress. Saudi J Biol Sci. 2023;30: 103664. https://doi.org/10.1016/j.sjbs.2023.103664.
Martin M, Dragos A, Holscher T, Maróti G, Bálint B, Westermann M, Kovács ÁT. De novo evolved interference competition promotes the spread of biofilm defectors. Nat Commun. 2017;8:15127. https://doi.org/10.1038/ncomms15127.
Martin M, Dragos A, Otto SB, Schafer D, Brix S, Maróti G, Kovács ÁT. Cheaters shape the evolution of phenotypic heterogeneity in Bacillus subtilis biofilms. ISME J. 2020;14:2302–12. https://doi.org/10.1038/s41396-020-0685-4.
Rosier A, Beauregard PB, Bais HP. Quorum quenching activity of the PGPR Bacillus subtilis UD1022 alters nodulation efficiency of Sinorhizobium meliloti on Medicago truncatula. Front Microbiol. 2021;11: 596299. https://doi.org/10.3389/fmicb.2020.596299.
Kaniz F, Zheng W, Bais HP, Jin Y. Plant growth-promoting rhizobacteria mediate soil hydro-physical properties: an investigation with Bacillus subtilis and its mutants. Vadose Zone J. 2023. https://doi.org/10.1002/vzj2.20274.
Kaur C, Fidanza M, Ervin E, Bais HP. Spo0A-dependent antifungal activity of a plant growth promoting rhizobacteria Bacillus subtilis strain UD1022 against the dollar spot pathogen (Clarireedia jacksonii). Biol Control. 2023;184: 105284. https://doi.org/10.1016/j.biocontrol.2023.105284.
Blake C, Christensen MN, Kovács AT. Molecular aspects of plant growth promotion and protection by Bacillus subtilis. MPMI. 2021;34(1):15–25. https://doi.org/10.1094/MPMI-08-20-0225-CR.
Web Soil Survey. USDA. https://websoilsurvey.sc.egov.usda.gov. Accessed 27 Jan 2023
Hol WHG, Boer W, Termorshuizen AJ, Meyer KM, Schneider JHM, van Dam NM, van Veen JA, van der Putten WH. Reduction of rare soil microbes modifies plant–herbivore interactions. Ecol Lett. 2010;13:292–301. https://doi.org/10.1111/j.1461-0248.2009.01424.x.
Wertz S, Degrange V, Posser JI, Poly F, Commeaux C, Freitag T, Guillaumaud N, Le Roux X. Maintenance of soil functioning following erosion of microbial diversity. Environ Microbiol. 2006;8(12):2162–9. https://doi.org/10.1111/j.1462-2920.2006.01098.x.
de Souza AJ, de Andrade PAM, Pereira APA, Andreote FD, Tornisielo VL, Regitano JB. The depleted mineralization of the fungicide chlorothalonil derived from loss in soil microbial diversity. Sci Rep. 2017;7:14646. https://doi.org/10.1038/s41598-017-14803-0.
Philippot L, Spor A, Hénault C, Bru D, Bizouard F, Jones CM, Sarr A, Maron PA. Loss in microbial diversity affects nitrogen cycling in soil. ISME J. 2013;7:1609–19. https://doi.org/10.1038/ismej.2013.34.
Rosier A, Pomerleau M, Beauregard PB, Samac DA, Bais HP. Surfactin and Spo0A-dependent antagonism by Bacillus subtilis strain UD1022 against Medicago sativa phytopathogens. Plants. 2023;12:1007. https://doi.org/10.3390/plants12051007.
Apprill A, Mcnally S, Parsons R, Weber L. Minor revision to V4 region SSU rRNA 806R gene primer greatly increases detection of SAR11 bacterioplankton. Aquat Microb Ecol. 2015;75:129–37. https://doi.org/10.3354/ame01753.
Parada AE, Needham DM, Fuhrman JA. Every base matters: assessing small subunit rRNA primers for marine microbiomes with mock communities, time series and global field samples. Environ Microbiol. 2015;18(5):1403–14. https://doi.org/10.1111/1462-2920.13023.
White TJ, Bruns T, Lee S, Taylor J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenies. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ, editors. PCR Protocols: A Guide to Methods and Applications. San Diego: Academic Press; 1990. p. 315–22.
Xie S, Yu H, Wang Q, Cheng Y, Ding T. Two rapid and sensitive methods based on TaqMan qPCR and droplet digital PCR assay for quantitative detection of Bacillus subtilis in rhizosphere. J Appl Microbiol. 2019;128:518–27. https://doi.org/10.1111/jam.14481.
Callahan BJ, McMurdie PJ, Rosen MJ, Han AW, Johnson AJA, Holmes SP. DADA2: high-resolution sample inference from Illumina amplicon data. Nat Methods. 2016;13:581–3. https://doi.org/10.1038/nmeth.3869.
Martin M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet J. 2011;17(1):10–2. https://doi.org/10.14806/ej.17.1.200.
Quast C, Pruesse E, Yilmaz P, Gerken J, Schweer T, Yarza P, Peplies J, Glockner FO. The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic Acids Res. 2013;41(1):590–6. https://doi.org/10.1093/nar/gks1219.
Yilmaz P, Parfrey LW, Yarza P, Gerken J, Pruesse E, Quast C, Schweer T, Peplies J, Ludwig W, Glockner FO. The SILVA and All-species Living Tree Project (LTP) taxonomic frameworks. Nucleic Acid Res. 2014;42:D643–8. https://doi.org/10.1093/nar/gkt1209.
Nilsson RH, Larsson KH, Taylor AFS, Bengtsson-Palme J, Jeppesen TS, Schigel D, Kennedy P, Pizard K, Glockner FO, Tedersoo L, Saar I, Kõljalg U, Abarenkov K. The UNITE database for molecular identification of fungi: handling dark taxa and parallel taxonomic classifications. Nucleic Acids Res. 2018;47(1):259–64. https://doi.org/10.1093/nar/gky1022.
Abarenkov K, Zirk A, Piirmann T, Pöhönen R, Ivanov F, Nilsson RH, Kõljalg U. UNITE general FASTA release for Fungi. Version 16.10.2022. UNITE Community. 2022. https://doi.org/10.15156/BIO/2483911
Kõljalg U, Nilsson HR, Schigel D, Tedersoo L, Larsson KH, May TW, Taylor AFS, Jeppesen TS, Frøslev TG, Lindahl BD, Põldmaa K, Saar I, Suija A, Savchenko A, Yatsiuk I, Adojaan K, Ivanov F, Piirmann T, Pöhönen R, Zirk A, Abarenkov K. The taxon hypothesis paradigm—On the unambiguous detection and communication of taxa. Microorganisms. 2020;8:12. https://doi.org/10.3390/microorganisms8121910.
Riggs MR, Haroldson KJ, Hanson MA. Analysis of covariance models for data from observational field studies. J Wildl Manag. 2008;72(1):34–43. https://doi.org/10.2193/2007-315.
Fernandes AD, Reid JN, Macklaim JM, McMurrough TA, Edgell DR, Gloor GB. Unifying the analysis of high-throughput sequencing datasets: characterizing RNA-seq, 16S rRNA gene sequencing and selective growth experiments by compositional data analysis. Microbiome. 2014;2:15. https://doi.org/10.1186/2049-2618-2-15.
Cao Y, Dong Q, Wang D, Zhang P, Liu Y, Niu C. microbiomeMarker: an R/Bioconductor package for microbiome marker identification and visualization. Bioinformatics. 2022. https://doi.org/10.1093/bioinformatics/btac438.
Smith SD. Phylosmith: an R-package for reproducible and efficient microbiome analysis with phyloseq-objects. J Open-Source Softw. 2019;4(38):1442. https://doi.org/10.21105/joss.01442.
Bastian M, Heymann S, Jacomy M. Gephi: an open source software for exploring and manipulating networks. In: International AAAI Conference on Weblogs and Social Media;2009.
Zhang X, Ling C, Wu X, Fan S, Liang Q, Zhou F. Bacterial diversity and function shift of strawberry root in different cultivation substrates. Rhizosphere. 2023. https://doi.org/10.1016/j.rhisph.2023.100696.
Shah A, Nazari M, Antar M, Msimbira LA, Naamala J, Lyu D, Rabileh M, Zajonc J, Smith DL. PGPR in agriculture: a sustainable approach to increasing climate change resilience. Front Sustain Food Syst. 2021;5: 667546. https://doi.org/10.3389/fsufs.2021.667546.
Singh BK, Trivedi P, Egidi E, Macdonald CA, Delgado-Baquerizo M. Crop microbiome and sustainable agriculture. Nat Rev Microbiol. 2020;18(11):601–2. https://doi.org/10.1038/s41579-020-00446-y.
Garrido-Sanz D, Čaušević S, Vacheron J, Heiman CM, Sentchilo V, van der Meer JR, Keel C. Changes in structure and assembly of a species-rich soil natural community with contrasting nutrient availability upon establishment of a plant-beneficial Pseudomonas in the wheat rhizosphere. Microbiome. 2023;11:214. https://doi.org/10.1186/s40168-023-01660-5.
Jones DL, Hinsinger P. The rhizosphere: complex by design. Plant Soil. 2008. https://doi.org/10.1007/s11104-008-9774-2.
Shi S, Nuccio EE, Shi ZJ, He Z, Zhou J, Firestone MK. The interconnected rhizosphere: high network complexity dominates rhizosphere assemblages. Ecol Lett. 2016;19:926–36. https://doi.org/10.1111/ele.12630.
Bulgarelli D, Schlaeppi K, Spaepen S, Ver Loren van Themaat E, Schulze-Lefert P. Structure and functions of the bacterial microbiota of plants. Ann Rev Plant Biol. 2013;64:807–38. https://doi.org/10.1146/annurev-arplant-050312-120106.
Chaparro JM, Badri DV, Vivanco JM. Rhizosphere microbiome assemblage is affected by plant development. ISME J. 2014;8(4):790–803. https://doi.org/10.1038/ismej.2013.196.
Mendes L, Mendes R, Raaijmakers JM, Tsai SM. Breeding for soil-borne pathogen resistance impacts active rhizosphere microbiome of common bean. ISME J. 2018;12:3038–42. https://doi.org/10.1038/s41396-018-0234-6.
Van Elsas JD, Chiurazzi M, Mallon CA, Elhottovã D, Krištufek V, Salles JF. Microbial diversity determines the invasion of soil by a bacterial pathogen. PNAS. 2012;109(4):1159–64. https://doi.org/10.1073/pnas.1109326109/-/DCSupplemental.
Elton C. Ecology of invasions by animals and plants. New York: Springer; 1958.
Mallon CA, Le Roux X, van Doorn GS, Andreote FD, Poly F, Salles JF. The impact of failure: unsuccessful bacterial invasions steer the soil microbial community away from the invader’s niche. ISME J. 2018;12:728–41. https://doi.org/10.1038/s41396-017-0003-y.
Mawarda PC, Lakke S, Van Elsas JD, Salles JF. Temporal dynamics of the soil bacterial community following Bacillus invasion. iScience. 2022;25:104185. https://doi.org/10.1016/j.isci.2022.104185.
Callaghan M, Ballard RA, Wright D. Soil microbial inoculants for sustainable agriculture: limitations and opportunities. Soil Use Manag. 2022;38:1340–69. https://doi.org/10.1111/sum.12811.
Zhu M, Wang Q, Mu H, Faz F, Wang Y, Dai X. A fitness trade-off between growth and survival governed by Spo0A-mediated proteome allocation constraints in Bacillus subtilis. Sci Adv. 2023;9:eadg9733. https://doi.org/10.1126/sciadv.adg9733.
Poppeliers SWM, Sánchez-Gil JJ, de Jonge R. Microbes to support plant health: understanding bioinoculant success in complex conditions. Curr Opin Microbiol. 2023;73:102286. https://doi.org/10.1016/j.mib.2023.102286.
Engelhardt IC, Patko D, Liu Y, Mimault M, Martinez GH, George TS, MacDonald M, Ptashnyk M, Sukhodub T, Stanley-Wall NR, Holden N, Daniell TJ, Dupuy LX. Novel form of collective movement by soil bacteria. ISME J. 2022;16:2337–47. https://doi.org/10.1038/s41396-022-01277-w.
Yan Y, Kuramae EE, Klinkhamer PG, van Veen JA. Revisiting the dilution procedure used to manipulate microbial biodiversity in terrestrial systems. Appl Environ Microbiol. 2015;81(13):4246–52. https://doi.org/10.1128/AEM.00958-15.
Ferrarezi JA, Defant H, Souza LF, Azevedo JL, Hungria M, Quecine MC. Meta-omics integration approach reveals the effect of soil native microbiome diversity in the performance of inoculant Azospirillum brasilense. Front Plant Sci. 2023;14:1172839. https://doi.org/10.3389/fpls.2023.1172839.
Mawarda PC, Mallon CA, Le Roux X, van Elsas JD, Salles JF. Interactions between bacterial inoculants and native soil bacterial community: the case of spore-forming Bacillus spp. FEMS Microbiol Ecol. 2022. https://doi.org/10.1093/femsec/fiac127.
Sangwan P, Chen X, Hugenholtz P, Janssen PH. Chthoniobacter flavus gen. nov., sp. Nov., the first pure-culture representative of subdivision two, Spartobacteria classis nov., of the phylum Verrucomicrobia. Appl Environ Microbiol. 2004;70(10):5875–81. https://doi.org/10.1128/AEM.70.10.5875-5881.2004.
Li M, Yang F, Wu X, Yan H, Liu Y. Effects of continuous cropping of sugar beet (Beta vulgaris L) on its endophytic and soil bacterial community by high-throughput sequencing. Ann Microbiol. 2020;70:39. https://doi.org/10.1186/s13213-020-01583-8.
Tshishonga K, Serepa-Dlamini MH. Draft genome sequence of Pseudarthrobacter phenanthrenivorans strain MHSD1, a bacterial endophyte isolated from the medicinal plant Pellaea calomelanos. Evolut Bioinf. 2020. https://doi.org/10.1177/1176934320913257.
Ham SH, Yoon AR, Oh HE, Park YG. Plant growth-promoting microorganism Pseudarthrobacter sp. NIBRBAC000502770 enhances the growth and flavonoid content of Geum aleppicum. Microorganisms. 2022;10:6. https://doi.org/10.3390/microorganisms10061241.
Khan AL, Asaf S, Khan AR, Al-Harrasi A, Al-Rawahi A, Lee IJ. First draft genome sequencing of indole acetic acid producing and plant growth promoting fungus Preussia sp. BSL10. J Biotechnol. 2016;225:44–5. https://doi.org/10.1016/j.jbiotec.2016.03.025.
Al-Hosni K, Shahzad R, Khan AL, Imran QM, Harrasi AA, Rawahi AA, Asaf R, Kang SM, Yun BW, Lee IJ. Preussia sp. BSL-10 producing nitric oxide, gibberellins, and indole acetic acid and improving rice plant growth. J Plant Interact. 2018;13(1):112–8. https://doi.org/10.1080/17429145.2018.1432773.
Ibrahim SRM, Mohamed SGA, Altyar AE, Mohamed GA. Natural products of the fungal genus Humicola: diversity, biological activity, and industrial importance. Curr Microbiol. 2021;78:2488–509. https://doi.org/10.1007/s00284-021-02533-6.
Steindorff AS, Serra LA, Formighieri EF, Faria FP, Poças-Fonseca MJ, Almeida JRM. Insights into the lignocellulose-degrading enzyme system of Humicola grisea var. thermoidea based on genome and transcriptome analysis. Microbiology Spectrum. 2021;9(2):e01088-e1121. https://doi.org/10.1128/Spectrum.01088-21.
Zhang C, Sirijovski N, Adler L, Ferrari BC. Exophiala macquariensis sp nov, a cold adapted black yeast species recovered from a hydrocarbon contaminated sub-Antarctic soil. Fung Biol. 2019;123:151–8. https://doi.org/10.1016/j.funbio.2018.11.011.
Boubekri K, Soumare A, Lyamlouli K, Ouhdouch Y, Hefidi M, Kouisni L. Improving the efficiency of phosphate rocks combined with phosphate solubilizing Actinomycetota to increase wheat growth under alkaline and acidic soils. Front Plant Sci. 2023;14:1154372. https://doi.org/10.3389/fpls.2023.1154372.
Ibrahimi M, Loqman S, Jemo M, Hafidi M, Lemee L, Ouhdouch Y. The potential of facultative predatory Actinomycetota spp. and prospects in agricultural sustainability. Front Microbiol. 2023. https://doi.org/10.3389/fmicb.2022.1081815.
Nguyen TP, Meng DR, Chang CH, Su PY, Ou CA, Hou PF, Sung HM, Chou CH, Ohme-Takagi M, Huang HJ. Antifungal mechanism of volatile compounds emitted by Actinomycetota Paenarthrobacter ureafaciens from a disease-suppressive soil on Saccharomyces cerevisiae. Antimicrob Chemother. 2023;8(5):e00324-e423. https://doi.org/10.1128/msphere.00324-23.
Yang Q, Song Z, Li X, Hou Y, Xu T, Wu S. Lichen-derived actinomycetota: novel taxa and bioactive metabolites. Int J Mol Sci. 2023;24:7342. https://doi.org/10.3390/ijms24087341.
Sun X, Xu Z, Xie J, Hesselberg-Thomsen V, Tan T, Zheng D, Strube ML, Dragos A, Shen Q, Zhang R, Kovács ÁT. Bacillus velezensis stimulates resident rhizosphere Pseudomonas stutzeri for plant health through metabolic interactions. ISME J. 2022;16:774–87. https://doi.org/10.1038/s41396-021-01125-3.
Molina-Santiago C, Pearson JR, Navarro Y, Berlanga-Clavero MV, Caraballo-Rodriguez AM, Petras D, García-Martín ML, Lamon G, Haberstein B, Cazorla FM, Vicente A, Loquet A, Dorrestein PC, Romero D. The extracellular matrix protects Bacillus subtilis colonies from Pseudomonas invasion and modulates plant co-colonization. Nat Commun. 1919;2019:10. https://doi.org/10.1038/s41467-019-09944-x.
Tilman D. Niche tradeoffs, neutrality, and community structure: a stochastic theory of resource competition, invasion, and community assembly. PNAS. 2004;101(30):10854–61. https://doi.org/10.1073/pnas.0403458101.
Tilman D, Isbell F, Cowles JM. Biodiversity and ecosystem functioning. Biodivers Ecosyst Funct. 2014;45:471–93. https://doi.org/10.1146/annurev-ecolsys-120213-091917.
Bogino PC, Oliva MM, Sorroche FG, Giordano W. The role of bacterial biofilms and surface components in plant-bacterial associations. Int J Mol Sci. 2013;14:15838–59. https://doi.org/10.3390/ijms140815838.
Penesyan A, Paulsen IT, Kjelleberg S, Gillings MR. Three faces of biofilms: a microbial lifestyle, a nascent multicellular organism, and an incubator for diversity. NPJ Biofilms Microbiomes. 2021;7:80. https://doi.org/10.1038/s41522-021-00251-2.
Kjeldgaard B, Listian SA, Ramaswamhi V, Richter A, Kiesewalter HT, Kovács ÁT. Fungal hyphae colonization by Bacillus subtilis relies on biofilm matrix components. Biofilm. 2019;1: 100007. https://doi.org/10.1016/j.bioflm.2019.100007.
Xiong W, Guo S, Jousset A, Zhao Q, Wu H, Li R, Kowalchuk GA, Shen Q. Bio-fertilizer application induces soil suppressiveness against Fusarium wilt disease by reshaping the soil microbiome. Soil Biol Biochem. 2017;114:238–47. https://doi.org/10.1016/j.soilbio.2017.07.016.
Acknowledgements
The authors acknowledge the University of Delaware, mainly Bais Lab, for the infrastructure and intellectual support.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study was supported by the São Paulo Research Foundation (FAPESP Processes No. 2021/14711-2, 2020/00469-2 and 2020/06077-9), the Brazilian National Council for Scientific and Technological Development (CNPq Process No. 302147/2022-5), and the USDA Multistate HACTH Grant.
Author information
Authors and Affiliations
Contributions
RM, HB, and CSN designed the project. CSN conducted the experiments, and CSN and JPV performed the data analysis. CSN and RM wrote the manuscript, and all the authors have read, revised, and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1:
The Supplementary Information contains additional data and results.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Nishisaka, C.S., Ventura, J.P., Bais, H.P. et al. Role of Bacillus subtilis exopolymeric genes in modulating rhizosphere microbiome assembly. Environmental Microbiome 19, 33 (2024). https://doi.org/10.1186/s40793-024-00567-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40793-024-00567-4