Abstract
Burkholderia sp. strain UYPR1.413 is an aerobic, motile, Gram-negative, non-spore-forming rod that was isolated from a root nodule of Parapiptadenia rigida collected at the Angico plantation, Mandiyu, Uruguay, in December 2006. A survey of symbionts of P. rigida in Uruguay demonstrated that this species is nodulated predominantly by Burkholderia microsymbionts. Moreover, Burkholderia sp. strain UYPR1.413 is a highly efficient nitrogen fixing symbiont with this host. Currently, the only other sequenced isolate to fix with this host is Cupriavidus sp. UYPR2.512. Therefore, Burkholderia sp. strain UYPR1.413 was selected for sequencing on the basis of its environmental and agricultural relevance to issues in global carbon cycling, alternative energy production, and biogeochemical importance, and is part of the GEBA-RNB project. Here we describe the features of Burkholderia sp. strain UYPR1.413, together with sequence and annotation. The 10,373,764 bp high-quality permanent draft genome is arranged in 336 scaffolds of 342 contigs, contains 9759 protein-coding genes and 77 RNA-only encoding genes.
Similar content being viewed by others
Introduction
Rhizobia are soil bacteria that have acquired the ability to establish symbiotic associations with plants, mainly from the Fabaceae family, and carry out the Biological Nitrogen Fixation (BNF) process. BNF is catalyzed by the rhizobial nitrogenase complex, whereby N2 is reduced to ammonium.
Well-known and studied rhizobia are those belonging to the α-proteobacteria (eg. Azorhizobium , Bradyrhizobium , Ensifer , Mesorhizobium and Rhizobium ). In 2001 symbiotic nitrogen fixing bacteria belonging to the group of Betaproteobacteria were reported as root nodule bacteria, introducing the term of Alpha and Beta-rhizobia to differentiate both groups of rhizobia [1, 2]. The Beta-rhizobia identified so far belong to only two genera: Burkholderia and Cupriavidus and the association seem to be mainly with plants from the Mimosoideae subfamily [3]. Additionally, studies indicate that the South American Mimosa genus is preferentially nodulated by Beta-rhizobia [4]. Different Beta-rhizobia species have been described belonging to the Burkholderia genus (eg. B. caballeronis , B. caribensis , B. diazotrophica , B. dilworthii , B. mimosarum , B. nodosa , B. phymatum , B. rhynchosiae , B. sabiae , B. sprentiae , B. symbiotica and B. tuberum ) but only two in the Cupriavidus genus ( C. taiwanensis and C. necator ) [2, 5–17].
Burkholderia sp. UYPR1.413 strain has been isolated from a root nodule of Parapiptadenia rigida (Benth.) Brenan found in an angico plantation in Artigas, Uruguay [18]. P. rigida belongs to the Mimosoideae subfamily and is a woody species, which can reach 30 m in height and a diameter of 60 to 80 cm [19]. The wood is of excellent quality, heavy, elastic, very hard and quite durable, rich in tannins and has medicinal properties [20]. There are six different species of Parapiptadenia in the Americas of which only P. rigida is present in Uruguay. A survey of symbionts of P. rigida in Uruguay demonstrated that this species is nodulated by rhizobia belonging to the genera Burkholderia , Cupriavidus and Rhizobium , of which the Burkholderia microsymbionts predominated [18]. Burkholderia sp. UYPR1.413 strain belongs to a group of microsymbionts that were able to nodulate and fix nitrogen with P. rigida [18]. In this work we present the description of the Burkholderia sp. UYPR1.413 high-quality permanent draft genome sequence and its annotation.
Organism information
Classification and features
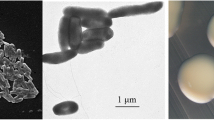
Burkholderia sp. strain UYPR1.413 is a motile, Gram-negative, non-spore-forming rod (Fig. 1 Left, Center) in the order Burkholderiales of the class Betaproteobacteria . The rod-shaped form varies in size with dimensions of 0.3–0.5 μm in width and 1.0–2.0 μm in length (Fig. 1 Left). It is fast growing, forming 0.5–1 mm diameter colonies after 24 h when grown on TY [21] at 28 °C. Colonies on TY are white-opaque, slightly domed, moderately mucoid with smooth margins (Fig. 1 Right).
Figure 2 shows the phylogenetic relationship of Burkholderia sp. strain UYPR1.413 in a 16S rRNA gene sequence based tree. This strain is phylogenetically most related to Burkholderia sabiae Br3407T, Burkholderia caribensis MWAP64T and Burkholderia phymatum STM815T with sequence identities to UYPR1.413 16S rRNA gene sequence of 98.96, 98.64 and 98.56 %, respectively, as determined using the EzTaxon-e server [22]. Burkholderia sabiae Br3407T was first isolated from root nodules of Mimosa caesalpiniifolia , a native tree to Brazil [6]. Burkholderia caribensis MWAP64T was first isolated from vertisol in Martinique [5] and related strains have been identified as a plant growth promoting bacteria for grain Amaranth and Mango trees [23, 24] and nitrogen fixing root nodule bacteria for several Mimosa species [25, 26]. Burkholderia phymatum STM815T is also known to nodulate effectively with several Mimosa species [27]. Minimum Information about the Genome Sequence (MIGS) [28] is provided in Table 1.
Phylogenetic tree highlighting the position of Burkholderia sp. strain UYPR1.413 (shown in blue print) relative to other type and non-type strains in the Burkholderia genus using 1046 bp internal region of the 16S rRNA gene. Several Alpha-rhizobia sequences were used as outgroup. All sites were informative and there were no gap-containing sites. Phylogenetic analyses were performed using MEGA, version 5.05 [47]. The tree was built using the maximum likelihood method with the General Time Reversible model. Bootstrap analysis with 500 replicates was performed to assess the support of the clusters. Type strains are indicated with a superscript T. Strains with a genome sequencing project registered in GOLD [30] have the GOLD ID provided after the strain number. Finished genomes are designated with an asterisk
Symbiotaxonomy
Burkholderia sp. strain UYPR1.413 was isolated from Parapiptadenia rigida , a Mimosoideae legume native to Uruguay [18]. This tree is native to South America, including south Brazil, Argentina, Paraguay, and Uruguay, and used by locals for timber and as a source of gums, tannins and essential oils [18]. Burkholderia sp. strain UYPR1.413 is able to renodulate its original host and is highly efficient in fixing nitrogen with this host [18]. A selection of host plants, including Trifolium repens , Medicago sativa , Peltophorum dubium and Mimosa pudica were investigated previously for their ability to nodulate with UYPR1.413 and only M. pudica plants were nodulated by UYPR1.413, albeit ineffectively [18].
Genome sequencing information
Genome project history
This organism was selected for sequencing on the basis of its environmental and agricultural relevance to issues in global carbon cycling, alternative energy production, and biogeochemical importance, and is part of the Genomic Encyclopedia of Bacteria and Archaea, The Root Nodulating Bacteria chapter (GEBA-RNB) project at the U.S. Department of Energy, Joint Genome Institute (JGI) for projects of relevance to agency missions [29]. The genome project is deposited in the Genomes OnLine Database [30] and the high-quality permanent draft genome sequence in IMG [31]. Sequencing, finishing and annotation were performed by the JGI using state of the art sequencing technology [32]. A summary of the project information is shown in Table 2.
Growth conditions and genomic DNA preparation
Burkholderia sp. strain UYPR1.413 was grown to mid logarithmic phase in TY rich media [21] on a gyratory shaker at 28 °C. DNA was isolated from 60 mL of cells using a CTAB (Cetyl trimethyl ammonium bromide) bacterial genomic DNA isolation method [33].
Genome sequencing and assembly
The draft genome of Burkholderia sp. UYPR1.413 was generated at the DOE Joint genome Institute (JGI) using state of the art technology [32]. An Illumina Std shotgun library was constructed and sequenced using the Illumina HiSeq 2000 platform which generated 23,255,298 reads totaling 3488.3 Mbp. All general aspects of library construction and sequencing performed at the JGI can be found at the JGI web site [34]. All raw Illumina sequence data was passed through DUK, a filtering program developed at JGI, which removes known Illumina sequencing and library preparation artifacts (Mingkun L, Copeland A, Han J. unpublished). The following steps were then performed for assembly: (1) filtered Illumina reads were assembled using Velvet version 1.1.04 [35] (2) 1–3 Kbp simulated paired end reads were created from Velvet contigs using wgsim [36] (3) Illumina reads were assembled with simulated read pairs using Allpaths-LG (version r41043) [37]. Parameters for assembly steps were: 1) Velvet (velveth: 63-shortPaired and velvetg: –very clean yes –exportFiltered yes –min contig lgth 500 –scaffolding no –cov cutoff 10) 2) wgsim (–e 0 –1 100 –2 100 –r 0 –R 0 –X 0) 3) Allpaths-LG (PrepareAllpathsInputs: PHRED 64 = 1 PLOIDY = 1 FRAG COVERAGE = 125 JUMP COVERAGE = 25 LONG JUMP COV = 50, RunAllpathsLG: THREADS = 8 RUN = std shredpairs TARGETS = standard VAPI WARN ONLY = True OVERWRITE = True). The final draft assembly contained 342 contigs in 336 scaffolds. The total size of the genome is 10.4 Mbp and the final assembly is based on 1214.2 Mbp of Illumina data, which provides an average of 117.1× coverage of the genome.
Genome annotation
Genes were identified using Prodigal [38], as part of the DOE-JGI genome annotation pipeline [39, 40] followed by a round of manual curation using GenePRIMP [41] for finished genomes and Draft genomes in fewer than 10 scaffolds. The predicted CDSs were translated and used to search the National Center for Biotechnology Information (NCBI) non-redundant database, UniProt, TIGRFam, Pfam, KEGG, COG, and InterPro databases. The tRNAScanSE tool [42] was used to find tRNA genes, whereas ribosomal RNA genes were found by searches against models of the ribosomal RNA genes built from SILVA [43]. Other non-coding RNAs such as the RNA components of the protein secretion complex and the RNase P were identified by searching the genome for the corresponding Rfam profiles using INFERNAL [44]. Additional gene prediction analysis and manual functional annotation was performed within the Integrated Microbial Genomes-Expert Review (IMG-ER) system [45] developed by the Joint Genome Institute, Walnut Creek, CA, USA.
Genome properties
The genome is 10,373,764 nucleotides with 62.28 % GC content (Table 3) and comprised of 336 scaffolds and 342 contigs (Fig. 3). From a total of 9836 genes, 9759 were protein encoding and 77 RNA only encoding genes. The majority of genes (75.92 %) were assigned a putative function whilst the remaining genes were annotated as hypothetical. The distribution of genes into COGs functional categories is presented in Table 4.
Graphical map of the four largest scaffolds of the genome of Burkholderia sp. strain UYPR1.413. From the bottom to the top of each scaffold: Genes on forward strand (color by COG categories as denoted by the IMG platform), Genes on reverse strand (color by COG categories), RNA genes (tRNAs green, sRNAs red, other RNAs black), GC content, GC skew
Conclusion
Burkholderia sp. UYPR1.413 belongs to a group of Beta-rhizobia isolated from Parapiptadenia rigida , a native tree from Uruguay belonging to the Mimosoideae legume group [18]. This tree is also native to the south of Brazil, Argentina and Paraguay [18]. Phylogenetic analysis revealed that UYPR1.413 is most closely related to Burkholderia sabiae Br3407T, Burkholderia caribensis MWAP64T and Burkholderia phymatum STM815T. Interestingly, Br3407T was isolated from nitrogen-fixing nodules on the roots of Mimosa caesalpiniifolia , a legume tree native to Brazil [6]. MWAP64T has not been reported to nodulate legume plants, however B. caribensis TJ182 is able to nodulate and fix nitrogen with Mimosa pigra [7]. STM815T was originally isolated from Macroptilium atropurpureum but could not be authenticated on this host [1]. Additional studies showed that STM815T is instead able to nodulate a wide range of Mimosa species [27]. Glasshouse experiments from previous studies have shown that Burkholderia sp. UYPR1.413 is also able to nodulate Mimosa pudica seedlings, albeit ineffectively [18]. However, it is different from the other microsymbiont in that it can form an effective association with Parapiptadenia rigida . The only other sequenced isolate to fix with this host is Cupriavidus sp. UYPR2.512 [46]. There are in total 13 Burkholderia strains that are known legume symbionts; four (WSM3556T, WSM4176, WSM5005T, STM678T) nodulate South African papilionoid species, in contrast to the other nine (BR3459, CCGE1002, DSM 21604, JPY251, JPY366, LMG 23256T, STM815, STM3621 and UYPR1.413) that are able to nodulate mimosoid species. A comparison of the mimosoid nodulating strains reveals that UYPR1.413 has the largest genome (10.4 Mbp), with the highest KOG count (1670) and the lowest GC (65.28 %) percentage in this group. All 13 of these genomes share the nitrogenase-RXN MetaCyc pathway catalyzed by a multiprotein nitrogenase complex. However, only Burkholderia sp. UYPR1.413 has been shown to fix effectively with Parapiptadenia rigida . The genome attributes of Burkholderia sp. UYPR1.413 will therefore be important for ongoing molecular analysis of the plant microbe interactions required for the establishment of leguminous tree symbioses with this host.
Abbreviations
- GEBA-RNB:
-
Genomic Encyclopedia of Bacteria and Archaea-Root Nodule Bacteria
- JGI:
-
Joint Genome Institute
- TY:
-
Trypton Yeast
- CTAB:
-
Cetyl trimethyl ammonium bromide
- WSM:
-
Western Australian Soil Microbiology
- BNF:
-
Biological Nitrogen Fixation
References
Moulin L, Munive A, Dreyfus B, Boivin-Masson C. Nodulation of legumes by members of the beta-subclass of Proteobacteria. Nature. 2001;411:948–50.
Chen WM, Laevens S, Lee TM, Coenye T, De Vos P, Mergeay M, et al. Ralstonia taiwanensis sp. nov., isolated from root nodules of Mimosa species and sputum of a cystic fibrosis patient. Int J Syst Evol Microbiol. 2001;51:1729–35.
Gyaneshwar P, Hirsch AM, Moulin L, Chen WM, Elliott GN, Bontemps C, et al. Legume-nodulating betaproteobacteria: diversity, host range, and future prospects. Mol Plant Microbe Interact. 2011;24:1276–88.
dos Reis Jr FB, Simon MF, Gross E, Boddey RM, Elliott GN, Neto NE, et al. Nodulation and nitrogen fixation by Mimosa spp. in the Cerrado and Caatinga biomes of Brazil. New Phytol. 2010;186:934–46.
Achouak W, Christen R, Barakat M, Martel MH, Heulin T. Burkholderia caribensis sp. nov., an exopolysaccharide-producing bacterium isolated from vertisol microaggregates in Martinique. Int J Syst Bacteriol. 1999;49:787–94.
Chen WM, de Faria SM, Chou J, James EK, Elliott GN, Sprent JI, et al. Burkholderia sabiae sp. nov., isolated from root nodules of Mimosa caesalpiniifolia. Int J Syst Evol Microbiol. 2008;58:2174–9.
Chen WM, James EK, Chou JH, Sheu SY, Yang SZ, Sprent JI. Beta-rhizobia from Mimosa pigra, a newly discovered invasive plant in Taiwan. New Phytol. 2005;168:661–75.
De Meyer SE, Cnockaert M, Ardley JK, Van Wyk B-E, Vandamme PA, Howieson JG. Burkholderia dilworthii sp. nov., isolated from Lebeckia ambigua root nodules. Int J Syst Evol Microbiol. 2014;64:1090–5.
Martinez-Aguilar L, Salazar-Salazar C, Mendez R, Caballero-Mellado J, Hirsch AM, Vasquez-Murrieta MS, et al. Burkholderia caballeronis sp nov., a nitrogen fixing species isolated from tomato (Lycopersicon esculentum) with the ability to effectively nodulate Phaseolus vulgaris. Antonie Leeuwenhoek. 2013;104:1063–71.
Sheu S-Y, Chou J-H, Bontemps C, Elliott GN, Gross E, dos Reis Junior FB, et al. Burkholderia diazotrophica sp. nov., isolated from root nodules of Mimosa spp. Int J Syst Evol Microbiol. 2013;63:435-441.
Sheu S-Y, Chou J-H, Bontemps C, Elliott GN, Gross E, James EK, et al. Burkholderia symbiotica sp. nov., isolated from root nodules of Mimosa spp. native to north-east Brazil. Int J Syst Evol Microbiol. 2012;62:2272–8.
Chen WM, de Faria SM, James EK, Elliott GN, Lin KY, Chou JH, et al. Burkholderia nodosa sp nov., isolated from root nodules of the woody Brazilian legumes Mimosa bimucronata and Mimosa scabrella. Int J Syst Evol Microbiol. 2007;57:1055–9.
Chen WM, James EK, Coenye T, Chou JH, Barrios E, de Faria SM, et al. Burkholderia mimosarum sp nov., isolated from root nodules of Mimosa spp. from Taiwan and South America. Int J Syst Evol Microbiol. 2006;56:1847–51.
De Meyer SE, Cnockaert M, Ardley JK, Maker G, Yates R, Howieson JG, et al. Burkholderia sprentiae sp. nov., isolated from Lebeckia ambigua root nodules. Int J Syst Evol Microbiol. 2013;63:3950–7.
De Meyer SE, Cnockaert M, Ardley JK, Trengove RD, Garau G, Howieson JG, et al. Burkholderia rhynchosiae sp. nov., isolated from Rhynchosia ferulifolia root nodules. Int J Syst Evol Microbiol. 2013;63:3944–9.
Vandamme P, Coenye T. Taxonomy of the genus Cupriavidus: a tale of lost and found. Int J Syst Evol Microbiol. 2004;54:2285–9.
Vandamme P, Goris J, Chen WM, de Vos P, Willems A. Burkholderia tuberum sp. nov. and Burkholderia phymatum sp. nov., nodulate the roots of tropical legumes. Syst Appl Microbiol. 2002;25:507–12.
Taule C, Zabaleta M, Mareque C, Platero R, Sanjurjo L, Sicardi M, et al. New betaproteobacterial rhizobium strains able to efficiently nodulate Parapiptadenia rigida (Benth.) Brenan. Appl Environ Microbiol. 2012;78:1692–700.
Jobson RW, Luckow M. Phylogenetic study of the genus Piptadenia (Mimosoideae : Leguminosae) using plastid trnL-F and trnK/matK sequence data. Syst Bot. 2007;32:569–75.
Izaguirre P, Beyhaut R. Las leguminosas en Uruguay y regiones vecinas. Uruguay: Agropecuaria Hemisferio Sur; 2003.
Beringer JE. R factor transfer in Rhizobium leguminosarum. J Gen Microbiol. 1974;84:188–98.
Kim O-S, Cho Y-J, Lee K, Yoon S-H, Kim M, Na H, et al. Introducing EzTaxon-e: a prokaryotic 16S rRNA gene sequence database with phylotypes that represent uncultured species. Int J Syst Evol Microbiol. 2012;62:716–21.
de los Santos-Villalobos S, de Folter S, Delano-Frier JP, Gomez-Lim MA, Guzman-Ortiz DA, Pena-Cabriales JJ. Growth promotion and flowering induction in mango (Mangifera indica L. cv “Ataulfo”) trees by Burkholderia and Rhizobium Inoculation: morphometric, biochemical, and molecular events. J Plant Growth Regul. 2013;32:615–27.
Parra-Cota FI, Pena-Cabriales JJ, de los Santos-Villalobos S, Martinez-Gallardo NA, Delano-Frier JP. Burkholderia ambifaria and B. caribensis promote growth and increase yield in grain amaranth (Amaranthus cruentus and A. hypochondriacus) by improving plant nitrogen uptake. PLoS One. 2014;9:14.
Chen W, de Faria SM, Straliotto R, Pitard RM, Simões-Araùjo JL, Chou J, et al. Proof that Burkholderia strains form effective symbioses with legumes: a study of novel Mimosa-nodulating strains from South America. Appl Environ Microbiol. 2005;71:7461–71.
Chen WM, Moulin L, Bontemps C, Vandamme P, Bena G, Boivin-Masson C. Legume symbiotic nitrogen fixation by beta-proteobacteria is widespread in nature. J Bacteriol. 2003;185:7266–72.
Elliott GN, Chen WM, Chou JH, Wang HC, Sheu SY, Perin L, et al. Burkholderia phymatum is a highly effective nitrogen-fixing symbiont of Mimosa spp. and fixes nitrogen ex planta. New Phytol. 2007;173:168–80.
Field D, Garrity G, Gray T, Morrison N, Selengut J, Sterk P, et al. Towards a richer description of our complete collection of genomes and metagenomes “Minimum Information about a Genome Sequence” (MIGS) specification. Nat Biotechnol. 2008;26:541–7.
Reeve W, Ardley J, Tian R, Eshraghi L, Yoon J, Ngamwisetkun P, et al. A genomic encyclopedia of the root nodule bacteria: assessing genetic diversity through a systematic biogeographic survey. Stand Genomic Sci. 2015;10:14.
Pagani I, Liolios K, Jansson J, Chen IM, Smirnova T, Nosrat B, et al. The Genomes OnLine Database (GOLD) v. 4: status of genomic and metagenomic projects and their associated metadata. Nucleic Acids Res. 2012;40:D571–9.
Markowitz VM, Chen I-MA, Palaniappan K, Chu K, Szeto E, Pillay M, et al. IMG 4 version of the integrated microbial genomes comparative analysis system. Nucleic Acids Res. 2014;42:D560–7.
Mavromatis K, Land ML, Brettin TS, Quest DJ, Copeland A, Clum A, et al. The fast changing landscape of sequencing technologies and their impact on microbial genome assemblies and annotation. PLoS One. 2012;7, e48837.
CTAB DNA extraction protocol. [http://jgi.doe.gov/collaborate-with-jgi/pmo-overview/protocols-sample-preparation-information/].
JGI Website. [http://www.jgi.doe.gov].
Zerbino D, Birney E. Velvet: algorithms for de novo short read assembly using de Bruijn graphs. Genome Res. 2008;18:821–9.
wgsim. [https://github.com/lh3/wgsim].
Gnerre S, MacCallum I, Przybylski D, Ribeiro FJ, Burton JN, Walker BJ, et al. High-quality draft assemblies of mammalian genomes from massively parallel sequence data. Proc Natl Acad Sci U S A. 2011;108:1513–8.
Hyatt D, Chen GL, Locascio PF, Land ML, Larimer FW, Hauser LJ. Prodigal: prokaryotic gene recognition and translation initiation site identification. BMC Bioinformatics. 2010;11:119.
Chen IM, Markowitz VM, Chu K, Anderson I, Mavromatis K, Kyrpides NC, et al. Improving microbial genome annotations in an integrated database context. PLoS One. 2013;8, e54859.
Mavromatis K, Ivanova NN, Chen IM, Szeto E, Markowitz VM, Kyrpides NC. The DOE-JGI standard operating procedure for the annotations of microbial genomes. Stand Genomic Sci. 2009;1:63–7.
Pati A, Ivanova NN, Mikhailova N, Ovchinnikova G, Hooper SD, Lykidis A, et al. GenePRIMP: a gene prediction improvement pipeline for prokaryotic genomes. Nat Methods. 2010;7:455–7.
Lowe TM, Eddy SR. tRNAscan-SE: a program for improved detection of transfer RNA genes in genomic sequence. Nucleic Acids Res. 1997;25:955–64.
Pruesse E, Quast C, Knittel K, Fuchs BM, Ludwig W, Peplies J, et al. SILVA: a comprehensive online resource for quality checked and aligned ribosomal RNA sequence data compatible with ARB. Nucleic Acids Res. 2007;35:7188–96.
INFERNAL. Inference of RNA alignments. [http://infernal.janelia.org].
Markowitz VM, Mavromatis K, Ivanova NN, Chen IM, Chu K, Kyrpides NC. IMG ER: a system for microbial genome annotation expert review and curation. Bioinformatics. 2009;25:2271–8.
De Meyer S, Fabiano E, Tian R, Van Berkum P, Seshadri R, Reddy T, et al. High-quality permanent draft genome sequence of the Parapiptadenia rigida-nodulating Cupriavidus sp. strain UYPR2.512. Stand Genomic Sci. 2015;10:13.
Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. MEGA5: Molecular Evolutionary Genetics Analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol. 2011;28:2731–9.
Field D, Amaral-Zettler L, Cochrane G, Cole JR, Dawyndt P, Garrity GM, et al. The Genomic Standards Consortium. PLoS Biol. 2011;9:e1001088.
Woese CR, Kandler O, Wheelis ML. Towards a natural system of organisms: proposal for the domains Archaea, Bacteria, and Eucarya. Proc Natl Acad Sci U S A. 1990;87:4576–9.
Chen WX, Wang ET, Kuykendall LD. The Proteobacteria. New York: Springer; 2005.
Validation of publication of new names and new combinations previously effectively published outside the IJSEM. Int J Syst Evol Microbiol. 2005;55:2235–38.
Garrity GM, Bell JA, Lilburn TE. Class II. Betaproteobacteria. In: Garrity GM, Brenner DJ, Krieg NR, Staley JT, editors. Bergey’s manual of systematic bacteriology, vol. 2. 2nd ed. New York: Springer; 2005.
Garrity GM, Bell JA, Lilburn TE. Order 1. Burkholderiales. In: Garrity GM, Brenner DJ, Krieg NR, Staley JT, editors. Bergey’s manual of systematic bacteriology, vol. 2. 2nd ed. New York: Springer; 2005.
Garrity GM, Bell JA, Lilburn TE. Family I. Burkholderiaceae. In: Garrity GM, Brenner DJ, Krieg NR, Staley JT, editors. Bergey’s manual of systematic bacteriology, vol. 2. 2nd ed. New York: Springer; 2005.
Palleroni NJ. Genus I. Burkholderia. In: Garrity GM, Brenner DJ, Krieg NR, Staley JT, editors. Bergey’s manual of systematic bacteriology, vol. 2. 2nd ed. New York: Springer; 2005.
Ashburner M, Ball CA, Blake JA, Botstein D, Butler H, Cherry JM, et al. Gene ontology: tool for the unification of biology. The Gene Ontology Consortium. Nat Genet. 2000;25:25–9.
Acknowledgements
This work was performed under the auspices of the US Department of Energy’s Office of Science, Biological and Environmental Research Program, and by the University of California, Lawrence Berkeley National Laboratory under contract No. DE-AC02-05CH11231, Lawrence Livermore National Laboratory under Contract No. DE-AC52-07NA27344, and Los Alamos National Laboratory under contract No. DE-AC02-06NA25396.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
EF supplied the strain and background information for this project, PVB supplied DNA to JGI, TR performed all imaging, SDM and WR drafted the paper, JH provided financial support and all other authors were involved in sequencing the genome and editing the final manuscript. All authors read and approved the final manuscript.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
De Meyer, S.E., Fabiano, E., Tian, R. et al. High-quality permanent draft genome sequence of the Parapiptadenia rigida-nodulating Burkholderia sp. strain UYPR1.413. Stand in Genomic Sci 10, 31 (2015). https://doi.org/10.1186/s40793-015-0018-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40793-015-0018-9