Abstract
Background
Interspecific competition is known to be strongest between those species that are both closely related and sympatric. Egrets are colonially nesting wetland birds that often overlap and can therefore be expected to compete in roosting and nesting habitat as well as in diet. According to the niche partitioning hypothesis, it is to be expected that these similar species would show differentiation in at least one of the main niche dimensions to reduce competition. We tested niche partitioning between the colonially nesting Little Egret (Egretta garzetta) and Cattle Egret (Bubulcus ibis) in temporal, spatial and trophic dimensions.
Methods
Field study was conducted in three mixed egret colonies in Yangxian County, southwest Shaanxi Province, central China. For each nest colony we recorded its spatial location, the height of nesting trees and of nests, the height of roosting trees and of roosting individuals within the trees. We determined the first egg-laying and first hatching dates of the two species. Craw dissection of storm-killed egret nestlings was used to measure the diet. Six transects were surveyed to study foraging habitat selection.
Results
We found that hatching time of Little Egrets peaked earlier (by about 1 month) than that of Cattle Egrets. Cattle Egrets nested and roosted higher than Little Egrets. The foraging habitats used by Little Egrets were dominated by river banks (73.49%), followed by paddy fields (13.25%) and reservoirs (10.84%), whereas Cattle Egret foraging sites were characterized by grasslands (44.44%), paddy fields (33.33%) and river banks (22.22%). Little Egrets consumed more fishes (65.66%) and Odonata larvae (13.69%) than Cattle Egrets, while Cattle Egrets were found feeding mainly on Coleoptera (29.69%) and Orthoptera (23.29%). Little Egrets preyed on larger mean biomasses of food items than Cattle Egrets.
Conclusions
Our results confirm the niche partitioning hypothesis as a mechanism for coexistence among ecologically similar species. In two coexisting egret species, niche partitioning is multidimensional, such that the two coexistent species occupy differing ecological space based on all three temporal, spatial and trophic niche dimensions.
Similar content being viewed by others
Background
How closely related sympatric species can coexist has long been a major issue in ecology (Hutchinson 1959; Pianka 2000; Beaulieu and Sockman 2012) and is essential for understanding the maintenance of diversity (Chesson 2000; Levine and HilleRisLambers 2009; Kent and Sherry 2020). Gause’s theorem states that two species cannot occur in the same place if the niche characteristics they occupy are extremely similar (Gause 1934). Niche theory predicts that similar species when in coexistence should show differentiation in at least one of the main niche dimensions (food, space and/or time) in order to escape the negative impacts of interspecific competition (MacArthur and Levins 1967; Schoener 1974; Hutchinson 1991; Julliard et al. 2006; Cameron et al. 2007). In mixed-species heronries, where hundreds or thousands of breeding birds of more than one species can coexist (Parsons 1995), nesting and foraging resources are critical for ardeid species during the breeding season (McCrimmon 1978; Fasola 1994; Kazantzidis et al. 1997). Competitive interactions are known to be stronger between closely related sympatric ardeid species (Bolton et al. 2019). There have been numerous studies on niche partitioning between ardeid species, but most of them have only investigated the diets of various herons (e.g., Trexler et al. 1994; Salazar et al. 2005; Boyle et al. 2012; Choi et al. 2016; Ashoori et al. 2017). And there were a few studies on nest site selection (Jenni 1969; McCrimmon 1978; Burger 1979; Parejo et al. 1999; Metallaoui et al. 2020) and temporal separation between species within heron colonies (Weber 1975; Burger 1978; Ashkenazi and Yom-Tov 1997). Despite those separate studies of diet, nest site selection and nesting phenology, we still do not have a comprehensive understanding of niche partitioning that integrates the temporal, spatial and trophic dimensions of sympatric ardeid species.
The Little Egret (Egretta garzetta) is widespread throughout Europe and Asia (Wong et al. 2000). It is closely related to the Cattle Egret (Bubulcus ibis) which was once native only to Africa, Europe and Asia, but dramatically expanded its geographic range to America and Australia during the last century (Arendt 1988; Massa et al. 2014). These two ardeid species often form mixed nesting colonies that may contain hundreds and thousands of breeding pairs (Kushlan and Hafner 2000), and may compete in terms of roosting and nesting habitat as well as diet (Snow et al. 1998). However, little study has yet tested potential niche partitioning between these species along the three main ecological dimensions simultaneously.
In this study, we combined reproductive time, habitat selection and diet to test the mechanism facilitating coexistence of Little and Cattle Egret during the breeding season. Specifically, the objectives of this study were to examine whether the two species partitioned their (1) onset of laying the first egg, (2) use of nesting sites, roosting trees and foraging habitats, and (3) dietary composition and biomass. We hypothesized that Little Egrets and Cattle Egrets would differ in these temporal, spatial and trophic niche dimensions.
Methods
Study area
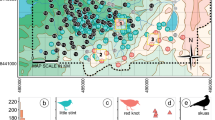
The study was conducted in Yangxian County (33° 06′–33° 36′ N, 107° 17′–108° 02′ E), southwest Shaanxi Province, central China (Fig. 1). The study area is approximately 600 km2. The mean annual temperature is between 12 and 14 °C, with maximum temperatures that can exceed 38.7 °C during summer. Average annual rainfall ranges from 900 to 1000 mm. The annual mean frostless season is 238 days. Local vegetation is dominated by Masson’s Pine (Pinus massoniana), Chinese Arborvitae (Platycladus orientalis), Pagoda Tree (Sophora japonica), Siberian Elm (Ulmus pumila), Sawtooth Oak (Quercus acutissima), Oriental Oak (Quercus variabilis), Black Locust (Robinia pseudoacacia), Chinese Chestnut (Castanea mollissima) and Ring-cupped Oak (Cyclobalanopsis glauca) (Ding 2004). In our study area, some of the Little Egrets are permanent residents but most are migratory and arrived in Yangxian County in late March. The entire Cattle Egret population is migratory and arrived in Yangxian County in late April. The breeding season of the two species occurs within the period from early April to late August. There were approximately 450 pairs of Little Egret and 500 pairs of Cattle Egret in three nesting colonies (Leichaogou Reservoir 180 pairs of Little Egret and 150 pairs of Cattle Egret; Donglian Village: 200 pairs of Little Egret and 240 pairs of Cattle Egret; Caoba Village: 70 pairs of Little Egret and 110 pairs of Cattle Egret). The three colonies were similar in structural appearance and vegetation (e.g., Oriental Oak, Sawtooth Oak, Siberian Elm were found within our sampled area). The elevation of the colonies ranged from 459 to 593 m above sea level.
Sampling design
Data were collected from March to September 2012 in all three colonies (Fig. 1). Dates of first egg-laying and dates of first nestlings in openly visible nests were checked in each colony three or four times per week from March to late June. A total of 108 nests were monitored including 54 of Cattle Egrets and 54 of Little Egrets. We randomly chose one nest per tree to minimise the impact of absolute tree height measurements on the nest height data. If there were nests of both species on a single tree, we randomly selected one nest of each species. In total, 27 Little Egret nests and 34 Cattle Egret nests were sampled. For each nest, we recorded its spatial location (eTrex20, Garmin, USA), measured the height of nesting trees, and the height of the nests above the ground (Diastimeter 202342, Bushnell, USA). Roost site characteristics of the two egret species were investigated in August: on locating a roosting tree containing both species, we firstly measured the height of the roosting trees (Diastimeter 202342, Bushnell, USA) and within each roosting tree, the height above ground of the roosting position of each species was measured by Diastimeter (Diastimeter 202342, Bushnell, USA). We randomly chose one individual per tree, and randomly selected one nest of each species if both species on a single tree. A total of 46 roost positions of Little Egrets and 57 of Cattle Egrets were measured.
Six transects were surveyed to study foraging habitat selection (Fig. 1). Each transect covered the major habitat types in the study area. Two investigators riding motorcycles surveyed along the transects at a speed of 10 km/h and censused the egrets using 10 × 42 binoculars. The habitats were classified into four types: grasslands (level marshes and mudflats with a vegetation cover of more than 30%), river banks (the river floodplain and bank), paddy fields (the area that farmers planted with rice and maintained water within the rice) and reservoirs (the natural or artificial lakes including small ponds with mudflats along their edges). We recorded the foraging habitat where each bird was sighted. All the surveys were conducted from 7:00 to 11:00 a.m. The length of each transect was between 8 and 20 km (total 85 km). We conducted the surveys once a month from April through June. Foraging habitat was recorded for 273 individuals including 196 Little Egrets and 77 Cattle Egrets.
The low reproductive success of colonial egrets and herons has often been reported (Teal 1965; Dusi and Dusi 1968; Maxwell and Kale 1977). Rainfall and strong wind are common causes of breeding failure for egret and heron species (Frederick and Collopy 1989; Baxter 1994; Zhu and Zou 2001). In our study, during heavy monsoon weather, strong winds and rain sometimes upset nests, so that some egret nestlings fell from nests and died naturally. Dead birds were sought after any day of heavy rain or strong winds, and dead egret nestlings were collected below the nests in order to avoid major disturbance to the colony. A total of 5 dead Little Egret nestlings and 17 Cattle Egret nestlings were collected during the breeding season. Dead birds were immediately stored in freezers until craw dissection. Prey items were identified and quantified by counting undigested pieces (Brown and Ewins 1996; Martínez 2004). To estimate the biomass of the prey items, measurements of total or partial length of prey were taken and compared with length-mass regression functions calculated for each taxon (Dorn et al. 2011).
Data analysis
The one-sample Kolmogorov–Smirnov test was used to determine whether data were normally distributed (Field 2009). We conducted a Mann–Whitney U test to test the difference in breeding dates between the two species, as data were not distributed normally even after being transformed. We made univariate comparisons using an independent-sample t-test for significance of differences in nest height of the two species as the data were normally distributed, but we used a Mann–Whitney U test on nest tree height, roost tree height and roost height since these data were not normally distributed. Chi-squared test was used to examine whether the two species used different foraging habitats (Zar 1999).
Prey items were classified by taxon (at family or order level) and mass (Martínez 2010). The frequencies of every prey class in the taxon categories over all the prey items were calculated as percentages. Niche overlap of the two species was estimated using:
where Oij denotes dietary overlap, Pij and Pik represent proportions use of food type i by species j and k. This equation gives values between 0 and 1 which signify no overlap and complete overlap respectively (Pianka 1973; Liordos and Kontsiotis 2020). Chi-squared test was used to compare the frequencies of prey items (Zar 1999). An independent-sample t-test was used to test different mean biomass of the prey for data that met the conditions of independence and normality (Zar 1999). All probabilities are two-tailed, and the significance level was set at α = 0.05. Values are presented with mean ± SE. For all statistical calculations we used software SPSS 18.0 for Windows (SPSS 2009).
Results
Reproductive time
Little Egrets started breeding on 7 April and ended by 4 July, and Cattle Egrets started breeding on 1 May and ended by 2 August. The overlap in breeding period was 72.7% (64 of 88 d) for Little Egrets and 68.8% (64 of 94 d) for Cattle Egrets.
The average egg-laying date of Little Egrets (19 April ± 8 days, n = 54) was significantly different from that of Cattle Egrets (16 May ± 8 days, n = 54; Mann–Whitney U test, Z = − 8.827, P < 0.001) (Fig. 2). By the time that Cattle Egrets began laying, 92.6% of Little Egret chicks had already hatched from the eggs.
Nesting and roosting height
Nesting tree height of Little Egrets (13.22 ± 1.18 m, n = 27) were similar in altitude with Cattle Egrets (13.07 ± 1.28 m, n = 34; Mann–Whitney U test, Z = − 0.408, P = 0.683). Nest height of Little Egret (10.87 ± 1.63 m, n = 27) differed significantly from that of Cattle Egret (11.79 ± 1.37 m, n = 34; Independent-sample t-test, t = − 2.473, P = 0.018).
Cattle Egrets (11.71 ± 1.88 m, n = 46) also preferred to roost significantly higher than Little Egrets (12.60 ± 1.31 m, n = 57; Mann–Whitney U test, Z = − 2.410, P = 0.016). However, roost tree height was not significantly different between Little egret (13.08 ± 1.54 m, n = 46) and Cattle egret (13.27 ± 1.17 m, n = 57; Mann–Whitney U test, Z = − 0.440, P = 0.660).
Foraging habitats
A Chi-squared test showed a significant difference in foraging habitats between the two egret species (χ2 = 85.422, df = 3, P < 0.001). The foraging habitats used by Little Egrets were dominated by river banks (73.49%), followed by paddy fields (13.25%) and reservoirs (10.84%) (Fig. 3), whereas Cattle Egret’s foraging habitats were dominated by grasslands (44.44%), paddy fields (33.33%) and river banks (22.22%) (Fig. 3).
Nestling dietary selection
The trophic niche overlap between nestlings of the two egret species was 0.377 calculated by prey taxon abundance and 0.257 by prey individual biomass. The degree of overlap was related to significant differences between the two species in their diet (Chi-squared test, χ2 = 6.926, df = 15, P = 0.008), which in both species consisted entirely of animal food provided by the parents. The Little Egret nestlings received fishes (6.20 ± 2.22 items/craw and average biomass 1.02 ± 0.32 g/item) and Odonata larvae (5.40 ± 3.34 items/craw and average biomass 0.24 ± 0.00 g/item) (Table 1). Cattle Egret nestlings did not receive any fish but were fed mainly Coleoptera (25.65 ± 4.72 items/craw and average biomass 0.20 ± 0.02 g/item) and Orthoptera (34.80 ± 9.81 items/craw and average biomass 0.18 ± 0.04 g/item) (Table 1). The mean biomasses of the prey fed to Little Egret nestlings (0.40 ± 0.45 g/item) were significantly higher than those fed to Cattle Egret nestlings (0.20 ± 0.54 g/item; Independent-sample t-test, t = − 4.116, df = 1639, P < 0.001). Taxonomic diversity of prey was greater in Cattle Egrets (14 categories in Table 1) than in Little Egrets (8 categories). Cattle Egrets took predominantly land-based prey, whereas the prey fed to Little Egrets included more aquatic taxa.
Discussion
As predicted, our results demonstrated significant differences in the timing of reproduction, habitat utilization, and diet fed to nestlings between two egret species. Thus they segregated in the use of the temporal, spatial and trophic niche dimensions, resulting in reduced interspecific competition during the breeding season. Our results are in agreement with the niche partition hypothesis, whereby morphologically, ecologically and closely related sympatric species segregate in at least one of the niche dimensions to allow coexistence.
Along the time dimension, our results underlined a clear pattern of niche partitioning in the mean clutch initiation date between the two species. Egg laying and hatching of the Cattle Egrets peaked about 1 month later than that of the Little Egrets. Such differentiation in reproductive timing can play an important role for coexisting species as it is related to the availability and choice of nesting sites and food resources (Ye et al. 2019), which could maximize their fitness (Sanz-Aguilar et al. 2015). The late arrival of Cattle Egrets enabled them to take over some abandoned nests of Little Egrets, saving time and energy in nest construction that would lead to low interspecific competition (Burger 1978). Another way of saving energy while building nests is probably by stealing nesting material, which was observed in this study (e.g., we observed 43 nest building events from May 14‒17, including 67.4% by stealing material and 32.6% events by picking twigs) mainly in Cattle Egrets and reported also by other studies (Burger 1978; Ashkenazi and Yom-Tov 1997).
The reproductive period is a critical time in the annual life cycle, when a lot of energy is expended and trophic resources may be limited and must be geared to meet the specific needs of the developing chick (Fasola 1994; Samraoui et al. 2012). By the time Cattle Egrets began nesting in Yangxian County, 92.6% of Little Egret chicks had already hatched. Variations in the growth rate of Little Egrets have been detected and at the age of 23 days, young Little Egrets have normally reached 82% of adult weight, beyond which growth is slow (Zhu et al. 2005). The mean incubation period of Cattle Egrets is 24.2 days (Zhu and Zou 2001), so that when Cattle Egrets in our study area first hatched, the peak in energy demand for chick development of Little Egrets has passed. Temporal partitioning may thus facilitate coexistence of these two species. Another factor related to the late arrival date of Cattle Egrets might be a benefit from the abundance of insects they consume. Cattle Egrets nestlings feed primarily on insects (Siegfried 1971; Si Bachir et al. 2001; Table 1), and insects are typically plentiful during June to August (e.g., Huang et al. 2005). Postponing reproduction might involve in the adaptation of Cattle Egret to local environments (Lack 1968; Sanz-Aguilar et al. 2015).
Nests sites are important for rearing offspring and nest height is a key characteristic that can influence nest survival and reproductive success (MacDonald et al. 2016; Jara et al. 2020; Overduijn et al. 2020). Competition for nesting space should be intense where nesting birds congregate in dense colonies during the breeding season (Kazantzidis et al. 1997). The advantages of nesting higher may relate to increased visibility of predators, increased ability to take flight quickly when predators approach, and decreased losses to ground predators (Burger 1982). Although Cattle Egrets in our study area arrived and began nesting later than Little Egrets, our results showed that Cattle Egret nests were located higher in trees by a small but statistically significant amount. There are theoretical reasons to suppose that higher nest positions should be preferred, such as unobstructed flight paths and nest hygiene (e.g., McCrimmon 1978; Parejo et al. 1999; Metallaoui et al. 2020). Therefore it is interesting that Little Egrets as first nesters seem not to choose the theoretically best positions. One possible reason is that the local vegetation structure (i.e., thin twigs located higher in the tree crown) could influence nest-site selection of ardeid birds (Zhu and Zou 2001). As Cattle Egrets are the smaller species, they are able to utilize nest positions among higher and thinner twigs that are not available to Little Egrets. Additionally, there can be aggressive contests between species (e.g., we observed 6 events when Cattle Egrets acted aggressively towards Little Egrets from May 14‒17, but only 2 events when Little Egrets attacked Cattle Egrets), and these can allow species to overcome the competitive disadvantage of small size (Hino 2005; Martin and Ghalambor 2014). Burger (1982) found that Cattle Egrets had the highest aggression rates and successfully defended higher perches compared to other egret species, which might account for their ability to nest higher up.
While the nestling diets of the two species showed partial niche overlap, there were striking dietary differences in exploiting food resources. There is general agreement that insects make up the most important dietary component for Cattle Egret nestlings (Siegfried 1971; Fogarty and Hetrick 1973; Ashoori et al. 2017). Most of the studies carried out to date show that the diet of Little Egret nestlings is mainly composed of fish (Zhou et al. 2000, 2003; Ashoori et al. 2017). Our sample size of only 5 Little Egret nestlings would normally be considered too small to give a stand-alone result—65.7% fish in the present study—but the close agreement with other studies quoted, and the fact that not a single fish was included in our sample of more than 1500 food items for Cattle Egret nestlings, indicating that our results reflect a real difference in nestling diet of the two species. One adaptive explanation for the dietary difference is that it allows resource partitioning between the two egrets, thereby increasing individual fitness through reducing interspecies competition (Ashoori et al. 2017; Nicolaus et al. 2019). In addition, Little Egrets examined in the present study preyed on larger mean biomasses of the food items than Cattle Egrets. It has been tested that prey size or biomass serves as a partitioning mechanism among herons (Britton and Moser 1982). Food type and size of a species is often closely related to foraging site selection (Kasahara and Katoh 2008; Martínez 2010; Jensen et al. 2017). Using different feeding sites may serve to subdivide the resource spectrum (Cody 1968). Our results demonstrated that the egrets used different foraging habitats contributing to their coexistence in an area of sympatry. Little Egrets foraged in shallow, open waters using river banks whereas Cattle Egrets foraged mainly in grasslands and paddy fields. The differential exploitation of food resources may be the result of foraging site selection (Samraoui et al. 2012; Choi et al. 2016).
Conclusions
Our results support the occurrence of niche partitioning in two closely related egret species, reducing competition and allowing them to coexist. Niche partitioning is multidimensional, for example, differences in preferred foraging sites (grassland versus river banks) are related to foraging methods (on land or in water) and food type (dryland insects or wetland fish and larvae). Niche differences are also related to chick provisioning with either a small taxonomic range of large-bodied prey or a wider taxonomic range of small-bodied prey.
References
Arendt WJ. Range expansion of the cattle egret (Bubulcus ibis) in the Greater Caribbean Basin. Colon Waterbirds. 1988;11:252–62.
Ashkenazi S, Yom-Tov Y. The breeding biology of the black-crowned night-heron (Nycticorax nycticorax) and the little egret (Egretta garzetta) at the Huleh Nature Reserve. Israel J Zool. 1997;242:623–41.
Ashoori A, Moradi HV, Rezaiee HR, Mahiny AS. Dietary segregation of four ardeid species breeding in Anzali International Wetland, Northern Iran. Waterbirds. 2017;40:377–89.
Baxter G. The influence of synchronous breeding, natal tree position and rainfall on egret nesting success. Colon Waterbirds. 1994;17:120–9.
Beaulieu M, Sockman KW. One meadow for two sparrows: resource partitioning in a high elevation habitat. Oecologia. 2012;170:529–40.
Bolton M, Conolly G, Carroll M, Wakefield ED, Caldow R. A review of the occurrence of inter-colony segregation of seabird foraging areas and the implications for marine environmental impact assessment. Ibis. 2019;161:241–59.
Boyle RA, Dorn NJ, Cook MI. Nestling diet of three sympatrically nesting wading bird species in the Florida Everglades. Waterbirds. 2012;35:154–9.
Britton RH, Moser ME. Size specific predation by herons and its effect on the sex-ratio of natural populations of the mosquito fish Gambusia affinis baird and girard. Oecologia. 1982;53:146–51.
Brown KM, Ewins PJ. Technique-dependent biases in determination of diet composition: an example with ring-billed gulls. Condor. 1996;98:34–41.
Burger J. Competition between Cattle Egrets and native North American herons, egrets, and ibises. Condor. 1978;80:15–23.
Burger J. Resource partitioning: nest site selection in mixed species colonies of herons, egrets and ibises. Am Midl Nat. 1979;101:191–210.
Burger J. On the nesting location of Cattle Egrets Bubulcus ibis in South African heronries. Ibis. 1982;124:523–9.
Cameron TC, Wearing HJ, Rohani P, Sait SM. Two-species asymmetric competition: effects of age structure on intra-and interspecific interactions. J Anim Ecol. 2007;76:83–93.
Chesson P. Mechanisms of maintenance of species diversity. Annu Rev Ecol Syst. 2000;31:343–66.
Choi YS, Kwon IK, Yoo JC. Nestling diet of three sympatric egret species: rice fields support breeding egret populations in Korea. Ornithol Sci. 2016;15:55–62.
Cody ML. On the methods of resource division in grassland bird communities. Am Nat. 1968;102:107–47.
Ding C. Research on the crested ibis. Shanghai: Shanghai Scientific and Technological Education Publishing House; 2004 (in Chinese).
Dorn NJ, Cook MI, Herring G, Boyle RA, Nelson J, Gawlik DE. Aquatic prey switching and urban foraging by the White Ibis Eudocimus albus are determined by wetland hydrological conditions. Ibis. 2011;153:323–35.
Dusi JL, Dusi RT. Ecological factors contributing to nesting failure in a heron colony. Wilson Bull. 1968;80:458–66.
Fasola M. Opportunistic use of foraging resources by heron communities in southern Europe. Ecography. 1994;17:113–23.
Field A. Discovering statistics using SPSS. Sage Publications Limited; 2009.
Fogarty MJ, Hetrick WM. Summer foods of Cattle Egrets in north central florida. Auk. 1973;90:268–80.
Frederick PC, Collopy MW. Nesting success of five Ciconiiform species in relation to water conditions in the Florida Everglades. Auk. 1989;106:625–34.
Gause GF. The struggle for existence. Baltimore: Williams and Wilkins; 1934.
Hino T. Resident males of small species dominate immigrants of large species in heterospecific, winter bird flocks. Ornithol Sci. 2005;4:89–94.
Huang B, Zou Y, Bi S, Li H, Zhu Q. Characteristics, dynamics and niche of insect community in plum orchard. Chin J Appl Ecol. 2005;2:307–12.
Hutchinson GE. Homage to Santa Rosalia or why are there so many kinds of animals? Am Nat. 1959;93:145–59.
Hutchinson GE. Population studies: animal ecology and demography. B Math Biol. 1991;53:193–213.
Jara RF, Crego RD, Samuel MD, Rozzi R, Jiménez JE. Nest-site selection and breeding success of passerines in the world’s southernmost forests. PeerJ. 2020;8:e9892.
Jenni DA. A study of the ecology of four species of herons during the breeding season at Lake Alice, Alachua County, Florida. Ecol Monogr. 1969;39:245–70.
Jensen H, Kiljunen M, Knudsen R, Amundsen PA. Resource partitioning in food, space and time between Arctic Charr (Salvelinus alpinus), Brown Trout (Salmo trutta) and European Whitefish (Coregonus lavaretus) at the southern edge of their continuous coexistenc. PLoS ONE. 2017;12:e0170582.
Julliard R, Clavel J, Devictor V, Jiguet F, Couvet D. Spatial segregation of specialists and generalists in bird communities. Ecol Lett. 2006;9:1237–44.
Kasahara S, Katoh K. Food-niche differentiation in sympatric species of kingfishers, the Common Kingfisher Alcedo atthis and the Greater Pied Kingfisher Ceryle lugubris. Ornithol Sci. 2008;7:123–34.
Kazantzidis S, Goutner V, Pyrovetsi M, Sinis A. Comparative nest site selection and breeding success in 2 sympatric ardeids, Black-crowned Night-Heron (Nycticorax nycticorax) and Little Egret (Egretta garzetta) in the Axios Delta, Macedonia, Greece. Colon Waterbirds. 1997;20:505–17.
Kent CM, Sherry TW. Behavioral niche partitioning reexamined: do behavioral differences predict dietary differences in warblers? Ecology. 2020;101:e03077.
Kushlan AJ, Hafner H. Heron conservation. New York: Academic Press; 2000.
Lack DL. Ecological adaptations for breeding in birds. London: Methuen; 1968.
Levine JM, HilleRisLambers J. The importance of niches for the maintenance of species diversity. Nature. 2009;461:254–7.
Liordos V, Kontsiotis VJ. Identifying important habitats for waterbird conservation at a Greek Regional Nature Park. Avian Res. 2020;11:39.
Macarthur R, Levins R. The limiting similarity, convergence, and divergence of coexisting species. Am Nat. 1967;101:377–85.
MacDonald EC, Camfield AF, Martin M, Wilson S, Martin K. Nest-site selection and consequences for nest survival among three sympatric songbirds in an alpine environment. J Ornithol. 2016;157:393–405.
Martin PR, Ghalambor CK. When David beats Goliath: the advantage of large size in interspecific aggressive contests declines over evolutionary time. PLoS ONE. 2014;9:e108741.
Martínez C. Food and niche overlap of the Scarlet Ibis and the Yellow-crowned Night Heron in a tropical mangrove swamp. Waterbirds. 2004;27:1–8.
Martínez C. Trophic niche breadth and overlap of three egret species in a neotropical mangrove swamp. Waterbirds. 2010;33:285–92.
Massa C, Doyle M, Callico FR. On how Cattle Egret (Bubulcus ibis) spread to the Americas: meteorological tools to assess probable colonization trajectories. Int J Biometeorol. 2014;58:1879–91.
Maxwell GR, Kale HW. Breeding biology of five species of herons in coastal Florida. Auk. 1977;94:689–700.
McCrimmon DA Jr. Nest site characteristics among five species of herons on the North Carolina coast. Auk. 1978;95:267–80.
Metallaoui S, Dziri H, Bousseheba A, Heddam S, Chenchouni H. Breeding ecology of the Cattle Egret (Bubulcus ibis) in Guerbes-Sanhadja wetlands of Algeria. Reg Stud Mar Sci. 2020;33:100979.
Nicolaus M, Barrault SCY, Both C. Diet and provisioning rate differ predictably between dispersing and philopatric pied flycatchers. Behav Ecol. 2019;30:114–24.
Overduijn KS, Handel CM, Powell AN. Does habitat partitioning by sympatric plovers affect nest survival? Auk. 2020;137:1–16.
Parejo D, Sanchez JM, Avilés JM. Factors affecting the nest heigth of three heron species in heronries in the South-West of Spain. Ardeola. 1999;46:227–30.
Parsons KC. Heron nesting at Pea Patch Island, upper Delaware Bay, USA: abundance and reproductive success. Colon Waterbirds. 1995;18:69–78.
Pianka ER. The structure of lizard communities. Annu Rev Ecol Syst. 1973;4:53–74.
Pianka ER. Evolutionary ecology. 6th ed. New York: Harper and Row; 2000.
Salazar RD, Riddiford NJ, Vicens P. A comparative dietary study of Cattle Egrets (Bubulcus ibis) and Little Egrets (Egretta garzetta) in S’ Albufera Natural Park, Mallorca. Boll Soc Hist Nat. 2005;48:153–62.
Samraoui F, Nedjah R, Boucheker A, Alfarhan AH, Samraoui B. Patterns of resource partitioning by nesting herons and ibis: how are odonata exploited? CR Biol. 2012;335:310–7.
Sanz-Aguilar A, Carrete M, Edelaar P, Potti J, Tella JL. The empty temporal niche: breeding phenology differs between coexisting native and invasive birds. Biol Invas. 2015;17:3275–88.
Schoener TW. Resource partitioning in ecological communities. Science. 1974;185:27–39.
Si Bachir A, Hafner H, Tourenq JN, Doumandji S, Lek S. Diet of adult Cattle Egrets Bubulcus ibis in a new North African colony (Soummam, Kabylie, Algeria): taxonomic composition and seasonal variability. Ardeola. 2001;48:217–23.
Siegfried W. The food of the cattle egret. J Appl Ecol. 1971;8:447–68.
Snow DW, Perrins CM, Gillmor R. The birds of the Western Palearctic. Oxford: Oxford University Press; 1998.
SPSS. SPSS statistics, v. 18. Chicago: SPSS, Inc.; 2009.
Teal JM. Nesting success of egrets and herons in Georgia. Wilson Bull. 1965;77:257–63.
Trexler J, Tempe R, Travis J. Size-selective predation of Sailfin Mollies by two species of heron. Oikos. 1994;69:250–8.
Weber WJ. Notes on Cattle Egret breeding. Auk. 1975;92:111–7.
Wong L, Corlett RT, Young L, Lee JS. Comparative feeding ecology of Little Egrets on intertidal mudflats in Hong Kong, South China. Waterbirds. 2000;23:214–25.
Ye P, Yang C, Liang W. Nest site availability and niche differentiation between two cavity-nesting birds in time and space. Ecol Evol. 2019;9:11904–10.
Zar JH. Biostatistical analysis. 4th ed. New Jersey: Prentice-Hall; 1999.
Zhou L, Ma Y, Song Y. Food diversity of three species of nesting herons Zipeng Mountains. Chin J Ecol. 2000;19:66–8 (in Chinese).
Zhou Z, Wu M, Chen D. Food diversity of three species nesting herons in Changshu Shang Lake. J Changshu Coll. 2003;17:59–60 (in Chinese).
Zhu X, Zou X. The herons of China. Beijing: China Forestry Publishing House; 2001 (in Chinese).
Zhu X, Li ZG, Chen W. A comparative study on asynchronous hatching and nestling growth of three heron species. Chin J Appl Ecol. 2005;16:125–8 (in Chinese).
Acknowledgements
We would like to thank the staff of Shaanxi Hanzhong Crested Ibis National Nature Reserve for their generous support.
Funding
This work was supported by National Natural Science Foundation of China (Nos. 31772483 and 31372218 to CD, No. 31900371 to YY).
Author information
Authors and Affiliations
Contributions
YY conceived and designed the experiments, performed the experiments, analyzed the data, and wrote the paper; HC and YJ designed and performed the experiments and contributed data analysis; GD wrote the paper and reviewed drafts of the paper. CD conceived and designed the experiments, wrote the paper and reviewed drafts of the paper. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All our study procedures were approved by the permission of the Shaanxi Hanzhong Crested Ibis National Nature Reserve.
Consent for publication
Not applicable.
Competing interests
All authors declare that they have no competing interests.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Ye, Y., Hu, C., Jiang, Y. et al. Three-dimensional niche partitioning between two colonially nesting ardeid species in central China. Avian Res 12, 33 (2021). https://doi.org/10.1186/s40657-021-00264-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40657-021-00264-7