Abstract
Birds often have to choose their nest site location along a food safety axis, balancing nest predation danger with the food requirements of themselves and their offspring. This is probably most important for precocial species, such as most shorebirds, in which both chicks and parents need access to food resources in the surroundings of the nest, at least during the first days of life of the chicks. In many Arctic ecosystems, shorebird nests are typically prone to predation by both avian and terrestrial predators, especially in lemming-poor years. Among other factors, the strength of the trophic interactions between shorebirds, their prey, and their predators depend on how all of these are distributed across space. During two breeding seasons in northern Taimyr, North-Central Russia, we investigated how the spatial distribution of red knot Calidris canutus and little stint Calidris minuta nests and broods overlaps with the local food landscape and also with the distribution of avian predators and their main prey, lemmings. We found that the two shorebird species use different habitats that vary in arthropod community structure in accordance with the birds’ diet: while little stints selected lower elevations where chironomid midges Chironomidae are more abundant, red knots selected higher elevations where crane flies Tipulidae are more abundant. Furthermore, little stints share low-elevation habitats with lemmings and predators, while red knots inhabit higher elevations averted by both lemmings and avian predators. We found higher nest predation for little stint nests than for red knots nests, especially in a low-lemming year. Our results thus support the idea that food web interactions are driven by landscape and community aspects.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Birds often face a trade-off between maximizing energy gain from foraging and minimizing predation risk (Stephens et al. 2007). Therefore, the presence of predators, or predation danger (Lank and Ydenberg 2003), impacts a bird’s decisions on foraging place, time, and food choice (e.g., McNamara and Houston 1987; Lima and Dill 1990; Verdolin 2006; Lameris et al. 2018). Models explaining habitat use under varying predation danger originate from the patch-use theory (Brown 1988) and have mainly been tested on relatively small temporal scales (e.g., Pitt 1999; Pomeroy et al. 2006; van den Hout et al. 2014). However, when birds decide where to make their nest, they need to choose a site that is safe for a fairly long period of time, as the nest cannot be moved in response to changes in predation danger (Lecomte et al. 2008; Hoy et al. 2016). For precocial species where the offspring will start to forage for themselves shortly after hatching, ample food availability in the vicinity of the nest is likely to be important for growth and survival at least in the first few days after hatching, after which chicks can move further from the nest (Schekkerman et al. 1998). Thus, the choice of nesting site is a critical decision that may define the fate of the offspring: the nest should be placed in an optimal location along the food safety axis, providing new-borns enough food while avoiding nest predation.
High-Arctic ecosystems are relatively simple and interactions between their trophic layers can readily be studied (Forchhammer et al. 2008; Wirta et al. 2015), therefore, they are convenient for studying food safety trade-offs. Strong trophic links exist between arthropods, insectivorous shorebirds, and their predators (including Arctic foxes Vulpes lagopus and skuas Stercorarius spp.). Among other reasons, shorebirds are likely to select their Arctic breeding territories aiming for as high as possible arthropod abundances and as infrequent as possible predator encounters. Arthropod availability becomes especially important during the brood-rearing period (Tulp and Schekkerman 2008), while predation occurs often during incubation (Liebezeit and Zack 2008) and can impact breeding success dramatically (Tomkovich 1998; Ganter and Boyd 2000).
Arthropods are considered a universal food resource for all Arctic shorebird species, both adults and chicks (Bolduc et al. 2013; Wirta et al. 2015). Nevertheless, diet composition may differ between species (Zhemchuzhnikov et al. 2022), e.g., depending on bill length and/or body size (Holmes and Pitelka 1968; Lifjeld 1984; Zhemchuzhnikov 2024), with larger species feeding on larger prey. As arthropod community composition varies between habitats, such as low-lying wet valleys and higher dryer hills (Koltz et al. 2018), differences in preferred diet between shorebird species may drive variation in nesting distribution along an elevational axis. Shorebirds may also consider other factors when choosing where to nest, such as predation risk. As such, diet composition may also be the result of habitat choice rather than the cause.
Arctic top-predators, such as arctic foxes and skuas which heavily rely on lemmings when these are abundant (Larson 1960; Angerbjörn et al. 1999; Wiklund et al. 1999; Ims et al. 2017), are expected to adjust their distribution so that it overlaps with lemming-rich areas at lower elevations (van Beckerath et al. 2021). Consequently, the distribution of predators may overlap with that of some shorebird species, but not all of them (Léandri-Breton and Bêty 2020). Therefore, shorebird species that share habitats with lemmings and, hence, predators, are likely to face higher predatory pressure, which was previously shown for shorebirds nesting within a goose colony, especially in low-lemming years (Robinson et al. 2014; Lamarre et al. 2017).
This predation danger may be highest in years when lemming populations crash in the course of their regular 3–4-year population cycles (but see also the “fading out” of these cycles (Nolet et al. 2013; Aharon-Rotman et al. 2015)), when bird nests become an important additional food source for predators (Summers et al. 1998; Wiklund et al. 1999; Gilg et al. 2006; Nolet et al. 2013). While arctic foxes and skuas almost exclusively feed on lemmings when these are abundant and depredate shorebird nests only opportunistically (Underhill et al. 1992; Wiklund et al. 1999; Elmhagen et al. 2000), they are known to broaden their diet in lemming-poor years using other food sources (Wiklund et al. 1999), including shorebird eggs (Eide et al. 2005). This gives rise to a large variation in predation danger between years (Werner and Hall 1974; Krebs 1977, Stephens and Krebs 1986), which may also impact the importance of predation danger as a driver of nest distribution.
In this study, we analyze the distribution of two species of Arctic-nesting shorebirds, little stint Calidris minuta and red knot Calidris canutus, in northern Taimyr, North-Central Russia, (1) to explore whether they are elevation-selective during nesting and chick rearing periods, and, if such selectivity exists, how it relates to the arthropod food landscape, and (2) to test how this elevation-selectivity of shorebirds is related to that of avian predators and lemmings. Finally, we (3) analyze nest survival of the two shorebird species in relation to their elevational distribution in both a lemming-rich and a lemming-poor year.
Methods
Study area
The study was conducted in the summer of 2018 and 2019 near Knipovich Bay (76°04' N, 98°32′ E), on the Taimyr Peninsula (the extreme north of central Siberia, Russia), within the Great Arctic State Nature Reserve. The study area (140 km2, Fig. 1a) can be defined as Arctic tundra with alternating valleys and hills with absolute heights up to 201 m above sea level (as described in Tomkovich and Soloviev 1994). According to the Circumpolar Arctic Vegetation Classification, this area belongs to bioclimatic subzone B or prostrate dwarf-shrub subzone (Walker et al. 2018). The hydrological network of the area is formed by a large number of small streams small lakes. The lower part of the tundra has a tendency to accumulate water in the period of snowmelt and as such can stay saturated with water for a large part of the summer season. This process may also lower soil temperatures as it protects the permafrost layer from melting. Upper parts of the tundra are drying up faster after the snow cover is gone, sometimes exposing large patches of bare ground, so-called medallion tundra. These differences in moisture levels and temperature regimes are possibly facilitating different arthropod communities and can as such affect the vertebrates that prey on them.
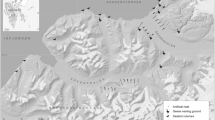
Distribution of shorebird and skua nests within the study area. a Map of the study area with locations of little stint Calidris minuta (green circles), red knot Calidris canutus (red circles), and skua Stercorarius spp. (black/gray circles) nests. The number inside each circle indicates the year when the nest was found. Yellow squares show the grids in which arthropod abundance was sampled, with grid 1, including the location of the study camp and both sampling transects. Grid 3 was only sampled in 2019. The UTM coordinate system (zone 47) is used for specifying the location of the map and objects. The location of the study site in the Arctic is shown on the inset in the top right corner. b Distribution of elevations inside the study area. c–e Elevational distribution of nests (circles) and broods (triangles) of little stints (c), red knots (d), and skuas (e)
Elevational distribution of the study area
The landscape of the study area was classed as zones between elevation isolines, with contour intervals of 20 m, using the topographic map of the Great Arctic State Nature Reserve, sector “Nizhnyaya Taimyra” (retrieved from Taimyr Nature Reserves website, 2020). Based on a digital elevation model ArcticDEM (Porter et al. 2018), we confirmed the high accuracy of this map (Online Resource 1). The proportion of each elevation zone in the study area was estimated (Fig. 1b) using ImageJ (Abràmoff et al. 2004). This data was used for the calculation of preference score for nesting (and brood-rearing) little stints, red knots, skuas Stercorarius spp., and lemmings (see below).
Shorebird and skua distribution
Little stint and red knot form the core of shorebird community in the area together with the curlew sandpiper Calidris ferruginea, red phalarope Phalaropus fulicarius, and grey plover Pluvialis squatarola. The little stint is the most abundant small uniparental species, with a body mass of 31.4 ± 0.4 g (mean ± SD) during incubation (Tulp et al. 2002) and in other study areas has been shown to prefer downhills and slopes with grassy or moss-sedge hillocky tundra for nesting (Tulp et al. 2009), often nesting in wet areas characterized by early snow melt (Kirikova et al. 2005). Sometimes females lay two clutches; one of these is incubated by the male and the second one by the female (Pitelka et al. 1974; Tomkovich et al. 1994; Tulp and Schekkerman 2006). Little stints often leave the nest to forage, and as such foraging birds can be relatively easily followed by human observers in order to locate their nest. The red knot is a medium-sized shorebird, with a body mass of 136.7 ± 7.3 g (mean ± SD) during the incubation (own data), often occurring in the upper parts of the slopes in the area (Soloviev et al. 2018). Both parents incubate the clutch, but females, as a rule, leave the nest before or at hatching and males take care of the chicks (Whitfield and Brade 1991; Bulla et al. 2016; Tomkovich et al. 2018). During incubation red knots rely on their camouflage and will flush from their nest only when an observer approaches within 1 m, which makes finding their nests a difficult task. The study area is also inhabited by three skua species: pomarine skua Stercorarius pomarinus, long-tailed skua Stercorarius longicaudus, and arctic skua Stercorarius parasiticus. Of these, pomarine skua was the most common species.
Active nest searching was performed from early until late June and broods were located (and captured) from late June till late July. Nest searching included (1) observation of potential pairs and females initiating the clutch, (2) observation of birds returning to the nest after foraging or after the bird was scared off the nest, (3) random nest encounters while walking across the tundra, (4) rope dragging in case of little stints, and (5) tracking red knot males to their nests with the aid of radio transmitters deployed to males; birds were caught by luring them into nets with playback of territorial male calls (van Gils et al. in prep). Incubation stage was determined using a flotation test (Liebezeit et al. 2007). Shorebird broods were either detected visually (little stints) or found by attracting males with playback of chick calls (red knots) (Johnson et al. 2020). We registered the location of nests and broods using handheld Garmin GPS devices. The elevations were determined afterward from the digital elevation model ArcticDEM. We tracked the fate of the nests of shorebirds by revisiting them at least once, a few days before expected hatching (based on egg floatation curves). If the nest was not predated (indicated by empty nests before the predicted hatch date), we revisited it again on the expected hatching date to determine its ultimate fate. Nests were considered to be successful when encountered with one or more eggs hatched or when encountered empty at or after the predicted hatching date but containing small egg-shell fragments indicative of successful hatch. Although our data appear to reflect the distribution of nests well, the area around the camp and inside the arthropod sampling grids may be over-explored compared to more remote areas, which may lead to some bias, e.g., a less accurate estimate of the preference score for red knots, which are distributed further away from the camp. In combination with relatively low detectability of red knot nests leading to overall low sample size, it could lead to overestimation of the preference score for the nesting sites at lower elevations and its underestimation at higher elevations.
Shorebird diet
In an earlier study (Zhemchuzhnikov et al. 2022), we determined the arthropods present in the diet of chicks of both of the present study species, using a meta-barcoding analysis on chick droppings. An important result from this previous study is that crane flies Tipulidae and chironomid midges Chironomidae form the most important part of the diet of red knot (n = 30) and little stint chicks (n = 33), with red knots mainly preying on crane flies (39.4%) and little stints eating mostly chironomid midges (43.7%). Moreover, the fraction of crane flies in the diet of chicks is negatively correlated with the fraction of chironomid midges: Spearman correlation test, r = − 0.59, p < 0.0001 (Zhemchuzhnikov et al. 2022). We used the fraction of crane flies in the following analyses.
Arthropod sampling
Arthropod abundance was measured from early June till early August, using yellow round pitfall traps (⌀ = 9 cm), following Reneerkens et al. (2016). Traps were filled with propylene glycol mixed with water in 1:1 ratio. The sampling was conducted in large-scale grids (number 1, 2, and 3), with grid 1 and 2 sampled in both years and grid 3 sampled only in 2019. Within the area of grid 1, we also sampled in one large-scale transect in 2019, and one small-scale transect in both years (Fig. 1a). In general, traps were located partly at low and partly at high elevations.
Large-scale grids consisted of ten traps each, distributed as a grid of 3 × 3 traps at 400-m intervals, with the tenth trap placed in a random location on one of the gridlines. Along the large-scale 0.9-km transect, ten traps were placed at 100-m intervals. Traps in large-scale grids and along the transect were emptied at five-day intervals. The small-scale 100-m transect consisted of five traps, with 25 m between them; traps in this transect were emptied every day between 18:00 and 21:00. For each trap, we recorded the elevation using a handheld Garmin GPS device. In total, 427 and 536 samples were collected in 2018 and 2019, respectively. All arthropods were extracted from traps, stored in ethanol, and taken to the laboratory, where they were identified up to the family level. For analyses, we totaled the number of chironomid midges and crane flies per trap per day.
Lemming distribution
Two lemming species (Siberian brown lemming Lemmus sibiricus and collared lemming Dicrostonyx torquatus) occur in our study area, where the former is by far the most abundant. No other rodents inhabit the area (Soloviev et al. 2018). One person, making daily trips with the purpose to cover the whole study area in both 2018 and 2019, recorded all lemmings encountered noting both coordinates and the number of lemmings. From the coordinates, we extracted elevation using the digital elevation model as described above.
Statistical analyses
Statistical analyses were performed in R, version 3.5.3 (R Core Team 2019) and MARK (White and Burnham 1999) in combination with RMark (Laake 2013).
For analyzing the preferred elevations for shorebird nests and brood-rearing locations, as well as the preferred elevations on which lemmings and skuas reside, we used the Ivlev index (Jacobs 1974). We calculated Ivlev index for each elevation category as a difference between the observed (O) and expected (E) proportional occupation divided by the sum of the observed and expected proportional occupation:
As our null-hypothesis was that organisms displayed no elevational selection, we assumed the expected proportional occupation to be equal to the available elevational range within the study area (Fig. 1b). This index ranges between 1 and − 1, with the value > 0 reflecting preference and the value < 0 indicating aversion. The residuals were not normally distributed, hence, we used the Mann–Whitney U test to evaluate the difference between elevations of nest and brood-rearing sites and to compare the number of lemmings encountered per day in 2018 and 2019.
We used beta regression models to analyze whether the proportion of crane flies in the diet of shorebirds’ chicks in 2019 (response variable) could be explained by elevation or species (little stint and red knot) as predictor variables. We used variance inflation factor (VIF) to test for issues with multicollinearity when running the model. VIF ≥ 10 indicates major problems with multicollinearity (Myers 1986; Kutner et al. 2005; Chatterjee and Hadi 2006), yet others state that values ≥ 4 (Fox 1997; Quinn and Holt 2008) or even ≥ 2 (Graham 2003) indicate potential problems. We selected the approach with the cut-off level of 2, where the value of VIF equal or above 2 would indicate issues with multicollinearity. In the model we tested, the VIF for elevation and species was 3.12 and 3.06, respectively. Therefore, we constructed a set of candidate models excluding those models which contained both variables simultaneously. Models were compared using Akaike’s information criterion correcting for small sample size (AICc) (Burnham and Anderson 2002). Hierarchically, more complex models (i.e., models with additional parameters) within 2 ΔAICc of the model with the lowest AICc were not considered informative/competitive (Arnold 2010).
We used linear multiple regression models to analyze whether the number of chironomid midges or crane flies caught per trap per day (response variable) could be explained by elevation and year (2018 and 2019) as predictor variables. We log transformed the response variables to satisfy the normality and homoscedasticity requirements. Then, we constructed a set of candidate models from all the possible combinations of predictor variables, including their interaction. We selected the final model based on AICc as described above.
Daily survival rate was analyzed using a nest survival model in MARK (Dinsmore et al. 2002). We tested if the daily survival rate (response variable) could be explained by the elevation, year, and species (red knot and little stint) as predictor variables. As indicated above, elevation and species identity were highly correlated, and we did not consider models that included both variables simultaneously. The final full model included species or elevation, the interaction of year and species or elevation, and year. We constructed the set of eight candidate models from all possible combinations of predictor variables, including the interactions. We selected the final model based on AICc as described above. Only predated or successful nests were used in the analysis; deserted nests or those with unknown fate were excluded. For some of those nests, it was not possible to identify the failure date, thus 173 out of 182 little stint nests and 14 out of 16 red knot nests were used to estimate the daily survival rate.
We used Pearson correlation to test (post hoc) if the elevation preferences for the nesting sites in skuas correlated with those in little stints and red knots and also with those of lemmings. We excluded the elevations above 160 m a. s. l. as no nests of shorebirds or skuas were observed there, which indicates that this area was unfavorable for these birds (Ivlev index = − 1.00).
Results
Distribution and elevation preferences of shorebirds
Over the entire study period, 227 little stint nests (116 in 2018 and 111 in 2019) and 18 red knot nests (12 and 6, respectively) were found. As explained above, nests of little stints are easier to find than nests of red knots, which probably explains the large difference in nests found for each species. Data on little stint broods were not collected in 2018; 33 broods were located in 2019. A total of 75 (32 in 2018 and 43 in 2019) red knot broods were located. Little stints had both their nests (median elevation 41.0 m [37.1–53.5 m]) and broods (46.1 m [39.0–54.9 m]) at lower elevations compared to red knots (nests: 105.8 m [90.9–119.8 m], U test: p < 0.001, Z = − 6.17; broods: 123.1 m [101.2–138.6 m], p < 0.0001, Z = − 8.01). In 2018, brood-rearing sites of red knots (124.1 m) were located at higher elevations than nests (109.7 m, U test: p = 0.034, Z = − 2.13). In 2019, elevations of nesting and brood-rearing sites did not differ, neither for little stints nor for red knots. (U test little stints: p = 0.9129, Z = − 0.11; U test red knots: p = 0.2868, Z = − 1.10; Fig. 1c and Fig. 1d).
Little stints seemed to prefer lower elevations for both placing nests and rearing chicks, as shown by a positive preference score for elevations between 20 and 60 m (Ivlev index > 0.0, Fig. 2a, b). The maximum preference score for nesting was found at an elevation range between 20 and 40 m in 2018 (Ivlev index = 0.60, Fig. 2A) and between 40 and 60 m in 2019 both for nests (Ivlev index = 0.52, Fig. 2b) and broods (Ivlev index = 0.55, Fig. 2b). Neither nests nor broods of little stints were found above 120 m in any year (Ivlev index = − 1.00, Fig. 2a, b). Red knots preferred elevations between 80 and 140 m for nesting (Ivlev index > 0.0) and between 100 and 160 m (with additional interval between 180 and 200 m) for brood rearing in 2018 (Ivlev index > 0.0, Fig. 2c). The highest preference was found at an elevational range between 120 and 140 m for nests (Ivlev index = 0.54, Fig. 2c) and between 180 and 200 m for broods (Ivlev index = 0.72, Fig. 2c). We found that red knots preferred three elevation intervals as nesting sites in 2019 (Ivlev index > 0, Fig. 2d): 40 to 60 m, 100 to 120 m, and 140 to 160 m, with the highest preference for the second one (Ivlev index = 0.56, Fig. 2d). The preferred elevations for broods were between 80 and 160 m and between 180 and 200 m in 2019 (Ivlev index > 0.0, Fig. 2d), with the highest preference for 140 to 160 m (Ivlev index = 0.55, Fig. 2d). Neither nests nor broods were found in the lowest (0 to 40 m) and the highest (200 to 220 m) parts of the study plot (Ivlev index = -1.00, Fig. 2C, d).
Preference score of shorebirds shown for different elevations, in relation to the abundance of the key arthropod food item for their chicks across elevation gradient. For little stints Calidris minuta (a, b, green) and red knots Calidris canutus (c, d, red), the preference score is based on the observation of nests (circles) and broods (triangles) in 2018 (a, c) and 2019 (b, d), averaged per 20 m. The marked window (white area) in a–d indicates the elevation range in which the arthropod abundance was measured. e, f Dependence of chironomid midges Chironomidae (gray) and crane flies Tipulidae (white) abundance on elevation in 2018 (e) and 2019 (f). Lines represent linear regression with 95% confidence intervals
Shorebird diet and distribution of arthropods
The proportion of crane flies in the diet of little stints and red knots was better explained by elevation than by species and showed an increase in proportion of crane flies with elevation (Table 1, Fig. 3). When analyzing the elevational distribution of chironomid midges and crane flies, we found that chironomid midges (the key food item for little stints) were more abundant in lower areas (Figs. 2e, f). The top-supported model (Table 2) included the interaction of elevation and year, showing a more pronounced trend in 2018 compared with 2019. Chironomid midges were 13.2 (2018) or 2.1 (2019) times more abundant at 40 m than at 120 m (odds ratio). The abundance of crane flies (the key food item for red knots) increased with elevation (Figs. 2e, f). The top-supported model explaining the abundance of crane flies included the effect of elevation and year (Table 2), but not the interaction, with crane flies being 3.6 (in 2018) or 6.3 (in 2019) times more abundant at 120 m compared with 40 m (odds ratio).
Dependence of the proportion of crane flies (measured as relative number of reads, see Zhemchuzhnikov et al. 2022) in the diet of the chicks from the elevation, where sample was collected
Distribution, elevation preference, and abundance of lemmings
The total number of lemmings observed was 30 specimens during 50 field days in 2018 and 226 specimens during 38 field days in 2019. The majority of these rodents were Siberian brown lemmings (100% of all identified lemmings in 2018 and 97.3% in 2019). Corrected for the number of field days, the abundance of lemmings was lower in 2018 with 0.0 median encounters per day (quartiles: 0.0–1.0) compared with a median number of 2.0 encounters (0.3–8.5) in 2019 (U test, p < 0.0001, Z = − 4.47, Fig. 4c). The mean number of encounters per day was 0.6 in 2018 and 5.9 in 2019, and the maximum number 3 and 28, respectively. Lemmings were observed at a median elevation (interquartile range) of 38.1 (28.8–48.0) m in 2018 and 38.1 (36.4–50.9) m in 2019 (Fig. 4a), with no difference between the two years (U test, p = 0.4340, Z = − 0.784). In both years, the elevation preference score was positive for elevations between 20 and 60 m (Ivlev index > 0.0, Fig. 4b) and negative for those exceeding 60 m (Ivlev index < 0.0, Fig. 4b). The highest preference was found for elevations of 20 to 40 m (Ivlev index = 0.58 in 2018, Ivlev index = 0.66 in 2019, Fig. 4b) and no lemmings were encountered above 80 m in 2018 and above 100 m in 2019 (Ivlev index = − 1.00, Fig. 4b).
Distribution, elevation preference, and abundance of lemmings and elevational preference of skuas Stercorarius spp. in 2018 (orange) and 2019 (blue). a Elevational distribution of lemmings. b Preference score of lemmings shown for different elevations. c Lemming abundance, measured as number of lemmings observed by one researcher during one field day. d Preference score of skuas shown for different elevations. In (a), (b), and (d) dark gray area indicates lower elevations (< 60 m) preferred by lemmings and skuas at least in one study year, and light gray area indicates higher elevations (> 60 m), strongly averted by lemmings and skuas in both years
Distribution and elevation preferences of skuas
In total, 113 (38 in 2018 and 75 in 2019) nests of skuas were found during the study period, the majority of them being pomarine skua nests (73.7% in 2018 and 93.3% in 2019). The median elevation of nest sites was 38.1 (quartiles: 26.9–51.2) m in 2018 and 39.8 (33.0–51.7) m in 2019 (Fig. 1e). In both years, the elevation preference score was positive for elevations between 20 and 60 m (Ivlev index > 0.0, Fig. 4d) and negative for the sites located higher than 60 m (Ivlev index < 0.0, Fig. 4d). The highest preference was found for elevations of 20 to 40 m (Ivlev index = 0.64 in 2018, Ivlev index = 0.59 in 2019, Fig. 4d) and no nests were found above 120 m in either year (Ivlev index = − 1.00, Fig. 4d).
Overlap of elevational ranges
In the elevational range between 0 and 160 m (thus n = 16), little stints showed a positive correlation with skuas in their preference for nesting habitats in both years (Spearman correlation R = 0.90, p = 0.0021 in 2018 and R = 0.81, p = 0.0150 in 2019, Fig. 5a, b). Correlation in nest site preference between red knots and skuas was not significant (Spearman correlation R = − 0.45, p = 0.2600 in 2018 and R = − 0.33, p = 0.4300 in 2019, Fig. 5a, b). In the elevation range between 0 and 160 m (n = 16), lemmings’ habitat preferences showed a positive correlation with skuas’ preferences for nesting habitats in both years (Spearman correlation R = 0.94, p = 0.0004 in 2018 and R = 0.96, p = 0.0001 in 2019, Fig. 5a, Fig. 5b).
a, b Correlation between elevations preferred by skuas Stercorarius spp. (nesting), by shorebirds (nesting), and by lemmings in 2018 (a) and 2019 (b). Green circles indicate the preference score of skuas versus little stints Calidris minuta, red circles indicate the preference score of skuas versus red knots Calidris canutus, and small white circles indicate the preference score of skuas versus lemmings. Lines represent linear regression. Significant correlations are shown by solid lines; non-significant correlations are shown by dotted lines
Shorebird nest predation
The sample included 84 nests of little stints (56.0% predated) in 2018 and 98 (39.8% predated) in 2019 and 10 nests of red knots (20.0% predated) in 2018 and 6 (0.0% predated) in 2019. The top-supported model included the effects of year and species on daily survival rate, elevation was not included (Table 3, Fig. 6). Based on the top-supported model output, irrespective of the study year, daily survival rate of little stint nests was lower than that of red knot nests. In both shorebird species, daily survival rate was lower in the year with a lower lemming abundance (2018) than in the year with a high abundance of these rodents (2019). Based on the function parameters of the best model, the daily survival rate of little stint nests was 0.928 (95% CI: 0.905–0.946) in 2018 and 0.963 (0.950–0.973) in 2019. The daily survival rate of red knot nests was 0.990 (0.962–0.998) in 2018 and 0.995 (0.980–0.999) in 2019 (Fig. 6). We did not find support for a significant effect of interaction between species and year (Table 3).
Discussion
We show that nesting and brood-rearing little stints displayed a preference for lower-lying valleys in an Arctic tundra ecosystem, whereas red knots preferred slopes at higher elevations. From a bottom-up perspective, elevational preferences put these species into areas with different arthropod communities, and we find that diet differences between species indeed correlate with elevation. From a top-down perspective, little stints share their habitat with lemmings and skuas. Although we did not directly measure predation risk, this could explain lower nest survival compared to red knots, especially in the years with a low abundance of lemmings (conceptualized in Fig. 7).
Proposed food web interactions between avian predators, shorebirds/lemmings, and arthropods in lemming-poor (2018) and lemming-rich (2019) years. The width of the arrows indicates the proposed interaction strength. Skua skua species Stercorarius spp., ReKn red knot Calidris canutus, LiSt little stint Calidris minuta, lem lemmings, Tip crane flies Tipulidae, Chi chironomid midges Chironomidae. Low low wet part of the tundra, high high dry part of the tundra
The fact that, in both species, nests and broods were located at similar elevations suggests that nests could be placed in habitats that is favorable for chicks. Food availability is of great importance for growth and survival of chicks (Schekkerman et al. 2003; Lameris et al. 2022), especially after hatching and during the first week of their life. In this period, chicks are brooded by their parents ca. 50% of the time (Tulp et al. 2009), thus restricting the time during which the chicks can forage and thus demanding relatively high food densities. In our study area, at least red knot chicks did not move to places located at different elevations during the brood-rearing period, as shown by no change in elevation for recaptured broods. This suggest that either the nesting habitat was also suitable for raising offspring or that traveling with broods up or down was too costly. At the same time, it has been shown that shorebird chicks can cover fairly long distances (Schekkerman et al. 1998). Preferences for specific prey items may be explained by the morphometry of bird species, e.g., relatively large red knots feed on a larger prey such as crane flies and therefore select breeding habitats in which these insects are abundant. This is in agreement with the results of previous studies in adult shorebirds, in which a positive correlation between the body size and the prey size was shown (Holmes and Pitelka 1968; Baker and Baker 1973; Baker 1977). As our data is mainly correlative, the reverse causal relationship cannot be excluded, i.e., it might be that diet composition is determined by the spatial distribution of birds rather than the bird distribution being driven by their diet preference (Andreeva and Tomkovich 1992).
Furthermore, nest site selection may also be optimized for foraging opportunities of incubating adults, which may be especially important for little stints. In the little stint, only one of the parents incubates the clutch, and the incubating parent needs to find enough food during short foraging trips in close vicinity of the nest (Tulp and Schekkerman 2006). Longer foraging trips are hampered, as the eggs need regular incubation in order to hatch successfully. In contrast, red knots shift incubation duties roughly every 14 h (Bulla et al. 2016), which allows parents to spend enough time in search of food and to travel to favorable foraging areas, even if these are located fairly far from the nest. In addition, other factors related to optimal incubation conditions, e.g., the selectivity for thermally favorable nesting microhabitats (Tulp et al. 2012), may also explain differences in nest site selection (Smith et al. 2007; Cunningham et al. 2016; Meyer et al. 2021).
Besides lower elevations providing more chironomid midges as prey for chicks, little stints also share their lower elevational habitat with lemmings and skuas. Although we did not directly measure predation danger, this may partly explain why little stints were subjected to lower nest survival compared to red knots in our study area. Video recordings of artificial nests at our study site show (A.B. Popovkina, unpublished data) that in both years most artificial nests were predated by pomarine skuas and other avian predators (other skua species and gulls), rather than by arctic foxes. In the presence of lemmings, predators in Arctic ecosystems feed primarily on these rodents, but opportunistically predate shorebird eggs and chicks (Larson 1960; Underhill et al. 1992; Summers et al. 1998). In our study area, lemmings were concentrated in lower-elevation habitats along the streams, which confirms the earlier findings for Lemmus sp. (Morris et al. 2000; Dupuch et al. 2014). Assuming that the distribution of predators across the landscape mirrors that of their main prey, the habitat preference of lemmings also increases the chances that the predators would find a shorebird nest at lower elevations, even if only accidentally. This may increase predation danger for little stints, while red knots may experience a much lower predation danger due to their preference for higher-elevation habitats. The little stint is also the most abundant species in our study area with the nest density of up to 40 pairs per km2 at low elevations (Soloviev et al. 2018). In fact, in years with a low abundance of lemmings, the high nesting density of shorebirds in lowland areas may become attractive to skuas and, possibly, Arctic foxes. In contrast, the higher elevations with lower nest densities of red knots and possibly other shorebirds could function as a ‘partial refuge’ to which predators are not attracted, although the lower nest densities might be affected by our ability to detect nests of red knots. Importantly, we recognize that our sample size is small, especially for red knot nests, and that elevation and shorebird species cannot be properly disentangled, and as such our conclusion on the causality of predation rates is speculative to some extent. Furthermore, differences in little stint and red knot behavior during the incubation could partly explain the differences in daily survival rate of their nest (Smith et al. 2012; Meyer et al. 2020). Red knots have a biparental breeding strategy and during incubation rely on their camouflage to stay put on their nest until a potential predator has approached to less than 1 m (Whitfield & Brade 1991). In contrast, little stints are uniparental breeders and fly off from their nest from a further distance upon approach of a predator, potentially increasing the predation danger. In our study area, lemming abundance was higher in 2019 than 2018 (Fig. 4c). As such, predation rate of shorebird nests was much lower in 2019 compared to 2018 for both red knots and little stints (Fig. 6). The higher predated rate in 2018 seemed even more pronounced for little stints (Fig. 6), although the interaction of species and elevation was not present in the best model. Years with an extremely low abundance of lemmings have been shown to have a dramatic effect on the breeding success of shorebirds, especially in combination with unfavorable weather conditions, when the nest failure rate may reach 90–100% (Tomkovich 1998). Besides differences in lemming abundance, our study years also differed in abundance of arthropods, with more chironomid midges in 2018 and more crane flies in 2019. Such differences could be explained by weather conditions, with 2018 being a colder year with more precipitation with wetter conditions potentially benefitting emergence of chironomid midges from water bodies, yet colder conditions possibly having a negative effect on emergence of crane flies where the larval growth is affected by temperature (MacLean Jr, 1973).
In this study, we describe the spatial position of little stints and red knots in the tundra food web, both from a top-down and a bottom-up perspective and suggest that these are related to shorebird diet and survival. The availability of key food items for chicks is related to their diet, and we find that both availability of different arthropod species as well as diet changes with elevation. It is not possible to disentangle cause and effect, so this could suggest that shorebirds either select elevations according to their diet or change their diet following availability of local arthropod prey at the elevation of their selected breeding site. Either way, the difference in the elevational distribution of shorebird nests impacts their relationships with predators. Skuas and, possibly, arctic foxes search for food in the lower, lemming-rich habitats and, therefore, are expected to predate primarily the nests located at the lower elevations. Thus, lowland-dwelling species may experience a higher predation rate, while those occupying nesting sites at higher elevations may stay in relative safety.
Data availability
This manuscript does not contain novel code. Data and script are available at the Royal Netherlands Institute for Sea Research (NIOZ) data repository: https://doi.org/https://doi.org/10.25850/nioz/7b.b.qd.
References
Abràmoff MD, Magalhães PJ, Ram SJ (2004) Image processing with imageJ. Biophotonics Int 11:36–41. https://doi.org/10.1201/9781420005615.ax4
Aharon-Rotman Y, Soloviev M, Minton C et al (2015) Loss of periodicity in breeding success of waders links to changes in lemming cycles in Arctic ecosystems. Oikos 124:861–870. https://doi.org/10.1111/oik.01730
Andreeva TR, Tomkovich PS (1992) On the role of the chick food in formation of the structure of shorebird population on the tundra. In: Coenotic interactions in tundra ecosystems. Nauka, Moscow, pp 70–78
Angerbjörn A, Tannerfeldt M, Erlinge S (1999) Predator-prey relationships: Arctic foxes and lemmings. J Anim Ecol 68:34–49. https://doi.org/10.1046/j.1365-2656.1999.00258.x
Arnold TW (2010) Uninformative parameters and model selection using Akaike’s Information Criterion. J Wildl Manage 74:1175–1178. https://doi.org/10.2193/2009-367
Baker MC (1977) Shorebird food habits in the Eastern Canadian Arctic. Condor 79:56–62. https://doi.org/10.2307/1367530
Baker MC, Baker AEM (1973) Niche relationships among six species of shorebirds on their wintering and breeding ranges. Ecol Monogr 43:193–212. https://doi.org/10.2307/1942194
Bolduc E, Casajus N, Legagneux P et al (2013) Terrestrial arthropod abundance and phenology in the Canadian Arctic: Modelling resource availability for Arctic-nesting insectivorous birds. Can Entomol 145:155–170. https://doi.org/10.4039/tce.2013.4
Brown JS (1988) Patch use as an indicator of habitat preference, predation risk, and competition. Behav Ecol Sociobiol 22:37–47
Bulla M, Valcu M, Dokter AM et al (2016) Unexpected diversity in socially synchronized rhythms of shorebirds. Nature 540:109–113. https://doi.org/10.1038/nature20563
Burnham KP, Anderson DR (2002) Model selection and inference: a practical information-theoretic approach, 2nd editio. Springer, New York
Chatterjee S, Hadi AS (2006) Regression analysis by example, 4th edn. John Wiley & Sons Inc, Hoboken
Cunningham JA, Kesler DC, Lanctot RB (2016) Habitat and social factors influence nest-site selection in Arctic-breeding shorebirds. Auk 133:364–377. https://doi.org/10.1642/AUK-15-196.1
Dinsmore SJ, White GC, Knopf FL (2002) Advanced techniques for modeling avian nest survival. Ecology 83:3476–3488. https://doi.org/10.2307/3072096
Dupuch A, Morris DW, Ale SB et al (2014) Landscapes of fear or competition? Predation did not alter habitat choice by Arctic rodents. Oecologia 174:403–412. https://doi.org/10.1007/s00442-013-2792-7
Eide NE, Eid PM, Prestrud P, Swenson JE (2005) Dietary responses of arctic foxes Alopex lagopus to changing prey availability across an Arctic landscape. Wildlife Biol 11:109–121. https://doi.org/10.2981/0909-6396(2005)11[109:DROAFA]2.0.CO;2
Elmhagen B, Tannerfeldt M, Verucci P, Angerbjörn A (2000) The arctic fox (Alopex lagopus): an opportunistic specialist. J Zool 251:139–149. https://doi.org/10.1017/S0952836900006014
Forchhammer MC, Schmidt NM, Høye TT et al (2008) Population dynamical responses to climate change. Adv Ecol Res 40:391–419. https://doi.org/10.1016/S0065-2504(07)00017-7
Fox J (1997) Applied regression analysis, linear models, and related methods. Sage Publications, Thousand Oaks
Ganter B, Boyd H (2000) A tropical volcano, high predation pressure, and the breeding biology of Arctic waterbirds: a circumpolar review of breeding failure in the summer of 1992. Arctic 53:289–305. https://doi.org/10.14430/arctic859
Gilg O, Sittler B, Sabard B et al (2006) Functional and numerical responses of four lemming predators in high arctic Greenland. Oikos 113:193–216. https://doi.org/10.1111/j.2006.0030-1299.14125.x
Graham MH (2003) Confronting multicollinearity in ecological multiple regression. Ecology 84:2809–2815. https://doi.org/10.1890/02-3114
Holmes RT, Pitelka FA (1968) Food overlap among coexisting sandpipers on northern Alaskan tundra. Syst Zool 17:305–318
Hoy SR, Millon A, Petty SJ et al (2016) Food availability and predation risk, rather than intrinsic attributes, are the main factors shaping the reproductive decisions of a long-lived predator. J Anim Ecol 85:892–902. https://doi.org/10.1111/1365-2656.12517
Ims RA, Killengreen ST, Ehrich D et al (2017) Ecosystem drivers of an Arctic fox population at the western fringe of the Eurasian Arctic. Polar Res. https://doi.org/10.1080/17518369.2017.1323621
Jacobs J (1974) Quantitative measurement of food selection: a modification of the forage ratio and Ivlev’s electivity index. Oecologia 14:413–417
Johnson JA, DeCicco LH, Hajdukovich NR (2020) Using playbacks of chick vocalizations to locate and capture breeding red knots. Wader Study 127:1–5. https://doi.org/10.18194/ws.00206
Kirikova TA, Kharitonov SP, Varlygina TI et al (2005) Distribution of breeding waders in tundra of the North-Western Taimyr according to the area and food capacity of habitats. Branta 8:54–79
Koltz AM, Schmidt NM, Høye TT (2018) Differential arthropod responses to warming are altering the structure of arctic communities. R Soc Open Sci 5:171503. https://doi.org/10.1098/rsos.171503
Krebs J (1977) Optimal foraging: theory and experiment. Nature 268:583–584. https://doi.org/10.1038/268583a0
Kutner MH, Nachtsheim CJ, Neter J, Li W (2005) Applied Linear Statistical Models, 5th editio. McGraw-Hill/Irwin, New York
Laake JL (2013) RMark: An R interface for analysis of capture-recapture data with MARK. Seattle
Lamarre JF, Legagneux P, Gauthier G et al (2017) Predator-mediated negative effects of overabundant snow geese on arctic-nesting shorebirds. Ecosphere 8:e01788. https://doi.org/10.1002/ecs2.1788
Lameris TK, Brown JS, Kleyheeg E et al (2018) Nest defensibility decreases home-range size in central place foragers. Behav Ecol 29:1038–1045. https://doi.org/10.1093/beheco/ary077
Lameris TK, Tomkovich PS, Johnson JA et al (2022) Mismatch-induced growth reductions in a clade of Arctic-breeding shorebirds are rarely mitigated by increasing temperatures. Glob Chang Biol 28:829–847. https://doi.org/10.1111/gcb.16025
Lank DB, Ydenberg RC (2003) Death and danger at migratory stopovers: problems with “predation risk.” J Avian Biol 34:225–228
Larson S (1960) On the influence of the arctic fox Alopex lagopus on the distribution of Arctic birds. Oikos 11:276–305
Léandri-Breton DJ, Bêty J (2020) Vulnerability to predation may affect species distribution: plovers with broader arctic breeding range nest in safer habitat. Sci Rep 10:1–8. https://doi.org/10.1038/s41598-020-61956-6
Lecomte N, Careau V, Gauthier G, Giroux JF (2008) Predator behaviour and predation risk in the heterogeneous Arctic environment. J Anim Ecol 77:439–447. https://doi.org/10.1111/j.1365-2656.2008.01354.x
Liebezeit JR, Zack S (2008) Point counts underestimate the importance of arctic foxes as avian nest predators: Evidence from remote video cameras in Arctic Alaskan oil fields. Arctic 61:153–161. https://doi.org/10.14430/arctic32
Liebezeit JR, Smith PA, Lanctot RB et al (2007) Assessing the development of shorebird eggs using the flotation method: Species-specific and generalized regression models. Condor 109:32–47. https://doi.org/10.1650/0010-5422(2007)109[32:ATDOSE]2.0.CO;2
Lifjeld JT (1984) Prey selection in relation to body size and bill length of five species of waders feeding in the same habitat. Ornis Scand 15:217–226. https://doi.org/10.2307/3675930
Lima SL, Dill LM (1990) Behavioral decisions made under the risk of predation: a review and prospectus. Can J Zool 68:619–640. https://doi.org/10.1139/z90-092
MacLean SF Jr (1973) Life cycle and growth energetics of the arctic cranefly Pedicia hannai antennata. Oikos 24:436–443
McNamara JM, Houston AI (1987) Starvation and predation as factors limiting population size. Ecology 68:1515–1519
Meyer N, Bollache L, Dechaume-Moncharmont FX et al (2020) Nest attentiveness drives nest predation in arctic sandpipers. Oikos 129:1481–1492. https://doi.org/10.1111/oik.07311
Meyer N, Bollache L, Galipaud M et al (2021) Behavioural responses of breeding arctic sandpipers to ground-surface temperature and primary productivity. Sci Total Environ 755:142485. https://doi.org/10.1016/j.scitotenv.2020.142485
Morris DW, Davidson DL, Krebs CJ (2000) Measuring the ghost of competition: Insights from density-dependent habitat selection on the co-existence and dynamics of lemmings. Evol Ecol Res 2:41–67
Myers RH (1986) Classical and modern regression with applications. Duxbury Press, Boston
Nolet BA, Bauer S, Feige N et al (2013) Faltering lemming cycles reduce productivity and population size of a migratory Arctic goose species. J Anim Ecol 82:804–813. https://doi.org/10.1111/1365-2656.12060
Pitelka FA, Holmes RT, MacLean SF (1974) Ecology and evolution of social organization in arctic sandpipers. Am Zool 14:185–204. https://doi.org/10.1093/icb/14.1.185
Pitt WC (1999) Effects of multiple vertebrate predators on grasshopper habitat selection: trade-offs due to predation risk, foraging, and thermoregulation. Evol Ecol 13:499–516. https://doi.org/10.1023/A:1006792726166
Pomeroy AC, Butler RW, Ydenberg RC (2006) Experimental evidence that migrants adjust usage at a stopover site to trade off food and danger. Behav Ecol 17:1041–1045. https://doi.org/10.1093/beheco/arl043
Porter C, Morin P, Howat I, et al (2018) ArcticDEM. Accessed on 31.08.2020
Quinn LD, Holt JS (2008) Ecological correlates of invasion by Arundo donax in three southern California riparian habitats. Biol Invasions 10:591–601. https://doi.org/10.1007/s10530-007-9155-4
R Core Team (2019) R: A language and environment for statistical computing. R foundation for statistical computing. Vienna, Austria
Reneerkens J, Schmidt NM, Gilg O et al (2016) Effects of food abundance and early clutch predation on reproductive timing in a high Arctic shorebird exposed to advancements in arthropod abundance. Ecol Evol 6:7375–7386. https://doi.org/10.1002/ece3.2361
Robinson BG, Franke A, Derocher AE (2014) The influence of weather and lemmings on spatiotemporal variation in the abundance of multiple avian guilds in the arctic. PLoS ONE. https://doi.org/10.1371/journal.pone.0101495
Schekkerman H, Van Roomen MWJ, Underhill LG (1998) Growth, behaviour of broods and weather-related variation in breeding productivity of Curlew Sandpipers Calidris ferruginea. Ardea 86:153–168
Schekkerman H, Tulp I, Piersma T, Visser GH (2003) Mechanisms promoting higher growth rate in arctic than in temperate shorebirds. Oecologia 134:332–342. https://doi.org/10.1007/s00442-002-1124-0
Smith PA, Gilchrist HG, Smith JNM (2007) Effects of nest habitat, food, and parental behavior on shorebird nest success. Condor 109:15–31. https://doi.org/10.1650/0010-5422(2007)109[15:EONHFA]2.0.CO;2
Smith PA, Tulp I, Schekkerman H et al (2012) Shorebird incubation behaviour and its influence on the risk of nest predation. Anim Behav 84:835–842. https://doi.org/10.1016/j.anbehav.2012.07.004
Soloviev MY, Golovnyuk V V., Popovkina AB, Sukhova MA (2018) Nesting conditions and abundance of birds in the area “Lower Taimyra” (Gusinnaya estuary): in Chronicles of Nature, 2018 of Great Arctic State Nature Reserve, Norilsk. 213–236
Stephens DW, Brown JS, Ydenberg RC (2007) Foraging: Behaviour and ecology. University of Chicago Press, Chicago
Summers RW, Underhill LG, Syroechkovski EEJ (1998) The breeding productivity of Dark-Bellied Brent geese and Curlew Sandpipers in relation to changes in the numbers of Arctic foxes and lemmings on the Taimyr peninsula, Siberia. Ecography (cop) 21:573–580
Tomkovich PS (1998) Breeding conditions for waders in Russian tundras in 1992. Int Wader Stud 10:117–123
Tomkovich PS, Soloviev MY (1994) Site fidelity in high arctic breeding waders. Ostrich 65:174–180. https://doi.org/10.1080/00306525.1994.9639680
Tomkovich PS, Johnson JA, Loktionov EY, DeCicco LH (2018) Brood attendance by female red knots. Wader Study 125:33–38. https://doi.org/10.18194/ws.00091
Tomkovich PS, Soloviev MY, Syroechkovsky Jr. EE (1994) Birds of the Arctic tundra of Northern Taimyr (Knipovich Bay area). In: Arctic tundra of Taimyr and the islands of the Kara Sea: Nature, fauna, and problems of their protection. Moscow, pp 44–110
Tulp I, Schekkerman H (2006) Time allocation between feeding and incubation in uniparental arctic-breeding shorebirds: energy reserves provide leeway in a tight schedule. J Avian Biol 37:207–218. https://doi.org/10.1111/j.2006.0908-8857.03519.x
Tulp I, Schekkerman H (2008) Has prey availability for arctic birds advanced with climate change? Hindcasting the abundance of tundra arthropods using weather and seasonal variation. Arctic 61:48–60. https://doi.org/10.14430/arctic6
Tulp I, Schekkerman H, Chylarecki P et al (2002) Body mass patterns of Little Stints at different latitudes during incubation and chick-rearing. Ibis 144:122–134. https://doi.org/10.1046/j.0019-1019.2001.00014.x
Tulp I, Schekkerman H, Bruinzeel LW et al (2009) Energetic demands during incubation and chick rearing in a uniparental and a biparental shorebird breeding in the high arctic. Auk 126:155–164. https://doi.org/10.1525/auk.2009.07181
Tulp I, Schekkerman H, de Leeuw J (2012) Eggs in the freezer: energetic consequences of nest site and nest design in arctic breeding shorebirds. PLoS ONE 7:1–9. https://doi.org/10.1371/journal.pone.0038041
Underhill LG, Prys-Jones RP, Syroechkovski EE Jr et al (1992) Breeding of waders (Charadrii) and Brent Geese Branta bernicla bernicla at Pronchishcheva Lake, northeastern Taimyr, Russia, in a peak and a decreasing lemming year. Ibis 135:277–292
van Beckerath X, Benadi G, Gilg O et al (2021) Long-term monitoring reveals topographical features and vegetation explain winter habitat use of an Arctic rodent. bioRxiv. https://doi.org/10.1101/2021.01.24.427984
van den Hout PJ, van Gils JA, Robin F et al (2014) Interference from adults forces young red knots to forage for longer and in dangerous places. Anim Behav 88:137–146. https://doi.org/10.1016/j.anbehav.2013.11.020
Verdolin JL (2006) Meta-analysis of foraging and predation risk trade-offs in terrestrial systems. Behav Ecol Sociobiol 60:457–464. https://doi.org/10.1007/s00265-006-0172-6
Walker DA, Daniëls FJA, Matveyeva NV et al (2018) Circumpolar Arctic vegetation classification. Phytocoenologia 48:181–201. https://doi.org/10.1127/phyto/2017/0192
Werner EE, Hall DJ (1974) Optimal foraging and the size selection of prey by the bluegill sunfish (lepomis Macrochirus). Ecology 55:1042–1052. https://doi.org/10.2307/1940354
White GC, Burnham KP (1999) Program mark: survival estimation from populations of marked animals. Bird Study 46:S120–S139. https://doi.org/10.1080/00063659909477239
Whitfield DP, Brade JJ (1991) The breeding behaviour of the Knot Calidris canutus. Ibis 133:246–255. https://doi.org/10.1111/j.1474-919X.1991.tb04566.x
Wiklund CG, Angerbjörn A, Isakson E et al (1999) Lemming predators on the Siberian tundra. Ambio 28:281–286
Wirta HK, Vesterinen EJ, Hambäck PA et al (2015) Exposing the structure of an Arctic food web. Ecol Evol 5:3842–3856. https://doi.org/10.1002/ece3.1647
Zhemchuzhnikov MK (2024) Reassessment of trophic mismatches in Arctic shorebirds. University of Groningen, Groningen. https://doi.org/10.33612/diss.908900225
Zhemchuzhnikov MK, Zhemchuzhnikova EA, Lameris TK et al (2022) Disentangling the diet composition of Arctic shorebirds ’ chicks provides a new perspective on trophic mismatches. bioRxiv. https://doi.org/10.1101/2022.10.10.511540
Acknowledgements
This work was supported by the Netherlands Polar Program, which is part of the Netherlands Organization for Scientific Research (ALWPP.2016.044 awarded to J. A. van Gils). We would like to thank the staff from the Great Arctic State Nature Reserve for providing access to the reserve and help in the organization of the expeditions. We thank Judith van Bleijswijk for help with the meta-barcoding analyses. We thank Camilo Carneiro, Zhijun Ma and three anonymous reviewer for commenting on an earlier version of this manuscript.
Funding
Nederlandse Organisatie voor Wetenschappelijk Onderzoek, ALWPP.2016.044.
Author information
Authors and Affiliations
Contributions
MZ: Conceived the idea for this study. MZ, TL, JtH, and JvG: Collected data on red knot nests and chicks in the field. MS, VG, AP, and MS: Collected data on little stint nests and chicks in the field. MZ: Collected data on arthropods in the field. VG: Collected data on skua nest distribution and lemming distribution in the field. MZ and DK: Identified trapped arthropods in the lab. EZ: Applied meta-barcoding methods to identify the diet of individual shorebird diet. MZ conducted the statistical analyses. MZ: Wrote the initial manuscript with significant input from TL and JvG. All authors commented on the manuscript and read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare to have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhemchuzhnikov, M.K., Lameris, T.K., Soloviev, M.Y. et al. Food web interactions of two breeding Arctic shorebird species, little stint Calidris minuta and red knot Calidris canutus, are shaped by their elevational distribution. Polar Biol 47, 247–261 (2024). https://doi.org/10.1007/s00300-024-03227-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00300-024-03227-y