Abstract
The behavior of COH fluids, their isotopes (hydrogen and carbon), and their interaction with magmatic liquids are at the core of understanding formation and evolution of the Earth. Experimental data are needed to aid our understanding of how COH volatiles affect rock-forming processes in the Earth’s interior. Here, I present a review of experimental data on structure of fluids and melts and an assessment of how structural factors govern hydrogen and carbon isotope partitioning within and between melts and fluids as a function of redox conditions, temperature, and pressure.
The solubility of individual COH components in silicate melts can differ by several orders of magnitude and ranges from several hundred ppm to several wt%. Silicate solubility in fluid can reach several molecular at mantle temperatures and pressures. Different solubility of oxidized and reduced C-bearing species in melts reflects different solution equilibria. These equilibria are 2CH4 + Qn = 2CH3− + H2O + Qn + 1 and 2CO32− + H2O + 2Qn + 1 = HCO3− + 2Qn, under reducing and oxidizing conditions, respectively. In the Qn-notations, the superscript, n, denotes the number of bridging oxygen in the silicate species (Q-species).
The structural changes of carbon and silicate in magmatic systems (melts and fluids) with variable redox conditions result in hydrogen and carbon isotope fractionation factors between melt, fluid, and crystalline materials that depend on redox conditions and can differ significantly from 1 even at magmatic temperatures. The ∆H of D/H fractionation between aqueous fluid and magma in silicate–COH systems is between − 5 and 25 kJ/mol depending on redox conditions. The ∆H values for 13C/12C fractionation factors are near − 3.2 and 1 kJ/mol under oxidizing and reducing conditions, respectively. These differences are because energetics of O–D, O–H, O–13C, and O–12C bonding environments are governed by different solution mechanisms in melts and fluids.
From the above data, it is suggested that (COH)-saturated partial melts in the upper mantle can have δD values 100%, or more, lighter than coexisting silicate-saturated fluid. This effect is greater under oxidizing than under reducing conditions. Analogous relationships exist for 13C/12C. At magmatic temperatures in the Earth’s upper mantle, 13C/12C of melt in equilibrium with COH-bearing mantle in the − 7 to − 30‰ range increases with temperature from about 40 to > 100‰ and 80–120‰ under oxidizing and reducing conditions, respectively.

Similar content being viewed by others
Introduction
The budget, recycling, and evolution of COH volatiles are central to our understanding of many geochemical and geophysical properties of the interior of the Earth. The behavior of COH volatiles, therefore, is a very important factor in the processes that governed the formation and evolution of the solid Earth and perhaps other terrestrial planets (Ardia et al. 2013; Armstrong et al. 2015).
The speciation and activity/concentration of volatiles are particularly relevant to melting and crystallization. For carbon-bearing species, for example, reduced and oxidized C behaves quite differently (Saxena and Fei 1988; Ulmer and Luth 1991). These factors can govern melt composition (see also Kushiro 1974, 1998; Eggler 1975; Eggler and Baker 1982; Gaetani and Grove 1998; Foley et al. 2009). For example, the clearly different effects of CO2 and CH4 on melting and crystallization of model magma systems are evident in the example in Fig. 1. The response of the liquidus boundary between forsterite and enstatite in the NaAlSiO4–Mg2SiO4–SiO2–COH system to changing volatile species at high pressure illustrates such effects (Taylor and Green 1987). This boundary defines the activity of SiO2 in the system and moves from SiO2 deficient to SiO2-enriched when carbon is reduced from CO2 to CH4 (Fig. 1). In fact, the effect of CH4 is not that different from the effect of H2O. This difference is also the underlying explanation for redox melting in the mantle (Song et al. 2009).
Simplified liquidus diagram showing the enstatite/forsterite liquidus boundary in portion of the system Mg2SiO4–NaAlSiO4–SiO2 in the presence of excess CO2, CH4, and H2O. Also shown (with dashed line) is the boundary under volatile-free conditions (data from Taylor and Green 1987)
The stable isotope behavior is critical to monitor budgets and recycling of volatiles (Van Soest et al. 1998; Dixon et al. 2002; Kingsley et al. 2002; Javoy 2004). Volatile components in the COH system, when dissolved in magmatic liquids, can affect stable isotope fractionation between the melt and coexisting fluids and crystalline materials (Dobson et al. 1989; Mattey et al. 1990; Deines 2002). Moreover, the redox state of the volatile components such as hydrogen, carbon, and perhaps sulfur can affect their influence on stable isotope fractionation in magmatic systems (Poulson 1996; Deines 2002).
In order to employ stable isotope behavior such as those of hydrogen and carbon to deduce processes of formation and evolution of the Earth’s interior, experimental data are necessary. With such tools, we can address how, in particular, COH volatiles and their isotopes fractionate between magmatic liquids, fluids, and crystallizing materials as a function of redox conditions temperature and pressure. Data from samples analyzed after quenching to ambient conditions have been reported (Dobson et al. 1988; Mattey et al. 1990; Mattey 1991). However, extrapolation of such data to conditions during equilibration of magmatic liquids with fluids and crystals at high temperature and pressure is quite challenging because both fluids and melts commonly alter their structure, and sometimes their composition, during quenching to ambient temperature and pressure conditions (Kuroda et al. 1982; Baker and Stolper 1994; Zhang and Frantz 2000; Mysen and Yamashita 2010). That nature of those alterations can vary depending on the temperature–pressure quenching path. It is, therefore, better to determine the isotope fractionation while the samples are at the high temperature and pressure under controlled redox conditions relevant to magmatic processes in the Earth’s interior. In this report, I will present and discuss recent experimental data relevant to these questions and illustrate with a few examples how the composition and redox state of COH volatiles affect carbon and hydrogen stable isotope fractionation between melts and fluids under conditions corresponding to those of the deep crust and upper mantle.
Review
A description and discussion of how carbon and hydrogen isotopes fractionate between fluids and melts at high temperature and pressure and with variable redox conditions is the focus of this presentation. Most of the experimental work on this subject have been carried out by using externally heated hydrothermal anvil cell method (Bassett et al. 1996) where the samples can be probed by vibrational spectroscopy while at the desired conditions. It should be noted that in these diamond cell experiments, temperature is an independent variable. Pressure is generated by the fluid in sample chambers of approximately constant volume and measured with probes such as the one-phonon shift of carbon-13 diamond embedded in the sample (Schiferl et al. 1997). It is for this reason that pressure is included as an upper horizontal axis in some of the figures in this presentation.
Vibrational spectroscopies, such as Raman and infrared not only can be used to probe structure but may also be employed to determine isotope ratios. Most of the experimental data discussed in this report were obtained by such methods. This technique can be used because the frequency ratio of vibrations of two oscillators that differ only in their mass (e.g., two isotopes) v1/v2, is proportional to the square root of their mass ratio, √m1/m2. Provided that the cross section for a given vibration is not dependent on the type of isotope, 1 and 2, the ratio of integrated areas of the vibrational spectra, A1/A2, equals their abundance ratio, X1/X2. Data in support of this conclusion exist for the oxygen isotopes, 16O and 18O, (McKay et al. 2013) and hydrogen isotopes (Dalou et al. 2015).
With those caveats in mind, isotope ratios can be obtained by both transmission infrared absorption and by Raman spectroscopy. Moreover, the samples can be probed with those methods while at the desired conditions. Raman spectroscopy has an advantage over infrared spectroscopy in that Raman spectroscopic intensities do not depend on sample density, whereas infrared absorption intensity does. Densities of coexisting phases at high temperature and pressure often are not available. Laser beams used in microRaman measurements typically are 1–2 μm across and with confocal optics penetrate less than 30 μm into the sample (depending on optical transparency). Measurements with transmission microinfrared absorption have less spatial resolution with a minimum aperture of approximately 30 × 30 μm. In addition, in infrared transmission spectroscopy, one has to ensure that only the sample of interest is in the optical path.
In addition to isotope ratios, it is also possible to ascertain the speciation and abundance ratio of the volatiles and the silicate components in silicate–COH systems from Raman and infrared spectroscopic data. Sometimes, it has also been possible to determine isotope fractionation between individual volatile species such as CO3 and HCO3 groups in silicate melts and coexisting, silicate-saturated melts, while these phases were at the temperature, pressure, and redox conditions of interest (Mysen 2015a, 2016, 2017). Further details of the experimental and analytical methods used for this purpose can be found in those papers and Dalou et al. (2015).
Although solubility of volatile components in silicate melts is not the focus of this report, solubility information is necessary in order to characterize the isotope behavior. Solubility of COH components can also be determined spectroscopically, using either infrared or Raman spectroscopy (Scholze 1960; Behrens et al. 2004, 2006). Traditionally, analyses have been conducted on quenched materials, but recent technical advances now allow infrared and Raman spectroscopy to be used to determine the concentration of at least some volatiles while the samples are at the desired temperatures and pressures (Behrens and Yamashita 2008; Le Losq et al. 2015).
Solubility and solution mechanisms
Fractionation of stable isotopes between coexisting melts and fluids can be linked to solubility and solution mechanisms of volatiles in melts and of silicate in coexisting COH fluids. The principles that govern solubility in coexisting melts and fluids have been reviewed extensively elsewhere (Paillat et al. 1992; Newton and Manning 2008) and will not be discussed further here.
COH volatiles in melts
Determination of the solubility of H2O and CO2 in silicate–COH melts requires sufficiently oxidizing conditions so that carbon-bearing species more reduced than that of carbon in CO2 are unimportant. Those conditions correspond to oxygen fugacities at or above that somewhere between that defined by the NNO (nickel-nickel oxide) and QFM (quartz-magnetite-fayalite) buffer (Mysen et al. 2011). In other words, CO2–H2O mixtures will be the principal species in environments such as those of subduction zone melting, for example (Wood et al. 1990; Arculus 1994).
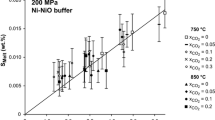
The solubilities of neither H2O nor CO2 are simple linear functions of the CO2/H2O abundance ratio of the system (Fig. 2a; see also Behrens et al. 2009). This non-linear solubility behavior is at least in part because of non-deal mixing behavior of CO2–H2O fluids (Eggler et al. 1979; Aranovich and Newton 1999). In addition, silicate melt–CO2–H2O mixtures also are non-ideal (Papale et al. 2006) which adds to the non-linear behavior of CO2 and H2O solubility in Fig. 2a.
Solubility of COH volatiles in silicate melts at high temperature and pressure. a Solubility under oxidizing conditions in ultrapotassic melts at 500 MPa and 1250 °C (data from (Behrens et al. 2009)). b Solubility of reduced C-species, shown as C, as a function of hydrogen fugacity at pressures or in pressure ranges indicated on individual curves (data from Botcharnikov et al. 2006; Morizet et al. 2010; Mysen et al. 2011; Ardia et al. 2013; Kadik et al. 2014)
Reduced carbon species may be encountered in the deep Earth where redox conditions approach those described by the IW (iron-wüstite) buffer and below (Frost and McCammon 2008; Rohrbach et al. 2011). Even lower oxygen fugacity appears to have existed during the early core-forming stages of the Earth (O'Neill 1991; Righter and Drake 1999). Under such conditions, the dominant C-bearing species in the COH system likely is methane, CH4. Molecular H2 can also play a significant role and contribute more than 10% of the volatile species under such very reducing conditions (Kadik et al. 2014). Its solubility in silicate at high temperature and pressure is greater than that which would be expected if hydrogen simply existed as molecular H2. There is some evidence to suggest that H2 interacts chemically with the silicate melt to form OH-groups (Luth and Boettcher 1986; Luth et al. 1987).
More experimental solubility data exist for carbon in melts as a function of redox conditions (Morizet et al. 2010; Mysen et al. 2011; Ardia et al. 2013). Some of the data are summarized for various melt and magma compositions in Fig. 2b. It is evident that as carbon undergoes reduction, its solubility in silicate melt decreases. However, there is considerable variation in available solubility data so that, for example, even the solubility of CH4 in basalt composition melt at several GPa pressure and upper mantle temperatures has been reported between about a hundred ppm C (Fig. 2b; see also Ardia et al. 2013; Armstrong et al. 2015) to several thousand ppm (Kadik et al. 2004, 2014). The reasons for this wide solubility range are not clear, but may be related to experimental difficulties controlling the speciation of COH volatiles under extremely reducing conditions.
Silicate solubility in fluid
Less is known about silicate solubility in COH composition fluids than about solubility of COH volatiles in silicate melts at high temperatures and pressures. Quantitative experimental data have been reported for silicates and aluminosilicates in pure H2O (Manning et al. 2010; Hunt et al. 2011; Manning and Aranovich 2014). The silicate solubility can reach several molecular (Newton and Manning 2008). There is, in fact, a second critical endpoint in the SiO2–H2 system between 0.9 and 1 GPa (Kennedy et al. 1962). Solubility of silica and other oxide components also depends on the presence of other components such as, in particular alkalies and alumina, which when occurring together, can cause major solubility enhancements because of formation of multicomponent complexes in the fluid (Pascal and Anderson 1989; Mysen and Armstrong 2002; Manning 2004; Mysen 2012).
Solution mechanisms
Water in melts and fluids
It has been known for some time that solution of water in silicate melts is in the form of molecular H2O and as OH-groups that form bonding to cations such as Si4+, Al3+, alkalis, and alkaline earths (Stolper 1982; Cody et al. 2005; Xue and Kanzaki 2008). The extent to which network-modifying cations such as alkalis and alkaline earths form bonds with OH-groups in aluminosilicate–H2O systems appears somewhat sensitive to the electronic properties of the cations as well as their proportion relative to network-forming Si4+ and Al3+ (Xue and Kanzaki 2008). Little is known about this latter feature in aluminosilicate–COH systems.
In silicate–H2O systems, water in aqueous fluids at high temperature and pressure does dissolve silicate components (Manning 2004) in which at least some of the oxygen in silicate tetrahedra are replaced by OH-groups (Zotov and Keppler 2002; Mibe and Bassett 2008; Mysen 2009). Such structural features recently also have been observed in Raman and infrared spectra of silicate-saturated COH fluid (Mysen, unpublished data). However, whether in silicate–H2O or silicate–COH systems at high temperature and pressure, the abundance ratio, OH/H2O, in melts always is greater than that in fluid (Mysen 2010). This difference is greater in silicate–COH fluids than in silicate–H2O fluids.
Carbon species
Under oxidizing conditions, carbon-bearing species in melts and fluids at high temperature and pressure are dominated by CO2, CO3, and HCO3. In hydrous C-bearing systems such as silicate-COH, the dominant species are of HCO3 and CO3 type with limited evidence for molecular CO2. The limited existence of CO2 is in part because it has been found that molecular CO2 becomes decreasingly important with increasing H2O content of silicate melts (King and Holloway 2002). The latter conclusion is also consistent with the general observation that abundance of molecular CO2 species diminishes as a silicate melt becomes depolymerized, which does, indeed happen with solution of H2O (Cody et al. 2005). It follows, therefore, that CO2 might be an unimportant species in silicate–COH melt, which is consistent with the lack of observed signals from molecular CO2 in such melts (Mysen 2015a, 2015b).
The relationship between carbonate stability (CO32− and HCO3−) and molecular CO2 in silicate melts and perhaps silicate-rich COH fluids in principle can be written as:
In Eq. (1) the Qn and Qn + 1 formalism expresses silicate species with in this case n and n + 1 bridging oxygen, respectively. Recent NMR-based evidence suggest that the carbonate groups form isolated clusters (Morizet et al. 2017) whereas other experimental data have been interpreted to suggest that oxygen in the CO32− complex is shared with the silicate network (Brooker et al. 2001).
Equation (1) obviously is a simplification. For example, this equation does not account for the observation that the HCO3/CO3 abundance ratio in both melts and fluids is temperature dependent (Fig. 3a) From the temperature dependence of the HCO3/CO3 abundance ratio in melts andin fluids and under the assumption of ideal mixing, the enthalpy change is 17 ± 3 and 29 ± 9 kJ/mol for melt and fluid, respectively. Equilibrium (1) likely also depends on temperature, and definitely on the activity of CO2 and protons (pH) but experimental data with which to address such questions have not been reported.
Abundance of C-bearing species in coexisting COH fluids and melts as a function of temperature and pressure as indicated. a Evolution of carbonate species under oxidizing conditions. b Evolution of C-bearing species under reducing conditions. c Evolution of fluid/melt exchange of carbonate species under oxidizing conditions. d Evolution of fluid/melt exchange of C-bearing reduced species under reducing conditions. Note that temperature is the independent variable with pressure evolving as a function of temperature as described briefly in the text and in more detail in the original sources (modified after Mysen 2015a, 2015b). The ∆H values derived from the linear regression of the data are discussed in the text. In calculated these ∆H values, it was assumed that there is no pressure effect on these equilibria
Methane is a stable species in COH fluid under reducing conditions (Kadik et al. 2004). In silicate-COH, this oxidation state was detected with carbon-13 MAS NMR in glasses formed by quenching of melts from 1450 °C and 2 GPa and with fH2 controlled by the magnetite-wüstite-H2O and more reducing conditions (Mysen et al. 2011). It was, furthermore, noted in the latter study that molecular CH4 coexists with methyl groups (CH3) in the melt similar to that more recently also reported by Ardia et al. (2013). Mysen et al. (2011) reported 13C NMR-based evidence that these CH3 groups were not linked to oxygen (methoxy groups). That conclusion may imply that the methyl groups actually replace oxygen in silicate tetrahedral with an equilibrium between CH4, CH3 and silicate species that in principle can be expressed as:
In this equation, the Qn(CH3)2 notation indicates that two of the n oxygens have been replaced by CH3 groups.
Even though in silicate–COH systems both HCO3/CO3 and CH3/CH4 abundance ratios vary in both melts and coexisting fluids, the exchange equilibria for oxidizing conditions (Fig. 3c);
and reducing conditions (Fig. 3d);
are such that under all conditions in Fig. 3, with increasing temperature under oxidizing conditions the CO3/HCO3 abundance ratio increases faster in melts than in coexisting fluid. The enthalpy change, ∆H, for reaction (3) is − 44 ± 9 kJ/mol with the assumption of ideal mixing (Mysen 2015a). Under reducing conditions, the CH4/CH3 abundance ratio in fluid increases faster than in coexisting melt with a ∆H for reaction (4) of 34 ± 3 kJ/mol (Mysen 2015b).
Hydrogen and carbon isotope behavior
In a situation where there is no interaction between the molecules, isotope fractionation factors can be calculated (Bigeleisen and Mayer 1947; Bottinga 1968). However, in most magmatic environments, there are indeed interactions between various silicate and COH complexes (Chacko et al. 2001). Changes in structural complexes in fluids and brines are known to affect isotope behavior (Horita 1988; O'Neil and Truesdell 1993; Horita et al. 1995; O'Neil et al. 2004). Isotope substitution also can affect materials properties (Horita et al. 2010). It should not be surprising, therefore, that significant isotope fractionation can be observed between melts or fluids with variable structural complexes. For example, hydrogen and carbon isotope ratios in melts in equilibrium with fluid of fixed composition vary as a function of changes on solution mechanisms of COH species in the melt (Fig. 4). The data in Fig. 4 were obtained by analyzing glasses along the join Na2O–SiO2 after quenching from equilibration with a COH fluid at 1400 °C and 2 GPa and with the hydrogen fugacity buffered with the iron-wüstite-H2O buffer. Isotope variations are expressed relative to composition, NS5 (Na2O · 5SiO2). Subsequent spectroscopic examination of the fluid, which remained of constant composition in all experiments, was a mixture of CH4, H2, and H2O. It can be seen quite clearly that the D/H and 13C/12C ratios respond to changes in water and methane speciation in the melt, respectively. Similar effects have been reported for D/H fractionation in melts formed in the silicate–H2O–D2O systems determined with proton and deuteron MAS NMR (Wang et al. 2015). Analogous experimental data were reported by Dobson et al. (1989) for D/H fractionation between fluid and rhyolite and feldspar melts and Mattey et al. (1990) and Mattey (1991) for carbon isotope fractionation between fluid, basalt, and sodamelilite melt. In light of the more recent data such as summarized in Fig. 4, those earlier data likely reflected variations in speciation of the volatiles in the synthetic and natural melts.
Changes in hydrogen and carbon isotope ratios of melts in equilibrium with fluid in melts equilibrated at 1400 °C and 2 GPa with the hydrogen fugacity controlled at the IW-H2O buffer. a D/H change expressed as a function of speciation of dissolved water relative to that of composition NS5 (Na2O · 5SiO2). (data from Mysen and Fogel 2010). b Changes in carbon isotope ratio in melts relative to composition NS5 (Na2O · 5SiO2) as a function of speciation of reduced carbon species (data from Mysen et al. 2009)
In studies such as those described in the previous paragraph, mass spectrometric measurements were made on glasses and fluids subsequent to quenching from high temperature and pressure. However, fluids quenched from pressures in the GPa pressure range and high temperature such as was the case with those experiments often comprise several molecular silicate components. These silicate-rich fluids are prone to precipitation of solid materials during quenching. There are also the problems that derive from exsolution of fluid from melts as these are cooled during quenching thus changing the content and possibly proportion of volatile components.
In order to circumvent quenching problems, more recent experiments have combined high-temperature/high-pressure experiments conducted in diamond anvil cells with vibrational spectroscopy as a means to obtain isotope ratios of elements whose isotopic mass differences are relatively large (e.g., D and H, 13 and 12C; see (Foustoukos and Mysen 2012; Mysen 2013; Dalou et al. 2015).
With this method, it is taken advantage of the fact that vibrational frequencies are discreet functions of oscillator mass:
where v1 and v2 are vibrational frequencies of a specific mode for isotopes 1 and 2. The m1 and m2 are the atomic masses of the 12C and 13C oscillator. In the case of an elemental phase such as graphite or diamond, m1 and m2 could be 12C and 13C.
We emphasize that the frequency difference between vibrations with two different isotopes does not change with abundance, but the vibrational intensity, expressed as integrated areas, A1 and A2, does. From vibrational spectra, the isotope ratio, X1/X2, of a sample then becomes
Such vibrational spectroscopic methods have been used to determine hydrogen and carbon isotope ratios in coexisting silicate melts and fluids in silicate–COH systems contained in hydrothermal diamond anvil cells at pressures and temperatures up to those of the Earth’s upper mantle. In some of these experiments, redox conditions were controlled with metal/oxide buffers (Re/ReO2, Ti/TiO2).
The method has the significant advantage that the analyses are conducted in situ while the samples are the conditions of interest, the method is non-destructive and with spatial resolution on the micrometer scale (see above). There is, therefore, no concern for sample changes during quenching because quenching is not involved. It can also probe quite small samples without interference from adjacent phases. Moreover, in this experimental environment, pressure/temperature conditions can be changed quickly (on the time scale of minutes) without changing the sample. A potential limitation is the sensitivity of the method to isotope abundances relevant to natural conditions. In an effort to assess whether or not this could be a problem, Dalou et al. (2015) conducted experiments with several series of fluid/melt D/H fractionation with different bulk D/H ratios in silicate–H2O–D2O systems, but did not find any concentration-dependent variations (Fig. 5).
D/H exchange equilibrium coefficient between coexisting fluid and melt in silicate–H2O as a function of temperature and bulk D/H abundance ratio (data from Dalou et al. 2015)
Hydrogen isotopes
In silicate–COH melt and fluid systems, the principal hydrogen-bearing species are H2O, OH, H2, CH4, CH3, and HCO3 where redox conditions and chemical composition of the fluids govern which species will dominate. The molecular species, H2O and H2, when present likely occupy 3-dimensional cavities in glass and melts much like that reported for noble gases, for example (Carroll and Stolper 1993; Zhang et al. 2010; Guillot and Sator 2012). Hydrogen isotopes in those molecular species likely will not show significant fractionation effects. As to bicarbonate, HCO3, no clear isotopic effects in either infrared or Raman spectra (Mysen 2015a) which may suggest that the C–O bond is not affected detectably whether H+ or D+ forms the bicarbonate complex.
For the exchange equilibrium,
under both oxidizing and reducing conditions, the dominant contribution is via OD/OH fractionation. The appearance of the vibrational modes assigned to O–D and O–H stretch vibrations is shown in the insert in Fig. 6, where the exchange equilibrium coefficient, \( {K}_{D/H}^{fluid/ melt} \), is defined as;
Fractionation factor, α, set equal to the fluid/melt exchange equilibrium coefficient (see text for discussion) for D/H exchange between coexisting fluid and melt as a function of temperature in silicate–COH systems under reducing and oxidizing conditions. Insert shows typical Raman spectrum (here from fluid) to illustrate the appearance of the Raman bands assigned to OD and OH stretch vibrations and used to extract the data themselves. The ∆H values from the two fits are given in Table 1. Also shown are the experimental data for rhyolite–H2O and feldspar–D2O glass and melt composition in the 5–20 MPa pressure range from Dobson et al. (1989) (data otherwise from Mysen 2015a)
where D/H denotes abundance ratio. From experiments conducted under oxidizing conditions, the D/H ratio simply is the ratio of integrated intensity such as indicated in the insert in Fig. 6, whereas for reducing conditions, information from deuterated methane and methyl complexes also is incorporated.
The D/H evolution with temperature in fluids and melts under oxidizing and reducing conditions differ significantly (Table 1). In general, under oxidizing conditions the ∆H is greater, whether in melts or coexisting fluids. The D/H abundance ratio in fluid always is greater than that in melt. This behavior leads to a D/H exchange coefficient that not only differs at given temperature and pressure, but the rate of change with temperature is different under oxidizing and reducing conditions (Fig. 6; see also Table 1). Notably, experimentally determined D/H fractionation data for rhyolite–H2O and feldspar composition–H2O (Dobson et al. 1989) are quite similar to those of silicate–COH under reducing conditions (Fig. 6). Furthermore, the temperature-dependent D/H fractionation in silicate–H2O–D2O, using the same silicate composition and working in similar temperature and pressure ranges, result in ∆H = 6.5 ± 0.7 kJ/mol (Mysen 2013). These groups have somewhat similar structural behavior (see discussion above—Eq. (2)) so a closer similarity between silicate–COH under reducing conditions and silicate–H2O than between silicate–COH under oxidizing conditions and silicate–H2O may not be so surprising.
The variations of D/H ratios in fluids and melts and, therefore, in the D/H exchange coefficient are because of changing silicate and COH speciation with changing redox conditions (Kadik et al. 2004; Mysen et al. 2011; Ardia et al. 2013). Additionally, speciation change as a function of temperature and pressure takes place partly because silicate solute concentration in the fluid increases and partly because of changing C-bearing species abundance in the melt (Manning 2004; Newton and Manning 2008; Mysen et al. 2009; Mibe and Bassett 2008; Mysen et al. 2013). Network-modifying cations, which include H+ and D+ (which form bonding with nonbridging oxygen), will show preference for specific silicate species depending on the ionization potential of the metal cation as has been demonstrated with 1H and 2H MAS NMR spectroscopy (Wang et al. 2015). These effects govern H+– and D+–oxygen bonding, with the temperature (and pressure)-dependent D/H ratio summarized in Fig. 6 as the result.
D/H behavior in COH-fluid-saturated magmatic systems
Variations in COH speciation in silicate–COH systems at high temperature and pressure affect melt polymerization. Silicate speciation and melt polymerization affect all physical and chemical properties of magmatic liquids, including D/H fractionation among species in the phases (Wang et al. 2015) which, in turn, will affect melt/mineral/fluid D/H fractionation behavior. This feature is evident in Fig. 6. Both redox conditions and abundance of COH volatiles are important.
Experimental data such as summarized in Fig. 6 (see also Table 1) can be employed to illustrate how temperature and redox conditions affect D/H fractionation factors, αD/Hfluid/melt, and how the fractionation factors, in turn, influence D/H evolution of melts and fluids in magmatic silicate–COH systems in the upper mantle and the deep crust. In doing this, I emphasize, however, that the data to be used are from model compositions in the Na2O–Al2O3–SiO2–COH system and should, therefore, not be applied quantitatively to natural magmatic processes. The behavior does, however, illustrate principles that should be kept in mind when applying hydrogen isotope data to deduce petrogenetic processes in the Earth’s interior.
The exchange equilibrium coefficient, \( {K}_{\mathrm{D}/\mathrm{H}}^{\mathrm{fluid}/\mathrm{melt}} \), in Eq. (8) equals the D/H fraction factor, of \( {\alpha}_{\mathrm{D}/\mathrm{H}}^{\mathrm{fluid}/\mathrm{melt}} \), provided that the \( {K}_{\mathrm{D}/\mathrm{H}}^{\mathrm{fluid}/\mathrm{melt}} \) is independent of bulk composition. According to the results in Fig. 5, this seems a reasonable assumption. Then, we can write its temperature-dependence;
where T is temperature (kelvin) and a and b are the regression coefficients in a plot such in Fig. 6. The fractionation factor is a strong non-linear function of temperature and is more sensitive to temperature under oxidizing conditions than under reducing conditions (Fig. 7). That fact that these curves do indeed pass through 1 as the result of changing silicate content in the fluid and COH fluid content in the melt with increasing temperature.
D/H fractionation factor between fluid and melt as a function of temperature and silicate–COH systems under reducing and oxidizing conditions (data from Mysen 2015a)
As an example of how the D/H relationships may affect the D/H evolution of magmatic liquids in (COH)-bearing mantle environments, let us start with the assumption that the COH fluid retains a fixed D/H = ratio, δD, of − 100‰. This value was chosen because it is reasonable near the δD value range of the Earth’s upper mantle (Bell and Ihinger 2000). The δD values of the melt in equilibrium with such a fluid is
In Eq. (10), αD/Hfluid/melt is defined in Eq. (9).
As can be seen from the calculated results (Fig. 8), this leads to the suggestion that a magmatic liquid in equilibrium with COH fluid under oxidizing conditions can be as much as 100% heavier than a melt equilibrated with COH under reducing conditions. The δDmelt difference is not significantly dependent on temperature of equilibration.
Carbon isotopes
For carbon isotopes, 13C and 12C, the relationship between vibrational frequency and oscillator mass in Eq. (5) would suggest a frequency difference near 4% if the masses simply were those of the carbon isotopes. However, this is not the case. For oxidized carbon, the frequency shift of C–O stretch vibrations, which for natural carbon (nearly pure 12C) occur near 1070 cm−1 in the Raman spectra (Frantz 1998) is only about 1.5%. Under reducing conditions, that of C–H stretch vibrations is less than 1% (see inserts in Fig. 9). This happens because oxygen and hydrogen vibrations, respectively, contribute to the oscillator mass.
Carbon isotope ratio evolution in coexisting melt and fluid as a function of temperature and pressure in silicate–COH systems. a. Under oxidizing conditions with carbon residing in CO32− and HCO3− groups. The bulk carbon isotope ratio was calculated with Eq. (11). Insert shows Raman spectrum of 0.7 m NaHCO3 solution with approximately 50:50 13C:12C at ambient temperature and pressure. b Under reducing conditions with carbon residing in CH4 and CH3 groups. The individual effect of these to complexes could not be resolved with Raman spectroscopy so the isotope ratio is reported in bulk. Insert shows Raman spectrum of fumaric acid with approximately 50:50 13C:12C. (data from Mysen 2016, 2017)
In an examination of carbon isotope behavior in silicate–COH systems under oxidizing conditions, the behavior of both CO32− and HCO3− complexes and their linkage to the silicate structure in fluids and melts need to be addressed (see Eq. (1)). This is so because the 13C/12C ratio and its temperature-dependence in coexisting CO32− and HCO3− differ (Table 2).
The variations with temperature of the 13C/12C ratios in the CO3 and HCO3 species also differ in fluids and melts (Table 2). In fluid, the 13C/12C ratio in HCO3 species decreases with temperature (and pressure) whereas for the CO3 groups, the opposite temperature trend is observed (Table 2). For (C–O–H)-saturated melt coexisting with fluid, on the other hand, the 13C/12C ratio of HCO3 complexes increases and that of CO3 complexes decreases with increasing temperature and pressure (Table 2). The absolute ∆H values for carbonate complex variations in melts is greater than for fluids, but do, of course, approach one another as the temperature (825 °C) and pressure (1303 MPa) conditions of supercritical phase stability field are reached (Mysen 2017).
The different temperature trajectories of the 13C/12C in fluids and melts probably reflect the much higher silicate and aluminosilicate content of melts compared with fluid. This difference is responsible for the presence of essentially only Q0 species in fluids compared with significant abundance of more polymerized aluminosilicate species in the melt (Mysen 2017). Given the interaction between the carbonate groups and oxygen in the Q-species in the melts and the fact that nonbridging oxygen in Q-species of different degree of polymerization likely are energetically diggerent (Kohn and Schofield 1994; Mysen 2007), it follows that the energetics of oxygen bonds associated with CO3 and HCO3 groups is also different. These difference, in turn, means that energetics of the C–O bonds in CO3 and HCO3 groups differ. As a result, the 13C/12C abundance evolution with temperature (and pressure) of these groups in silicate melt will differ from that in fluid. These differences in silicate speciation in fluid and melt change, however, with temperature and pressure. In particular, the silicate content of the fluid likely increases, which will cause changes in Q-speciation in the fluid (Mysen et al. 2013). As this occurs, the influence of fluid composition on its 13C/12C ratio will also become increasingly important, a conclusion which is similar to that made for temperature- and pressure-dependent changes D/H fractionation in silicate–H2O and silicate–C–O–H systems (Wang et al. 2015; Dalou et al. 2015; Le Losq et al. 2016).
The bulk 13C/12C, ∑ 13C/∑12C, comprises the contributions from both the CO32− and HCO3− groups, 13CO32−, 12CO32−, 13HCO32−, and 12HCO32− together with the molecular fraction of the species, XCO3 and XHCO3, where XHCO3 = 1 − XCO3, so that
and with a similar expression for fluid. The KDmelt and KDfluid differ (Fig. 9a; Table 2) because the values and temperature-dependence of 13C/12C of fluid and melt differ.
Therefore, for the carbon isotope exchange equilibrium between melt and fluid under oxidizing conditions we have the exchange equilibrium
for which,
where KDmelt and KDfluid are defined in Eq. (11) for melt and fluid, respectively.
The temperature-dependence of this KDmelt/fluid (Fig. 10b) leads to ∆H = 9.5 ± 0.8 kJ/mol. Under the assumption that the carbon isotope ratio is not dependent on total carbon concentration, the KDfluid/melt values equal the fractionation factors. The carbon isotope fractionation factor under reducing conditions is about three times as sensitive to temperature than under oxidizing conditions. This means the carbon isotope behavior also differs from the D/H fractionation behavior where oxidizing conditions are those under which the D/H fractionation factor is the most sensitive to temperature.
Carbon isotope melt/fluid exchange equilibrium coefficient in silicate–COH systems as a function of temperature and pressure. a Under oxidizing conditions with all carbon residing in CO32− and HCO3− groups. Note that these redox conditions are similar to those of Mattey (1991) and Mattey et al. (1990) who reported log values in the 0.09 to − 0.83 range in the 1200–1400 °C range depending on silicate composition. b Under reducing conditions with all carbon residing in CH4 and/or CH3 groups. The ∆H values from these slopes are reported in Table 1. Data otherwise from Mysen (2016, 2017)
Carbon isotope behavior in COH-fluid-saturated magmatic systems
Before addressing an example of how redox conditions during melting and crystallization may affect the carbon isotope behavior, the reader is reminded that the data to be used for this illustration were obtained in simple three component systems and with only one C/O/H ratio of the fluid component. We must also remember that the melts examined in this study were (C–O–H)-saturated and the coexisting (C–O–H) fluids were silicate-saturated. The speciation behavior is not, therefore, the same as in silicate-free carbonate–H2O system such as in the in situ studies of Facq et al. (2014) and Foustoukos and Mysen (2015).
The example below, therefore, is intended to illustrate principles, but not necessarily quantitative behavior. Calculated δ13C-evolutions in a melt in equilibrium with a (C–O–H)-bearing source with δ13C of fixed at − 7 and − 30 ‰ and in a temperature corresponding to those of magmatic processes in the deep crust and upper mantle are shown in Fig. 11. The − 7 and − 30 ‰ δ13C values correspond to the range in δ13C of mantle-derived peridotite and eclogite nodules in basalt and kimberlite (see Deines 2002, for review).
For a melt, its δ13C value can differ from that of the source region by more than 100‰ and this difference increases with increasing temperature (Fig. 11). This also means that exsolution of C–O–H fluid from a melt in the upper mantle and deep crust, the δ13C of an evolving CO2-rich fluid can be equally enriched in carbon-13 relative to the magma. This isotope behavior is, however, quite different under reducing and oxidizing conditions regardless of the δ13C value of the source rock (Fig. 11). Under reducing conditions, (COH)-saturated melts are isotopically heavier than silicate-saturated fluids. Under oxidizing conditions, the δ13C of the magmatic liquid at lower temperature is also heavier than fluid, but the temperature effect shifts this value to become isotopically lighter at higher temperature. This different behavior under reducing and oxidizing conditions reflects the different structural roles and effects on melt and fluid structure depending on the oxidation state of carbon (Mysen 2015a) (see also Eqs. (1) and (2)). It follows that changing redox conditions during melting and crystallization of (C–O–H)-bearing deep crust and upper mantle can have profound effect on the carbon isotopic signature of the melt.
Conclusions
The behavior of COHN fluids, their isotopes, and their interaction with magmatic liquids are at the core of understanding formation and evolution of the Earth.
The solubility of individual COHN components in silicate melts can differ by several orders of magnitude and ranges from several hundred ppm to several wt% at upper mantle pressure and temperature. Silicate solubility in fluid can reach several molecular. Different solubility of oxidized and reduced C-bearing species in melts reflects different solution equilibria. The oxidized species are H2O, OH−, CO32−, HCO3−, CO2, and N2, whereas reduced species are H2O, OH−, H2, CH4, CH3, NH3, and NH2. The C-solubility decreases and N-solubility increases with fO2 below the QFM oxygen buffer.
The structural changes with variable redox conditions of carbon and silicate in magmatic melts and fluids result in D/H, 13C/12C, and 15N/14N fractionation between melt, fluid, and crystalline materials that depend on redox conditions and can differ significantly from 1 even at magmatic temperatures. The D/H fractionation between aqueous fluid and magma yields ∆H between − 5 and 25 kJ/mol depending on redox conditions. The ∆H values for 13C/12C fractionation factors are near − 3.2 and 1 kJ/mol under oxidizing and reducing conditions, respectively. These differences are because energetics of O–D, O–H, O–13C, and O–12C bonding environments are governed by different solution mechanisms in melts and fluids.
It is suggested that (COH)-saturated partial melts in the upper mantle can have δD values of 100‰, or more, lighter than coexisting silicate-saturated fluid. This effect is greater under oxidizing than under reducing conditions. Analogous relationships exist for 13C/12C. For example, at magmatic temperatures in the Earth’s upper mantle, 13C/12C of melt in equilibrium with COH-bearing mantle in the − 7 to − 30‰ range increases with temperature from about 40 to > 100‰ and 80–120 ‰ under oxidizing and reducing conditions, respectively.
References
Aranovich LY, Newton RC (1999) Experimental determination of CO2-H2O activity-composition relations at 600-1000°C and 6-14 kbar by reversed decarbonation and dehydration reactions. Am Mineral 84:1319–1332
Arculus RJ (1994) Aspects of magma genesis in arcs. Lithos 33:189–208
Ardia P, Hirschmann MM, Withers AC, Stanley BD (2013) Solubility of CH4 in a synthetic basaltic melt, with applications to atmosphere-magma ocean-core partitioning of volatiles and to the evolution of the Martian atmosphere. Geochim Cosmochim Acta 114:52–71. https://doi.org/10.1016/j.gca.2013.03.028
Armstrong LS, Hirschmann MM, Stanley BD, Falksen EG, Jacobsen SD (2015) Speciation and solubility of reduced C-O-H-N volatiles in mafic melt: implications for volcanism, atmospheric evolution, and deep volatile cycles in the terrestrial planets. Geochim Cosmochim Acta 171:283–302. https://doi.org/10.1016/j.gca.2015.07.007
Baker MB, Stolper EM (1994) Determination of high-pressure mantle melts using diamond aggregates. Geochim Cosmochim Acta 58:2811–2828
Bassett, W. A., T.-C. Wu, I.-M. Chou, T. Haselton, J. D. Frantz, B. O. Mysen, W.-L. Huang, S. K. Sharma, Schiferl, D. (1996) The hydrothermal diamond anvil cell (DAC) and its applications, in Mineral Spectroscopy: a Tribute to Roger G. Burns, edited by M. D. Dyar, C. A. McCammon and M. Schaefer, pp. 261–272, The Geochemical Society, Houston.
Behrens H, Misiti V, Freda C, Vetere F, Botcharnikov RE, Scarlato P (2009) Solubility of H2O and CO2 in ultrapotassic melts at 1200 and 1250 degrees C and pressure from 50 to 500 MPa. Am Mineral 94(1):105–120. https://doi.org/10.2138/am.2009.2796
Behrens H, Ohlhorst S, Holtz F, Champenois M (2004) CO2 solubility in dacitic melts equilibrated with H2O-CO2 fluids: implications for modeling the solubility of CO2 in silicic melts. Geochim Cosmochim Acta 68(22):4687–4703. https://doi.org/10.1016/j.gca.2004.04.019
Behrens H, Roux J, Neuville DR, Siemann M (2006) Quantification of dissolved H2O in silicate glasses using confocal microRaman spectroscopy. Chem Geol 229(1–3):96–112. https://doi.org/10.1016/j.chemgeo.2006.01.014
Behrens H, Yamashita Y (2008) Water speciation in hydrous sodium tetrasilicate and hexasilicate melts: constraint from high temperature NIR spectroscopy. Chem Geol 256(3–4):306–315. https://doi.org/10.1016/j.chemgeo.2008.06.053
Bell DR, Ihinger PD (2000) The isotopic composition of hydrogen in nominally anhydrous mantle minerals. Geochim Cosmochim Acta 64:2109–2118
Bigeleisen J, Mayer MG (1947) Calculation of equilibrium constants for isotopic exchange reactions. J Chem Phys 15:261–267
Botcharnikov RE, Behrens H, Holtz F (2006) Solubility and speciation of C-O-H fluids in andesitic melt at T=1100-1300 degrees C and P=200 and 500MPa. Chem Geol 229(1–3):125–143. https://doi.org/10.1016/j.chemgeo.2006.01.016
Bottinga Y (1968) Calculation of fractionation factors for carbon and oxygen isotopic exchange in the system calcite-carbon dioxide-water. J Phys Chem A 72:800–808
Brooker RA, Kohn SC, Holloway JR, McMillan PF (2001) Structural controls on the solubility of CO2 in silicate melts Part II: IR characteristics of carbonate groups in silicate glasses. Chem Geol 174(1–3):241–254. https://doi.org/10.1016/s0009-2541(00)00318-1
Carroll MR, Stolper EM (1993) Noble gas solubilities in silicate melts and glasses: new experimental results for argon and the relationship between solubility and ionic porosity. Geochim Cosmochim Acta 57(23–24):5039–5052
Chacko T, Cole DR, Horita J (2001) Equilibrium oxygen, hydrogen, and carbon isotope fractionation factors applicable to geologic systems. In: Valley JW, Cole DR (eds) Stable isotope fractionation. Mineralogical Society of America, Washington DC, pp 1–81
Cody GD, Mysen BO, Lee SK (2005) Structure vs. composition: a solid state 1H and 29Si NMR study of quenched glasses along the Na2O-SiO2-H2O join. Geochim Cosmochim Acta 69:2373–2384
Dalou C, Le Losq C, Mysen BO (2015) In situ study of the fractionation of hydrogen isotopes between aluminosilicate melts and coexisting aqueous fluids at high pressure and high temperature - implications for the delta D in magmatic processes. Earth Planet Sci Lett 426:158–166. https://doi.org/10.1016/j.epsl.2015.06.032
Deines P (2002) The carbon isotope geochemistry of mantle xenoliths. Earth Sci Rev 58:247–278
Dixon JE, Leist L, Langmuir C, Schilling J-G (2002) Recycled dehydrated lithosphere observed in plume-influenced mid-ocean-ricge basalt. Nature 420:385–389
Dobson PF, Epstein S, Stolper, EM (1988) Hydrogen isotope fractionation between coexisting vapor and silicate glasses and melts at low pressure. In: Anonymous (Ed.), Geological Society of America, 1988 centennial celebration. Geological Society of America (GSA), Boulder, p. 190.
Dobson PF, Epstein S, Stolper EM (1989) Hydrogen isotope fractionation between coexisting vapor and silicate glasses and melts at low pressure. Geochim Cosmochim Acta 53:2723–2731
Eggler DH (1975) Peridotite-carbonatite relations in the system CaO-MgO-SiO2-CO2, Carnegie Institution of Washington, Year Book, 74, pp 468–474
Eggler DH, Baker DR (1982) Reduced volatiles in the system C-H-O: implications to mantle melting, fluid formation and diamond genesis. In: Reidel D (ed) High-pressure research in geophysics. Kluwer Academic Publishers, Boston / Dordrecht, pp 237–250
Eggler DH, Kushiro I, Holloway JR (1979) Free energies of decarbonation reactions at mantle pressures; I, stability of the assemblage forsterite-enstatite-magnesite in the system MgO-SiO2-CO2-H2O to 60 kbar. Am Mineral 64(3–4):288–293
Facq S, Daniel I, Montagnac G, Cardon H, Sverjensky DA (2014) In-situ Raman study and thermodynamic model of aqueous carbonate speciation in equilibrium with aragonite under subduction zone conditions. Geochim Cosmochim Acta 132:375–390
Foley SF, Yaxley GM, Rosenthal A, Buhre S, Kiseeva ES, Rapp RP, Jacob DE, E D (2009) The composition of near-solidus melts of peridotite in the presence of CO2 and H2O between 40 and 60 kbar. Lithos 112:274–283. https://doi.org/10.1016/j.lithos.2009.03.020
Foustoukos DI, Mysen BO (2012) D/H isotopic fractionation in the H2-H2O system at supercritical water conditions: composition and hydrogen bonding effects. Geochim Cosmochim Acta 86:88–102
Foustoukos DI, Mysen BO (2015) The structure of water-saturated carbonate melts. Am Mineral 100(1):35–46. https://doi.org/10.2138/am-2015-4856
Frantz G (1998) Raman spectra of potassium carbonate and bicarbonate aqueous fluids at elevated temperatures and pressures: comparison with theoretical simulations. Chem Geol 152:211–225
Frost DJ, McCammon CA (2008) The redox state of the Earth’s mantle. Annu Rev Earth Planet Sci 36:389–420
Gaetani GA, Grove TL (1998) The influence of water on melting of mantle peridotite. Contrib Mineral Petrol 131(4):323–346
Guillot B, Sator N (2012) Noble gases in high-pressure silicate liquids: a computer simulation study. Geochim Cosmochim Acta 80:51–69. https://doi.org/10.1016/j.gca.2011.11.040
Horita J (1988) Hydrogen isotope analyses of natural waters using an H2-water equilibration method: a special implication to brines. Chem Geol 72:89–94
Horita J, Cole DR, Wesolowski DJ (1995) The activity-composition relationship of oxygen and hydrogen isotopes in aqueous salt solutions; III, vapor-liquid water equilibration of NaCl solutions to 350°C. Geochim Cosmochim Acta 59:1139–1151
Horita J, dos Santos AM, Tulk CA, Chakaoumakos BC, Polyakov VB (2010) High-pressure neutron diffraction study on H–D isotope effects in brucite. Phys Chem Miner 37:741–749
Hunt JD, Kavner A, Schauble EA, Snyder D, Manning CE (2011) Polymerization of aqueous silica in H2O-K2O solutions at 25-200 degrees C and 1 bar to 20 kbar. Chem Geol 283(3–4):161–170. https://doi.org/10.1016/j.chemgeo.2010.12.022
Javoy M (2004) The early earth evolution. Geochim Cosmochim Acta 68:A749–A749
Kadik A, Pineau F, Litvin Y, Jendrzejewski N, Martinez I, Javoy M (2004) Formation of carbon and hydrogen species in magmas at low oxygen fugacity. J Petrol 45(7):1297–1310. https://doi.org/10.1093/petrology/egh007.
Kadik AA, Koltashev VV, Kryukova EB, Plotnichenko VG, Tsekhonya TI, Kononkova NN (2014) Solution behavior of C-O-H volatiles in FeO-Na2O-Al2O3-SiO2 melts in equilibrium with liquid iron alloy and graphite at 4 GPa and 1550 degrees C. Geochem Int 52(9):707–725. https://doi.org/10.1134/s0016702914090067
Kennedy GC, Wasserburg GJ, Heard HC, Newton RC (1962) The upper three-phase region in the system SiO2-H2O. Amer J Sci 260:501–521
King PL, Holloway JR (2002) CO2 solubility and speciation in intermediate (andesitic) melts: the role of H2O and composition. Geochim Cosmochim Acta 66(9):1627–1640. https://doi.org/10.1016/s0016-7037(01)00872-9
Kingsley RH, Schilling J-G, Dixon JE, Swart P, Poreda R, Simons K (2002) D/H ratios in basalt glasses from the Salas y Gomez mantle plume interacting with the East Pacific Rise: water from old D-rich recycled crust or primordial water from the lower mantle? Geochm Geophys Geosyst 3:1–26. https://doi.org/10.1029/2001GC000199
Kohn SC, Schofield PF (1994) The importance of melt composition in controlling trace-element behaviour: an exprimental study of Mn and Zn partitioning between forsterite and silicate melt. Chem Geol 117:73–87
Kuroda Y, Kariya Y, Suzukoki T, Matsuo S (1982) D/H fractionation between water and the melts of quartz, K-feldspar, albite and anorthite at high temperature and pressure. Geochem J 16:73–78
Kushiro I (1974) The system forsterite-anorthite-albite-silica-H2O at 15 kbar and the genesis of andesitic magmas in the upper mantle, in geophysical laboratory; igneous petrology, experimental and field studies; volatiles in ultrabasic and derivative rock systems. Carnegie Institution of Washington, Washington, DC, pp 244–248
Kushiro I (1998) Compositions of partial melts formed in mantle peridotites at high pressures and their relation to those of primitive MORB. In: Fujii T, Dingwell DB, Mysen BO (eds) Magmatology; the role of magmas in the evolution of the earth. Elsevier, Amsterdam, pp 103–110
Le Losq C, Mysen BO, Cody GD (2015) Water and magmas: insights about the water solution mechanisms in alkali silicate melts from infrared, Raman, and 29Si solid-state NMR spectroscopies. Progr Earth Planet Sci 2:22. https://doi.org/10.1186/s40645-015-0052-7.
Le Losq C, Mysen BO, Cody GD (2016) Intramolecular fractionation of hydrogen isotopes in silicate quenched melts. Geochem Perspect Lett 2:87–94
Luth RW, Boettcher AL (1986) Hydrogen and the melting of silicates. Am Mineral 71:264–276
Luth RW, Mysen BO, Virgo D (1987) Hydrogen in silicate liquids: solution mechanisms of hydrogen in liquids in the system Na2O - Al2O3 - SiO2 -H2. Am Mineral 72:481–487
Manning CE (2004) Polymeric silicate complexing in aqueous fluids at high pressure and temperature, and its implications for water-rock interaction. In: Wanty RB, Seal RR II (eds) Water-rock interaction. Balkema, New York, pp 45–49
Manning CE, Antignano A, Lin HA (2010) Premelting polymerization of crustal and mantle fluids, as indicated by the solubility of albite plus paragonite plus quartz in H2O at 1 GPa and 350-620 degrees C. Earth Planet Sci Lett 292(3–4):325–336. https://doi.org/10.1016/j.epsl.2010.01.044.
Manning CE, Aranovich LY (2014) Brines at high pressure and temperature: thermodynamic, petrologic and geochemical effects. Precambrian Res 253:6–16. https://doi.org/10.1016/j.precamres.2014.06.025
Mattey DP (1991) Carbon dioxide solubility and carbon isotope fractionation in basaltic melt. Geochim Cosmochim Acta 55(11):3467–3473. https://doi.org/10.1016/0016-7037(91)90508-3
Mattey DP, Taylor WR, Green DH, Pillinger CT (1990) Carbon isotopic fractionation between CO2 vapour, silicate and carbonate melts: an experimental study to 30 kbar. Contrib Mineral Petrol 104:492–505
McKay NP, Dettman DL, Downs DL, Overpeck JT (2013) The potential of Raman-spectroscopy-based carbonate mass spectrometry. J Raman Spectrosc 44:469–474
Mibe K, Bassett WA (2008) In situ Raman spectroscopic investigation of the structure of subduction-zone fluids. J Geophys Res 113. https://doi.org/10.1029/2007JB005179
Morizet Y, Florian P, Paris M, Gaillard F (2017) 17O NMR evidence of free ionic clusters Mn+CO3 2− in silicate glasses: precursors for carbonate-silicate liquids immiscibility. Am Mineral 102:1561–1564
Morizet Y, Paris M, Gaillard F, Scaillet B (2010) C-O-H fluid solubility in haplobasalt under reducing conditions: an experimental study. Chem Geol 279(1–2):1–16. https://doi.org/10.1016/j.chemgeo.2010.09.011.
Mysen BO (2007) Partitioning of calcium, magnesium, and transition metals between olivine and melt governed by the structure of the silicate melt at ambient pressure. Am Mineral 92:844–862
Mysen BO (2009) Solution mechanisms of silicate in aqueous fluid and H2O in coexisting silicate melts determined in-situ at high pressure and high temperature. Geochim Cosmochim Acta 73(19):5748–5763. https://doi.org/10.1016/j.gca.2009.06.023
Mysen BO (2010) Structure of H2O-saturated peralkaline aluminosilicate melt and coexisting aluminosilicate-saturated aqueous fluid determined in-situ to 800 C and 800 MPa. Geochim Cosmochim Acta 74(14):4123–4139. https://doi.org/10.1016/j.gca.2010.04.024
Mysen BO (2012) High-pressure/−temperature titanium solution mechanisms in silicate-saturated aqueous fluids and hydrous silicate melts. Am Mineral 97:1241–1251
Mysen BO (2013) Effects of fluid and melt density and structure on high pressure and temperature experimental studies of hydrogen isotope partitioning between coexisting melt and aqueous fluid. Am Mineral 98:1754–1764
Mysen BO (2015a) Hydrogen isotope fractionation and redox-controlled solution mechanisms in silicate-COH melt plus fluid systems. J Geophys Res-Solid Earth 120(11):7440–7459. https://doi.org/10.1002/2015jb011954
Mysen BO (2015b) Carbon speciation in silicate-C-O-H melt and fluid as a function of redox conditions: an experimental study, in situ to 1.7 GPa and 900 degrees C. Am Mineral 100(4):872–882. https://doi.org/10.2138/am-2015-4976.
Mysen BO (2016) Experimentally-determined carbon isotope fractionation in and between methane-bearing melt and fluid to upper mantle temperatures and pressures. Earth Planet Sci Lett 445:28–35
Mysen BO (2017) Experimental, in-situ carbon solution mechanisms and isotope fractionation in and between (C–O–H)-saturated silicate melt and silicate-saturated (C–O–H) fluid to upper mantle temperatures and pressures. Earth Planet Sci Lett 459:352–361
Mysen BO, Armstrong L (2002) Solubility behavior of alkali aluminosilicate components in aqueous fluids and silicate melts at high pressure and temperature. Geochim Cosmochim Acta 66:2287–2298
Mysen BO, Fogel ML (2010) Nitrogen and hydrogen isotopic compositions and solubility in silicate melts in equilibrium with reduced (N+H)-bearing fluids at high pressure and temperature: effects of melt structure. Am Mineral 95:987–999
Mysen BO, Fogel ML, Cody GD, Morrill PL (2009) Solution behavior of reduced C-O-H volatiles in silicate melts at high pressure and temperature. Geochim Cosmochim Acta 73:1696–1710
Mysen BO, Kumamoto K, Cody GD, Fogel ML (2011) Solubility and solution mechanisms of C-O-H volatiles in silicate melt with variable redox conditions and melt composition at upper mantle temperatures and pressures. Geochim Cosmochim Acta 75(20):6183–6199. https://doi.org/10.1016/j.gca.2011.07.035
Mysen BO, Mibe K, Chou IM, Bassett WA (2013) Structure and equilibria among silicate species in aqueous fluids in the upper mantle: experimental SiO2-H2O and MgO-SiO2-H2O data recorded in situ to 900 degrees C and 5.4GPa. J Geophys Res-Solid Earth 118(12):6076–6085. https://doi.org/10.1002/2013jb010537
Mysen BO, Yamashita S (2010) Speciation of reduced C-O-H volatiles in coexisting fluids and silicate melts determined in-situ to ~1.4 GPa and 800°C. Geochim. Cosmochim. Geochim Cosmochim Acta 74:4577–4588
Newton RC, Manning CE (2008) Thermodynamics of SiO2-H2O fluid near the upper critical end point from quartz solubility measurements at 10 kbar. Earth Planet Sci Lett 274(1–2):241–249. https://doi.org/10.1016/j.epsl.2008.07.028
O'Neil JR, Truesdell AE (1993) Oxygen isotope fractionation studies of solute water interactions. In: Taylor HP, O'NEil JR, Kaplan IR (eds) Stable isotope geochemistry: a tribute to Samuel Epstein. The Geochemical Society, Calgary, pp 17–25
O'Neil JR, Vennemann TW, McKenzie WF (2004) Effect of speciation on equilibrium fractionations and rates of oxygen isotope exchange between (PO4)aq and H2O. Geochim Cosmochim Acta 67:3135–3144
O'Neill HSC (1991) The origin of the Moon and the early history of the Earth - a chemical model. Part 2: The Earth. Geochem Cosmochim Acta 55:1159–1172
Paillat O, Elphick EC, Brown WL (1992) The solubility behavior of H2O in NaAlSi3O8 melts: a re-examination of Ab-H2O phase relationships and critical behavior at high pressure. Contrib Mineral Petrol 112:490–500
Papale P, Moretti R, Barbato D (2006) The compositional dependence of the saturation surface of H2O + CO2 fluids in silicate melts. Chem Geol 229:78–95. https://doi.org/10.1016/j.chemgeo.2006.01.013
Pascal ML, Anderson GM (1989) Speciation of Al, Si, and K in supercritical solutions: experimental study and interpretation. Geochim Cosmochim Acta 53:1843–1856
Poulson SR (1996) Equilibrium mineral-fluid stable isotope fractionation factors in graphitic metapelites. Chem Geol 131:207–217
Righter K, Drake MJ (1999) Effect of water on metal-silicate partitioning of siderophile elements: a high pressure and temperature terrestrial magma ocean and core formation. Earth Planet Sci Lett 171:383–399
Rohrbach A, Ballhaus C, Ulmer P, Golla-Schindler U, Schonbohm D (2011) Experimental evidence for a reduced metal-saturated upper mantle. J Petrol 52:717–731. https://doi.org/10.1093/petrology/egq101
Saxena SK, Fei Y (1988) Fluid mixtures in the C-H-O system at high pressure and temperature. Geochim Cosmochim Acta 52:505–512
Schiferl D, Nicol M, Zaug JM, Sharma SK, Cooney TF, Wang S-Y, Anthony TR, Fleischer JF (1997) The diamond 13C/12C isotope Raman pressure sensor system for high temperature/pressure diamond-anvil cells with reactive samples. J Appl Phys 82:3256–3265
Scholze F (1960) Über die quantitative IR-Spektrosskopische Wasser-Bestimmung in Silikaten. Fortschr Mineral 38:122–123
Song S, Li S, Niu Y, Zhang L (2009) CH4 inclusions in orogenic harzburgite; evidence for reduced slab fluids and implication for redox melting in mantle wedge. Geochim Cosmochim Acta 73:1737–1754
Stolper EM (1982) The speciation of water in silicate melts. Geochim Cosmochim Acta 46(12):2609–2620
Taylor WR, Green DH (1987) The petrogenetic role of methane: Effect on liquidus phase relations and the solubility mechanisms of reduced C-H volatiles. In: Mysen BO (ed) Magmatic Processes: Physicochemical Principles, pp 121–138
Ulmer P, Luth RW (1991) The graphite-COH fluid equilibrium in P, T, fO2 space: An experimental determination to 30 kbar and 1600°C. Contrib Mineral Petrol 106:265–272
Van Soest MC, Hilton DR, Kreulen R (1998) Tracing crustal and slab contributions to arc magmatism in the Lesser Antilles Arc using helium and carbon relationships in hydrothermal fluids. Geochim Cosmochim Acta 62:3323–3335
Wang YB, Cody GD, Cody SX, Foustoukos DI, Mysen BO (2015) Very large Intramolecular D-H partitioning in hydrated silicate melts synthesized at upper mantle pressures and temperatures. Am Mineral 100:1182–1189
Wood BJ, Bryndzia LT, Johnson KE (1990) Mantle oxidation state and its relationship to tectonic environment and fluid speciation. Science 248(4953):337–345
Xue X, Kanzaki M (2008) Structure of hydrous aluminosilicate glasses along the diopside-anorthite join: a comprehensive one- and two-dimensional H-1 and Al-27 NMR study. Geochim Cosmochim Acta 72(9):2331–2348. https://doi.org/10.1016/j.gca.2008.01.022
Zhang C, Duan Z, Li M (2010) Interstitial voids in silica melts and implication for argon solubility under high pressures. Geochim Cosmochim Acta 74:4140–4149. https://doi.org/10.1016/j.gca.2010.04.017
Zhang Y-G, Frantz JD (2000) Enstatite-forsterite-water equilibria at elevated temperatures and pressures. Am Mineral 85:918–925
Zotov N, Keppler H (2002) Silica speciation in aqueous fluids at high pressures and high temperatures. Chem Geol 184:71–82
Acknowledgements
Instrument, electronics, and library support was provided by Geophysical Laboratory technical staff. Two reviewers of the manuscript are gratefully acknowledged.
Funding
The research in this review was supported in by grants EAR-1212754, EAR-1250449, and EAR-0707861 from the National Science Foundation.
Availability of data and materials
This is a review paper. All data discussed here are in the original papers cited in the text.
Author information
Authors and Affiliations
Contributions
All research of the manuscript was by the author. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Mysen, B. Redox-controlled mechanisms of C and H isotope fractionation between silicate melt and COH fluid in the Earth’s interior. Prog Earth Planet Sci 5, 46 (2018). https://doi.org/10.1186/s40645-018-0203-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40645-018-0203-8