Abstract
Background
Malachite green (MG) is a triphenyl methane cationic dye which is used to color fabrics and is employed as food additive, food coloring agent and medical disinfectant. MG is found to be toxic to aquatic organisms, animals including humans. Copper is a commonly found metal in environment due to anthropogenic activities. Most of the microorganisms show sensitivity toward it. This adversely affects their growth and activity. In the present study, biodegradation of MG by a copper-tolerant bacterium has been investigated. Biodegradation was confirmed by UV–Vis and FTIR spectroscopy. The metabolites generated after degradation of MG were identified by LC/MS and a plausible pathway of MG degradation has been elucidated. Microbial and phyto toxicity of generated metabolites were also evaluated.
Results
A strain belonging to Ochrobactrum pseudogrignonense strain GGUPV1 was discovered from copper mine waste water. This bacterium could tolerate as high as 50 mM copper sulfate in minimal medium. It was observed that this bacterium could degrade 400 mg/L of MG in minimal medium. Decolorization of MG was also observed in presence of copper sulfate in the medium. Degradation of MG was confirmed by UV–Vis and FTIR spectroscopy. GC/MS study indicated that metabolites generated after degradation of MG were nontoxic to Staphylococcus aureus.
Conclusions
This is the first report showing degradation of MG by Ochrobactrum pseudogrignonense. This strain can be successfully employed for degradation of MG.
Similar content being viewed by others
Background
Ever-increasing demand for colored products has lead to widespread use of dyestuffs (Daneshwar et al. 2007). Various industries discharge effluents containing unused dyes directly into the water bodies causing serious threat to the environment (Zollinger 1987). In an estimate, textile dyeing effluents discharge approximately 280,000 tons of dyes worldwide every year (Jin et al. 2007). Most of the dyes are stable and thus recalcitrant to biodegradation (Robinson et al. 2001). These dyes exhibit potential toxicity to human and animals. Hence, treatment of dyeing wastewater is of utmost importance before its safe discharging into environment (Hazrat 2010). A large number of physiochemical methods are available for treatment of dye wastewater but these methods posses a constraint due to their limited versatility, high cost, low efficiency and interference by other wastewater constituents (Banat et al. 1996). These physiochemical methods also produce a lot of sludge posing a threat as secondary pollutant (Du et al. 2011). However, biological methods are available which are eco-friendly and completely mineralize organic pollutant (Pandey et al. 2007). These methods are inexpensive, have wide range applicability, low running cost, complete mineralization of dye to a nontoxic compound and eco-friendly (Forgacs et al. 2004). Malachite green (MG) is a water-soluble triphenylmethane cationic dye which is used to color fabrics (Zhou et al. 2015). It is also utilized in food and medical industry (Chowdhury et al. 2011). Apart from its toxicity on aquatic and terrestrial animals, it also elicits cytotoxicity on mammalian cell and causes formation of liver tumors (Srivastava et al. 2004). Due to its ill effects, it has been banned in many countries, but in some developing countries including India, it is still being used (Hameed and El-Khaiary 2008). Therefore, there is an urgent need to develop efficient and effective method which can treat MG dye from wastewater up to an extent which is nontoxic and can be easily disposed off to the environment in an eco-friendly way.
Recent thrust in the field of bioremediation is the use of multifaceted microorganisms. These organisms perform different roles in the environment viz. apart from biodegradation, they may exhibit plant growth-promoting activities, they may degrade multiple pollutants, or they show resistance toward toxic metals (Chaturvedi et al. 2013). Copper is one of the metals, which is normally found in high amounts in water bodies due to anthropogenic activities (Giller et al. 1998). Most of the microorganisms are susceptible to high doses of copper. Thus, presence of copper in water systems severely affects growth of microorganisms, leading to a loss in their biodegradation capabilities (De La Iglesia et al. 2006). In the present investigation, a copper-tolerant bacterial strain was discovered from copper mine waste water by employing enrichment technique. Potential of the isolate to degrade MG in presence and absence of copper ions was evaluated.
Methods
Microorganisms and culture conditions
Water samples were collected from the copper mines located at Balaghat, Malanjkhand Madhya Pradesh, India. It is an open-pit-type mine owned by Hindustan Copper limited. Geographical coordinates of Balaghat are 21° 47′ 23.5248″ north, 80° 47′ 28.3344″ east. Bacteria were isolated by enrichment technique. 5 mL of water sample was inoculated into 50 mL BSMY1 (Aksornchu et al. 2008) containing 5 mM CuSO4 and incubated at 30 °C temperatures on rotary shaker at 120 rpm for 3 days. Fresh grown 1 mL of culture was transferred into BSMY1 medium containing 10, 20, 30, 40 and 50 mM CuSO4 in different flasks. Finally, 0.1 mL of fresh culture growing in 50 mM CuSO4 was spread on BSMY1 agar containing 50 mM CuSO4 and incubated at 30 °C for 3 days. The most tolerant bacterial colony was observed and then they were used to further obtain the pure culture. Bacteria were isolated that could tolerate the highest CuSO4 concentration. For identification, the selected strain was sent to Chromas Biotech Pvt. Ltd, for 16S rDNA sequence analysis. The sequencing of rDNA was performed on payment basis. The sequence was matched against nucleotide sequences present in Gen Bank at NCBI database using BLASTn program. The nucleotide sequences showing similarity to the sequence of strain GGUPV1 were retrieved and a neighbor-joining phylogenetic tree was created using phylogeny.fr program (Dereeper et al. 2008).
BSMY1 medium contained the following (g/L); yeast extract–1, (NH4)2SO4—0.3, MgSO4·7H2O—0.14, CaCl2·2H2O—0.2, NaCl—0.1, KH2PO4—0.05, K2HPO4—0.05, H3BO3—0.6, CoCl2·6H2O—0.17, CuCl2·2H2O—0.09, MnCl2—0.1, ZnCl2—0.22, glucose—1.0 pH-7.5
Decolorization of MG
MG decoloriza’tion experiments 2.5 % (containing approximately 1 × 108 CFU/mL) inoculum was transferred under sterile conditions to 50 mL BSMY1 medium taken in a 150-mL erlenmeyer flask supplemented with varying concentration of MG (50–400 mg/L). The flasks were incubated in static conditions at 30 °C for 96 h. A comparative study was also performed under shaking conditions at 120 rpm under similar conditions. Flasks containing 50 mL BSMY1 medium supplemented with 50–400 mg/L MG, without inoculum were designated as control and were kept under identical conditions. After incubation of 24 h, 10 mL culture was retrieved and centrifuged at 8000 rpm for 10 min to remove bacterial cells. Decolorization was analyzed in a UV–Vis spectrophotometer by taking absorbance at 620 nm (Parshetti et al. 2006). Percent decolorization was calculated according to Ayed et al. (2009). To study the effect of different concentrations of Cu2+ ions on decolorization of MG, 10 and 20 mM CuSO4 was supplemented to 50 mL BSMY1 medium containing 50 and 100 mg/L MG. The decolorization experiment was performed as stated above.
UV–Vis spectral and FTIR analysis
Decolorization of MG was studied in UV–Vis spectroscopic analysis using Shimadzu UV–Vis spectrophotometer (UV-1800), the spectrum of MG (50 mg/L) and its degraded metabolites (50 mg/L) arising after 8 h of incubation was recorded in the range of 200–1000 nm (Chaturvedi et al. 2013). Biodegradation of MG was also monitored by Fourier transform infrared (FTIR) spectroscopy, for analysis samples were send to STIC, Cochin, Kerala, India. The analysis was carried out on payment basis (Chaturvedi et al. 2013).
GC/MS analysis
The degraded metabolites arising after 24 h of incubation of strain GGUPV1 in BSMY1 medium containing MG (50 mg/L) were extracted with equal volume of ethyl acetate (Du et al. 2011). The ethyl acetate fraction was evaporated to dryness and was sent to SAIF, IIT Bombay, Mumbai, India for analysis. The analysis was performed on payment basis.
Microbial and phytotoxicity studies of MG and its degraded metabolites
Microbial toxicity of MG and its degraded metabolites was studied on Staphylococcus aureus MTCC 737. A single colony of the strain was inoculated in 10 mL LB medium and incubated at 120 rpm at 30 °C. After overnight incubation, 100 µL culture was spread on the surface of nutrient agar plate. After spreading, four sterile filter paper discs were placed at four corners of the plates. MG (50 mg/L) and its degraded metabolites were extracted with equal volume of ethyl acetate (50 mL). The ethyl acetate fraction was evaporated to dryness. The solid residues were dissolved in 500 µL Milli-Q (MQ) water. 10 and 15 µL of MG and metabolites were placed on separate paper discs. The plates were incubated in a biological oxygen demand (BOD) incubator at 30 °C and were visually inspected (Chaturvedi et al. 2013).
Phytotoxicity of MG and its degraded metabolites was accessed by estimating the germination of seeds of Vigna radiata in presence of ethyl acetate extract of MG and its degraded metabolites (after evaporation of ethyl acetate, the solid residues were suspended in 20 mL MQ water). The seeds were surface sterilized with 2 % mercuric chloride for 20 min, and were washed thrice with double distilled water. Twenty seeds were placed on the surface of sterile cotton containing 20 mL MG and its metabolites separately and all plates were incubated at 20 °C in presence of 70 % humidity. In control plates, MQ water (20 mL) was used in place of MG solution. After 7 days of incubation, seed germination percentage and radical/plumule lengths were calculated (Du et al. 2011).
Results and discussion
Isolation of copper-tolerant microorganism
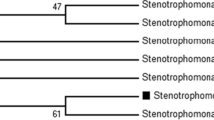
By employing enrichment technique in BSMY1 medium containing varying concentrations of CuSO4. A single bacterial colony was recovered which could tolerate as high as 50 mM CuSO4. Growth of this isolate in presence of varying concentration of copper sulfate is represented in Fig. 1a. It is evident that with increasing concentration of CuSO4, a gradual decrease in growth was observed. The decrease in growth was more pronounced in higher concentration of CuSO4, i.e., 40 and 50 mM. The possible mechanism of bacterial resistance to copper ions has been attributed to retention of copper ions in periplasmic space of the bacterium and its removal from cytoplasm with the help of various enzymes (Anand et al. 2006). The strain was sent for identification on the basis of 16S rDNA sequencing; it was identified as Ochrobactrum pseudogrignonense strain GGUPV1 (Accession No. KC145266). The phylogenetic tree of the isolate is represented in Fig. 1b.
Decolorization of MG
Synthetic dyes when discharged in water systems impart color to the water thus inhibit penetration of light in the water body (Daneshwar et al. 2007; Jin et al. 2007). They also interfere with transfer of oxygen in water leading to a decrease in dissolved oxygen (DO), and increase in chemical oxygen demand (COD), biological oxygen demand (BOD). This situation often leads to death of aquatic organisms (Banat et al. 1996; Chowdhury et al. 2011). Therefore, identification of microorganisms, which decolorize these dyes, is of utmost importance (Du et al. 2011). Decolorization potential of the strain GGUPV1 was studied in minimal medium BSMY1 containing varying concentration of MG (50–400 mg/L). Initially, comparison in decolorization was made under shaking and static conditions. The results are depicted in Fig. 2a. It is evident that under static conditions, decolorization of 50 and 100 mg/L MG after 24 h of incubation of dye was highest as compared to under shaking conditions. There are many reports which confirm that bacterial isolates show high rates of MG decolorization under static conditions as compared to shaking conditions. Parshetti et al. (2006) have reported complete decolorization of 50 mg/L MG by Kocuria rosea MTCC 1532 under static conditions. The apparent reason for this observation is availability of reduced electron carriers such as NADH2 to reduce MG under static conditions, whereas under shaking conditions both O2 and MG competes with these electron carriers leading to a decrease in decolorization (Du et al. 2011; Parshetti et al. 2006).
A time course study of MG (50–400 mg/L) decolorization was performed under static conditions (Fig. 2b). It is evident that 50–200 mg/L MG was completely decolorized after 48 h of incubation, whereas 300 mg/L MG was completely decolorized after 72 h of incubation and 400 mg/L of MG was incompletely decolorized after 96 h of incubation. There are several reports of degradation of high levels of MG by bacterial strains. This strain was able to mineralize 400 mg/L of MG in minimal medium, which was higher than previous reports made by Parshetti et al. (2006) in which K. rosea could mineralize 50 mg/L of MG in semisynthetic medium. Also, a strain of Sphingomonas paucimobilis was able to decolorize 50 mg/L of MG in minimal medium (Ayed et al. 2009). Decolorization of MG was also studied in presence of 10 and 20 mM CuSO4 after 24 h of incubation (Fig. 2c). This concentration was selected because reduction in bacterial growth was less as compared to higher concentrations. It was observed that in presence of copper ions, significant reduction in decolorization of MG took place. The reduction of decolorization was more prominent at 20 mM concentration as compared to 10 mM. The apparent reason for this observation could be toxicity of CuSO4 to bacterial cells. Similar result was also reported by Du et al. (2011) in Pseudomonas sp. strain DY1 in which pronounced reduction in MG decolorization was observed in presence of Cu2+ ions.
UV–Vis and FTIR analysis
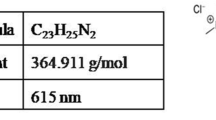
In biological systems, decolorization of MG occurs by two processes, absorption or adsorption by bacterial/fungal cells and degradation. UV–visible spectroscopy is a valuable technique to differentiate between these two processes. UV–Vis spectrum of MG shows a single prominent peak at 615 nm. During absorption or adsorption, the intensity of this peak decreases with course of time but during degradation, there is disappearance of this peak with concomitant appearance of some new peaks, which correspond to various metabolites produced during degradation of MG. MG degradation was studied by recording UV–Vis spectra (200–1000 nm) of ethyl acetate extract of MG (100 mg/L) and its degraded metabolites (100 mg/L) extracted after 24, 48 and 72 h of degradation (Fig. 3). The spectra of MG showed a characteristic major peak at 615 nm and two minor peaks at 420 and 305 nm, respectively. The spectra of degraded metabolites showed disappearance of the major–minor peaks and appearance of new peaks at 355 nm, respectively, indicating degradation of MG by strain GGUPV1. These results are in accordance with previous reports (Du et al. 2011), which demonstrate that after degradation of MG by Pseudomonas strain DY1, two new peaks appeared in the UV–Vis spectrum.
Degradation of MG was also confirmed by FTIR analysis of MG and its degraded metabolites (Fig. 4a, b). FTIR spectrum of MG showed distinct peaks in the fingerprint region (1500–500 cm−1), which corresponds to mono- and para-substituted benzene rings and were distinct to MG. The peaks were observed at 714, 828 cm−1, corresponding to aromatic ring structure, 937 cm−1, corresponding to amine group, and 1514 cm−1, which corresponds to nitro aromatic group, respectively. Also, peaks at 1167 cm−1 corresponds aromatic C–N stretching vibrations. The peaks at 1365 and 828 cm−1 were observed for CH2 scissoring and C55O stretching band (Fig. 4a).
The degraded metabolites showed characteristic peak at 3431 cm−1 corresponding to C55O overtone, 1654 cm−1 for aromatic ketones, and 1458 cm−1 for aromatic group. Reduction of peaks at 714, 937, and 1514 cm−1 indicated loss of aromaticity of metabolites (Fig. 4b). These results are in accordance with previous reports of Chaturvedi et al. (2013), Ayed et al. (2009), and Kalyani et al. (2009), and confirming degradation of MG by strain.
GC/MS study
The metabolites generated after degradation of 50 mg/mL of MG were identified by GC/MS analysis (Fig. 5). The metabolites methanone, [4-(dimethyl amino)pheny]phenyl (m/z 148), phenol, 3-(demethylamino) (m/z 136) and phenol, 3,5,-demethoxy (m/z 154) were identified. Previous studies have demonstrated that degradation of MG is facilitated by action of multiple enzymes such as MG reductase, Mn peroxidase and lignin peroxidase. (Parshetti et al. 2006; Ayed et al. 2009). Based on these available data, a pathway was proposed (Fig. 6). According to this pathway, MG is first reduced to lueco MG, which then is cleaved to produce methanone, [4-(dimethyl amino)pheny]phenyl and phenol, 3-(demethylamino).
Microbial and phytotoxicity of MG and its degraded metabolites
The toxicity of MG and its degradation products are major environmental concern. Microbial toxicity was studied by employing the conventional plate assay technique in the bacterium S. aureus MTCC 737. The results clearly showed that in the presence of MG (5 and 10 µL), inhibitory zones were formed indicating toxicity of MG to the bacterium. When the bacterium was grown in presence of ethyl acetate extract of degraded metabolites (5 and 10 µL), no zone of inhibition was observed. It indicated nontoxic nature of degraded metabolites to the bacterium. Similar results have also been reported by Chaturvedi et al. (2013), Ayed et al. (2009), which prove that the metabolites produced after degradation of MG are less toxic to microorganisms.
Usually, synthetic dyes and their degraded metabolites are disposed in water bodies without any treatment. Often this water is used for irrigation purposes. Therefore, phytotoxicity of MG and its degradation products was also evaluated. Phytotoxicity studies of MG and its degraded metabolites were performed with mung bean (Vigna radiata), which is a most widely grown pulse crop in India. The results are depicted in Table 1. It is clearly evident that MG is toxic to V. radiata, in presence of MG seed germination was significantly reduced (40 ± 0.57) when compared to control (86 ± 1.53). In the presence of degraded metabolites, the germination was higher (70 ± 0.57) indicating reduced toxicity of metabolites. Apart from germination, radical/plumule length also showed a drastic reduction in presence of MG. In presence of degraded metabolites, the reduction was not significant. These results demonstrate that MG is potentially toxic to V. radiata whereas its degraded metabolites are to a lesser extent. Our findings are consistent with previous reports showing that MG is toxic to microbes and plants, its degradation by microbes leads to a significant reduction in its toxicity (Chaturvedi et al. 2013; Parshetti et al. 2006; Ayed et al. 2009). The results of cytotoxicity and phytotoxicity analysis are in accordance with earlier findings. Du et al. (2011) reported that degraded product formed by biodegradation of MG by Pseudomonas aeruginosa NCIM 2074 are non toxic. Similarly Parshetti et al. (2006) reported that biodegradation product of MG formed by action of Kocuria rosea MTCC 1532 is nontoxic.
Conclusions
In the present study, a copper-tolerant bacterium identified as Ochrobactrum pseudogrignonense strain GGUPV1 was discovered from copper mine waste water. This strain was able to degrade 400 mg/L MG in minimal medium. This bacterium was also able to decolorize 100 mg/L MG in presence of 10 and 20 mM CuSO4. Biodegradation was confirmed by UV–Vis and FTIR spectroscopy. GC/MS analysis led to propose a pathway of MG degradation operative in this bacterium, which involved formation of metabolites such as methanone, [4-(dimethyl amino) pheny]phenyl (m/z 148) and phenol, 3-(demethylamino) (m/z 136). Further, the degraded metabolites were nontoxic to S. aureus MTCC 737 and V. radiata.
References
Aksornchu P, Prasertsan P, Sobhon V (2008) Isolation of arsenic-tolerant bacteria from arsenic-contaminated soil. Songklanakarin J Sci Technol 30(Suppl. 1):95–102
Anand P, Isar J, Saran S, Saxena RK (2006) Bioaccumulation of copper by Trichoderma viride. Bioresour Technol 97:1018–1025
Ayed L, Chaieb K, Cheref A (2009) Biodegradation of triphenylmethane dye malachite green by Spingomonas paucimobilis. World J Microbial Biotechnol 25:705–711
Banat IM, Nigam P, McMullan G, Marchant R, Singh D (1996) Microbial decolorization of textile dye containing effluents: a review. Bioresour Technol 58:217–227
Chaturvedi V, Bhange K, Bhatt R, Verma P (2013) Biodetoxification of high amounts of malachite green by a multifunctional strain of Pseudomonas mendocina and its ability of metabolize dye adsorbed chicken feathers. J Env Chem Eng 1:1205–1213
Chowdhury S, Mishra R, Saha P, Kushwaha P (2011) Adsorption thermodynamics, kinetics and isosteric heat of adsorption of malachite green onto chemically modified rice husk. Desalination 265:159–168
Daneshwar N, Ayazloo M, Khataee AR, Pourhassan M (2007) Biological decolourization of dye solution containing malachite green by microalgae Cosmarium sp. Bioresour Technol 98(6):1176–1182
De La Iglesia R, Castro D, Ginocchio R, Van Der Lelie D, Gonzalez B (2006) Factors influencing the composition of bacterial communities found at abandoned copper-tailings dumps. J Appl Microbiol 100:537–544
Dereeper A, Guignon V, Blanc G, Audic S, Buffet S, Chevenet F, Dufayard JF, Guindon S, Lefort V, Lescot M, Claverie JM, Gascuel O (2008) Phylogeny.fr: robust phylogenetic analysis for the non-specialist. Nucleic Acids Res 1:36
Du LN, Wang S, Li G, Wang B, Jia XM, Zhao YH, Chen YL (2011) Biodegradation of malachite green by Pseudomonas sp. strain DY1 under aerobic condition: characteristics, degradation products, enzyme analysis and phytotoxicity. Ecotoxicology 20(2):438–446
Forgacs E, Cserhati T, Oros G (2004) Removal of synthetic dyes from wastewaters: a review. Env Int 30:953–971
Giller K, Witter E, McGrath SP (1998) Toxicity of heavy metals to microorganisms and microbial processes in agricultural soils: a review. Soil Biol Biochem 30:1389–1414
Hameed BH, El-Khaiary MI (2008) Malachite green adsorption by rattan sawdust: isotherm, kinetic and mechanism modeling. J Hazard Mater 154:237–244
Hazrat A (2010) Biodegradation of synthetic dyes: a review. Water Air Soil Pollut 213:251–273
Jin X, Liu G, Xu Z, Yao W (2007) Decolorization of a dye industry effluent by Aspergillus fumigatus XC6. Appl Microbiol Biotechnol 74:239–243
Kalyani DC, Telke AA, Dhanve RS, Jadhav JP (2009) Ecofriendly biodegradation and detoxification of reactive red 2 textile dye by newly isolated Pseudomonas sp. SUK1. J Hazard Mater 163(2):735–742
Pandey A, Singh P, Iyengar L (2007) Bacterial decolorization and degradation of azo dyes. Int Biodeter Biodeg 59(2):73–84
Parshetti G, Kalme S, Saratale G, Govinndwar S (2006) Biodegradation of malachite green by Kocuria rosea MTCC 1532. Acta Chim Slov 53:492–498
Robinson T, McMullan G, Marchant R, Nigam P (2001) Remediation of dyes in textile effluent: a critical review on current treatment technologies with a proposed alternative. Bioresour Technol 77:247–255
Srivastava S, Sinha R, Roy D (2004) Toxicological effects of malachite green. Aquat Toxicol 66:319–329
Zhou Y, Min Y, Qiao H, Huang Q, Wang E, Ma T (2015) Improved removal of malachite green from aqueous solution using chemically modified cellulose by anhydride. Int J Biol Macromol 74:271–277
Zollinger H (1987) Synthesis, properties and applications of organic dyes and pigments. Color chemistry. VCH Publisher, New York, pp 92–102
Authors’ contributions
VC carried out the isolation of bacterium, and degradation, microbial and phytotoxicity experiments. PV designed the whole study and prepared the manuscript. Both authors read and approved the final manuscript.
Acknowledgements
PV is thankful to DBT for providing the financial support (Grant No. BT/304/NE/TBP/2012).
Competing interests
The authors declare that they have no competing interests.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Chaturvedi, V., Verma, P. Biodegradation of malachite green by a novel copper-tolerant Ochrobactrum pseudogrignonense strain GGUPV1 isolated from copper mine waste water. Bioresour. Bioprocess. 2, 42 (2015). https://doi.org/10.1186/s40643-015-0070-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40643-015-0070-8