Abstract
Background
The venous excess ultrasound (VExUS) score is a multi-organ Doppler approach to assess venous congestion. Despite growing use of VExUS in research and clinical practice, other veins can be visualized to assess for venous hypertension, which may overcome acquisition barriers of the VExUS exam. In this pilot, observational study, we used a wearable Doppler ultrasound to assess the relationship between jugular venous Doppler and the VExUS score under different preload conditions. We hypothesized that jugular Doppler morphology would accurately distinguish preload conditions, that it would most closely relate to the hepatic venous Doppler morphology in the fully supine position and that the VExUS score would be influenced by preload condition.
Results
We recruited 15 healthy volunteers with no cardiovascular history. Preload change was achieved using a tilt-table with three positions: supine, fully upright, and 30-degree head-down tilt. In each position, a VExUS score was performed; furthermore, inferior vena collapsibility and sphericity index were calculated. At the same time, jugular venous Doppler was captured by a novel, wireless, wearable ultrasound system. A continuous jugular venous Doppler morphology was 96% accurate for detecting the low preload condition. The jugular venous Doppler morphology was highly correlated with the hepatic vein, but only in the supine position. Gravitational position did not significantly affect the sphericity index or the VExUS score.
Conclusions
The jugular vein Doppler morphology was able to accurately distinguish low from high preload conditions in healthy volunteers. Comparisons between VExUS Doppler morphologies and other veins should occur in the supine position when gravitational pressure gradients are minimized; finally, different preload conditions in healthy subjects did not affect the VExUS score.
Similar content being viewed by others
Background
A criticism of the ‘liberal’ intravenous (IV) fluid resuscitative approach is that excessive IV volume risks raising venous pressure, which may impair organ perfusion [1,2,3]. Accordingly, associations between positive fluid balance and poor outcome may be partly explained by ‘congestive’ pathophysiology [4]. Historically, intensivists have monitored venous hypertension invasively by measuring the central venous pressure [5]. However, there is a trend in the twenty-first century to monitor hemodynamics non-invasively, especially via ultrasound [6]. With increasing awareness of venous Doppler ultrasound and its relationship to venous congestion, the venous excess ultrasound (VExUS) was developed [3].
The VExUS score has been associated with adverse outcomes in a number of critically ill populations, particularly with respect to acute kidney injury [7,8,9]. Though the VExUS score focuses on the spectral Doppler morphology of three intra-abdominal veins (i.e., hepatic, portal and intra-renal), venous spectral Doppler of the superior and inferior vena cavae [10, 11], femoral vein [12] and internal jugular vein [13,14,15] have all previously been linked to right heart function. Regarding the jugular vein, we have described rapid spectral Doppler changes with preload [6, 16, 17]—observations facilitated by a wireless, wearable Doppler ultrasound system that continuously displays both the common carotid arterial and internal jugular venous Doppler spectra [16,17,18,19,20]. Given the simplicity of acquiring jugular waveforms using a wearable Doppler ultrasound system, we wondered how jugular Doppler would compare to the VExUS score during preload changes in healthy subjects.
In this pilot investigation, we evaluated three basic hypotheses. For our primary outcome, we expected that the internal jugular vein Doppler morphology would be highly accurate for detecting gravity-induced changes in preload. For our secondary outcome, we predicted that the Doppler morphologies of the internal jugular and hepatic veins would correlate most in the supine position given their anatomical proximities to the right atrium and the absence of a gravitational pressure gradient between them when lying flat. For our tertiary outcome, we anticipated that gravity-induced changes in the right atrial pressure would move the jugular venous Doppler and the VExUS score in physiologically opposing directions given that the jugular vein is anatomically above the right atrium while the 3 veins in the VExUS score are anatomically below the right atrium.
Methods
Clinical setting
We recruited healthy, adult, volunteers who were clinically euvolemic. We used a convenience sample in a local physiology lab, a power calculation was not performed as this was a pilot study. Written and informed consent was obtained for all subjects and the study was reviewed and approved by the Research Ethics Board of Health Sciences North (IRB number and date of approval: CR00351324, Mar. 21, 2022). The procedures followed were in accord with the local ethical standards of the committee on human experimentation and with the Helsinki Declaration of 1975. Exclusion criteria were known cardiovascular history and/or taking regular cardiovascular medications.
Venous excess ultrasound score
The VExUS score has been previously described [3]; it comprises measurements of the end-expiratory inferior vena cava (IVC) dimension in addition to Doppler signals from the hepatic, portal and intra-renal veins. A normal score consists of an IVC diameter less than 2 cm, a systolic greater than diastolic wave in the hepatic vein Doppler, a continuous portal vein Doppler (i.e., with pulsatility less than 30%) and a continuous intra-renal vein pattern.
Internal jugular vein Doppler
Internal jugular vein Doppler was obtained using a novel, wireless, wearable Doppler ultrasound system. As previously described [18, 21, 22], the common carotid artery and internal jugular vein Doppler signals are obtained by simultaneously-acquired visual and audio cues from the wearable system. Once common carotid and internal jugular venous Doppler signals were seen and heard, the wearable Doppler was adhered in place on the subject’s neck; jugular vein Doppler was monitored throughout the protocol. Doppler morphology was judged at end-expiration and synchronous with the hepatic vein recordings.
Internal jugular vein Doppler morphology was stored for blind, independent assessment by R.P., P.R., and K.H. The jugular morphology was scored based upon its qualitative pattern, a modification of that described by Iida et al. and Tang for the intra-renal vein [23, 24]. The jugular Doppler scoring was as follows: 0 = continuous; 1 = pulsatile fused or S wave > D wave or; 2 = D wave > S wave (Fig. 1). Any disagreement was resolved by consensus between the 3 experts in venous Doppler.
Tilt table protocol
Each protocol consisted of three gravitational positions: supine, fully upright and head-down tilt, 30 degrees below the horizon. At each position, a full VExUS score was obtained by one expert in the field (R.P., P.R., K.H.) and completed within 3–5 min of each preload change. Each protocol began in the supine position with baseline measurements obtained; these included the VExUS score, the inferior vena cava sphericity index and continuous internal jugular venous Doppler from the wearable ultrasound. After supine measurements were complete, the second gravitational position for all subjects was the fully upright position; all ultrasound measures were then repeated and the subject returned to the supine position for at least a 3 min ‘wash-out’ period. Thereafter, the subject was tilted to 30 degrees head-down and all ultrasound measures repeated. Thus, the participant had VExUS scores, sphericity indices (i.e., the ratio of the short-to-long axes of the IVC in cross section) [25] and internal jugular vein Doppler measurements repeated in each tilt-table position.
Analysis of measurements
The analysis was completed in three basic parts. First, preload was dichotomized into low (i.e., fully upright) and high (i.e., head-down tilt) preload conditions; the blinded jugular vein evaluations were matched to these conditions, and the accuracy of the continuous jugular pattern for detecting low preload was calculated. To do this, we defined the following matrix:
(A) true positive occurred when there was low preload and continuous jugular Doppler; (B) true negative occurred when there was high preload with a pulsatile jugular Doppler; (C) false negative occurred when there was low preload with a pulsatile jugular morphology and (D) false positive occurred when there was high preload with continuous Doppler morphology. Overall accuracy was calculated as the true positives plus true negatives divided by the total number of observations. Second, the internal jugular vein Doppler morphology was compared to the hepatic vein within each individual at each gravitational position (i.e., supine, upright and head down). Finally, the total VExUS score for each subject in each gravitational position was also calculated.
Statistical analysis
A Fleiss Kappa was calculated to assess agreement of jugular vein Doppler morphology between the blinded observers. The effect of position on end-expiratory IVC diameter, sphericity index and portal vein pulsatility index were all compared using a Wilcoxon signed-rank test with significance defined as p < 0.05.
Results
15 adult volunteers were studied; 8 were women. One male subject was entirely excluded because of lost ultrasound image uploads; therefore, the analysis comprises 14 total adults. The baseline characteristics of the healthy volunteers included in the final analysis are listed in Table 1.
Diagnostic accuracy of jugular vein Doppler ultrasound for detecting low preload
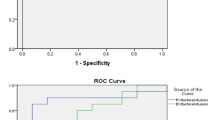
Based on the matrix defined above, there were 12 true positives, 14 true negatives and 27 total observations. Therefore, the total accuracy was 96% (Fig. 2A). There was a single false negative which occurred when a single subject had a pulsatile jugular vein in the upright position. The Fleiss Kappa inter-observer coefficient was very good (0.83) for the three blinded experts.
Effect of gravitational preload change on jugular and hepatic veins. A 2 × 2 diagnostic accuracy matrix for jugular morphology versus preload, FN is false negative, TN is true negative, TP is true positive and FP is false positive B agreement between the jugular vein and hepatic vein morphologies at different preload conditions. C Examples of jugular and hepatic vein Doppler at low (left most) versus high (right most) preload conditions
Effect of gravitational position on jugular and hepatic vein Doppler
The jugular vein Doppler morphology was scored as ‘continuous’ in 92% of subjects in the fully upright position, whereas this fraction fell to 7% and 0% in the supine and head-down positions, respectively. By contrast, the hepatic vein Doppler morphology was scored as ‘continuous’ in 21% of subjects in the fully upright position, as well as 21% in and 29% in the supine and head-down positions, respectively. Per the scoring classification scheme described above, the jugular and hepatic vein morphologies agreed 93%, 15% and 71% in the supine, fully upright and head-down positions, respectively (Fig. 2B, C). In one subject, we performed B-mode imaging of the internal jugular vein relative to the common carotid artery at all three gravitational positions (Fig. 3). Figure 4 shows the hepatic, portal, renal and jugular veins during the high preload (i.e., head-down) condition in one subject.
Example of venous scoring during high preload condition. A The hepatic vein Doppler at the bottom with labeled a, s and d waves showing an S > D morphology. B The portal vein showing the maximum and minimum velocities use to calculate the portal pulsatility index. C The renal artery and vein revealing a ‘pulsatile fused’ or ‘short end-diastolic pause’ morphology. D The carotid artery and jugular venous spectra from the wearable Doppler. The jugular has an S > D morphology most comparable to the hepatic vein
Effect of gravitational position on VExUS and its components
A full VExUS score was obtained in all subjects. In all but two subjects, the end-expiratory IVC diameter remained below 2 cm in all gravitational positions; thus, the VExUS score remained zero throughout for 86% of subjects. In one of the two subjects in whom IVC was above 2 cm, it remained so across all gravitational positions; this subject had normal hepatic and intra-renal vein Doppler morphologies across all positions, though the subject’s portal vein did become more pulsatile during head-down position, generating a VExUS = 1. In the second subject, IVC was greater than 2 cm only the in upright and head down position with otherwise normal venous Doppler throughout.
There was no statistically significant change in the sphericity index, or IVC collapsibility index across gravitational changes. There was a statistically significant increase in the end-expiratory IVC diameter from supine to upright (Table 2 and Fig. 5).
Discussion
In this pilot, physiology study assessing the impact of gravitationally modified preload conditions on venous Doppler patterns in healthy volunteers, we noted several clinically relevant findings. First, we found that jugular vein Doppler morphology had high diagnostic accuracy to detect gravity-induced preload condition. Second, the Doppler morphologies of the internal jugular and hepatic veins were most closely related in the supine position. Finally, and contrary to expectation, positional change had very little effect on the overall VExUS score, though end-expiratory IVC diameter increased very slightly in the upright position.
The Doppler morphology of the jugular vein had high diagnostic accuracy to dichotomize pre-load into “high” and “low” categories, which could aid in the rapid phenotyping of shock for acutely decompensating patients. A clinical extrapolation of our ‘true positive’ definition is a patient with low preload (e.g., normal, upright physiology, volume loss, septic venodilation) who is also observed to have continuous jugular Doppler morphology. On the other hand, a ‘true negative’ is a patient with high preload (e.g., volume expansion, cardiac dysfunction) who is observed to have at least an S > D wave jugular morphology. Furthermore, jugular Doppler could help clinicians assess the effect of preload during functional hemodynamic monitoring, as we have previously reported [6, 16, 17, 19]. These observations are facilitated by a wireless, wearable Doppler ultrasound system that continuously insonates the internal jugular vein. That over 90% of healthy volunteers displayed a continuous waveform, which fell to 7% when supine and 0% when head-down, suggested that jugular vein pulsatility qualitatively estimates preload. Notably, none of the intra-abdominal venous Doppler signals correlated with gravity-mediated preload change. For instance, the hepatic vein remained pulsatile in roughly three-quarters of all subjects in each position. This fits previous data that suggest hepatic vein Doppler does not correlate well with right atrial pressure [26]. We cannot answer the mechanism for these observations, though we note that the degree to which a vein’s Doppler signal assumes the right atrial pressure trace is mediated by its volume, compliance, transmural pressure, as well as physical and gravitational relation to the right heart. Given that the surrounding pressure of the jugular vein is atmosphere (rather than intra-hepatic or intra-renal), we suspect that jugular transmural pressure is closest to what would be measured by a central venous catheter zeroed to atmosphere [27]. The continuous nature of the jugular signal in the upright position likely reflects a collapsed or ellipsoid vein at the level of the wearable transducer (Fig. 3). Then, as the central venous pressure rises and the vein rounds out, a pulsatile waveform is observed. Fundamentally, the jugular venous Doppler morphology is a sonographic transduction of the venerable jugular venous pressure (JVP) examination.
Second, as anticipated, the morphology of the internal jugular vein was closely related to the hepatic vein in healthy volunteers, but only when supine. When lying flat, there was only a single disagreement between the jugular and hepatic vein morphologies. In this particular instance, the hepatic Doppler was continuous while the jugular had features consistent with inspiratory collapse, but with pulsatile qualities during expiration. Overall, the greatest disagreement between the hepatic and jugular veins was in the upright position, when the jugular vein was almost always continuous and the hepatic vein pulsatile. This makes sense given the anatomical relationships between the right atrium and the jugular and hepatic veins when upright. Clinically, these observations are important for further venous Doppler research; that is, relating venous morphologies is best achieved in the supine position when gravitational pressure gradients are minimized.
Finally, and somewhat unexpectedly, the overall VExUS score was resistant to gravitational preload changes in healthy volunteers. The primary reason for this was that the vast majority of subjects maintained an IVC diameter below 2 cm in all positions. Nevertheless, the Doppler vein morphologies changed very little individually as well. The hepatic vein Doppler was pulsatile in approximately 75% of subjects in each of the upright, supine and head-down positions. Neither the intra-renal nor hepatic veins showed any clinically significant qualitative changes with gravitational preload variation; in line with this, IVC collapse, portal vein pulsatility and the IVC sphericity indices were not significantly different between positions. While we cannot be sure that these results would be replicated in patients with pathological changes in volume status, intra-abdominal pressure or cardiac function [28], the clinical implication is that the overall VExUS score is resistant to preload change induced by gravity in clinically euvolemic healthy subjects with intact cardiovascular reflexes (i.e., not anesthetized or on cardiovascular medications). Because our observations cannot be generalized to critically-ill patients, gravitational position should still be considered when interpreting the VExUS score, especially in light of the work of Hermansen and colleagues as described below [28]. Nevertheless, our data remain relevant for intensivists because understanding normal, expected, physiology is the foundation for interpreting pathophysiology. As described in a recent, narrative review, the absence of significant change in the VExUS score could have predictive value in the critically-ill, for example, by delineating specific hemodynamic phenotypes defined by a “Doppler Starling curve” [6]. Our data in healthy volunteers support this framework [6]. More specifically, a PLR or Trendelenburg position coupled with the absence of change in the VExUS score (and/or a jugular Doppler pattern increasing beyond an S > D wave) could represent a good hemodynamic phenotype (e.g., low risk of weaning-induced cardiogenic edema), especially in conjunction with measures of rising stroke volume [6]. Additional study in the critically-ill population is planned.
Our study has several important limitations. We did not actually measure the central venous pressure or objectively qualify preload. Nevertheless, gravitational changes are well-accepted methods for increasing right heart filling; indeed, this is the rationale for both passive leg raising [29] and the Trendelenburg position [30,31,32]. Furthermore, in one subject, we performed simultaneous B-mode imaging of the internal jugular vein and common carotid artery in all three positions (Fig. 3). As expected, the area of the jugular vein increased relative to the carotid from upright to supine to head down; the ratio of the jugular-to-carotid area is known to correlate with central venous pressure [33,34,35]. Second, this study was in healthy volunteers with all but 1 participant having VExUS zero at baseline limiting our ability to apply these results to patients with hemodynamic derangement. Because the normal heart seeks to keep the right atrial pressure low, our observations are somewhat expected. This is in distinction to Hermansen and colleagues who performed VExUS in ventilated, sedated, post-cardiac surgery patients during the semi-recumbent and legs raised positions [28]. Contrary to our findings, they did observe a clinically significant change in renal and hepatic Doppler morphology consistent with increasing venous congestion. Nevertheless, given that the jugular vein Doppler morphology revealed robust changes in our healthy volunteer study, we suspect that jugular Doppler would also disclose preload changes in the post-surgical group. Third, interpretation of the jugular Doppler waveform does have a subjective element. We tried to minimize this effect by having three experts in venous Doppler interpretation blindly and independently score the signals. Of 41 files, there were only 5 instances where the 3 reviewers were not unanimous in their blind and independent interpretation. All five of these instances had agreement between two reviewers and the third reviewer judged the jugular morphology differently by only a single ordinal value (i.e., 0 versus 1 or 1 versus 2). This was also reflected in the Fleiss Kappa coefficient (i.e., 0.83) which indicated high inter-rater agreement between the three blinded experts. Finally, we did not measure stroke volume (SV) during preload changes in this healthy cohort. Previous work using venous measures (e.g., IVC collapse and central venous pressure) to infer ‘preload responsiveness’ show false positive rates of 20–30% [36,37,38]. In other words, a collapsing IVC or low right atrial pressure is observed in 1-to-2 out of 5 patients who are, nevertheless, fluid unresponsive; such physiology is anticipated in patients with a shallow cardiac function curve [6, 39]. Thus, we are skeptical that pure venous measures will exactly predict SV response. Furthermore, some investigators have observed high preload unresponsive rates in healthy volunteers [40], which is contrary to our earlier work. In over 30 healthy volunteers and 70 preload augmentations, 100% of healthy subjects increased SV by at least 10% [18, 21]. In our previous studies, the change in SV was monitored over thousands of cardiac cycles which may be a limitation of other investigations [41]. With respect to the current study, relating change in carotid arterial Doppler (i.e., as a surrogate for changing SV [42]) to venous measures in healthy subjects during gravity-induced preload change is an active avenue of investigation.
Conclusions
In healthy volunteers, jugular Doppler waveforms measured using a wearable Doppler device had high diagnostic accuracy for gravity-induced preload changes. In addition, the supine position showed the highest agreement between the jugular and hepatic Doppler waveforms. Finally, patient position had little effect on the overall VExUS score. The clinical implications of these findings are that internal jugular vein Doppler morphology may serve as a rapid method to phenotype patients in shock, and determine the physiological effect of preload during functional hemodynamic monitoring. Furthermore, patient position in the hospital bed does not significantly affect VExUS score, at least in relatively healthy, euvolemic, subjects with intact cardiovascular reflexes. Further investigation in patients with hemodynamic pathology is needed to confirm these preliminary observations.
Availability of data and materials
The data sets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- IV:
-
Intravenous
- VExUS:
-
Venous excess ultrasound score
- IVC:
-
Inferior vena cava
- S-wave:
-
Systolic wave
- D-wave:
-
Diastolic wave
- B-mode:
-
Brightness mode
- JVP:
-
Jugular venous pressure
References
Meyhoff TS, Hjortrup PB, Wetterslev J, Sivapalan P, Laake JH, Cronhjort M, Jakob SM, Cecconi M, Nalos M, Ostermann M, Malbrain M, Pettilä V, Møller MH, Kjær MN, Lange T, Overgaard-Steensen C, Brand BA, Winther-Olesen M, White JO, Quist L, Westergaard B, Jonsson AB, Hjortsø CJS, Meier N, Jensen TS, Engstrøm J, Nebrich L, Andersen-Ranberg NC, Jensen JV, Joseph NA, Poulsen LM, Herløv LS, Sølling CG, Pedersen SK, Knudsen KK, Straarup TS, Vang ML, Bundgaard H, Rasmussen BS, Aagaard SR, Hildebrandt T, Russell L, Bestle MH, Schønemann-Lund M, Brøchner AC, Elvander CF, Hoffmann SKL, Rasmussen ML, Martin YK, Friberg FF, Seter H, Aslam TN, Ådnøy S, Seidel P, Strand K, Johnstad B, Joelsson-Alm E, Christensen J, Ahlstedt C, Pfortmueller CA, Siegemund M, Greco M, Raděj J, Kříž M, Gould DW, Rowan KM, Mouncey PR, Perner A (2022) Restriction of intravenous fluid in ICU patients with septic shock. N Engl J Med 386:2459–2470
Self WH, Semler MW, Bellomo R, Brown SM, deBoisblanc BP, Exline MC, Ginde AA, Grissom CK, Janz DR, Jones AE (2018) Liberal versus restrictive intravenous fluid therapy for early septic shock: rationale for a randomized trial. Ann Emerg Med 72:457–466
Beaubien-Souligny W, Rola P, Haycock K, Bouchard J, Lamarche Y, Spiegel R, Denault AY (2020) Quantifying systemic congestion with Point-Of-Care ultrasound: development of the venous excess ultrasound grading system. Ultrasound J 12:1–12
Argaiz ER (2021) VExUS nexus: bedside assessment of venous congestion. Adv Chronic Kidney Dis 28:252–261
Berlin DA, Bakker J (2015) Starling curves and central venous pressure. Crit Care 19:55
Kenny J-ES (2022) Assessing fluid intolerance with Doppler ultrasonography: a physiological framework. Med Sci 10:12
Spiegel R, Teeter W, Sullivan S, Tupchong K, Mohammed N, Sutherland M, Leibner E, Rola P, Galvagno SM, Murthi SB (2020) The use of venous Doppler to predict adverse kidney events in a general ICU cohort. Crit Care 24:1–9
Denault A, Couture EJ, De Medicis É, Shim JK, Mazzeffi M, Henderson RA, Langevin S, Dhawan R, Michaud M, Guensch DP, Berger D, Erb JM, Gebhard CE, Royse C, Levy D, Lamarche Y, Dagenais F, Deschamps A, Desjardins G, Beaubien-Souligny W (2022) Perioperative Doppler ultrasound assessment of portal vein flow pulsatility in high-risk cardiac surgery patients: a multicentre prospective cohort study. Br J Anaesth 129:659
Guinot PG, Bahr PA, Andrei S, Popescu BA, Caruso V, Mertes PM, Berthoud V, Nguyen M, Bouhemad B (2022) Doppler study of portal vein and renal venous velocity predict the appropriate fluid response to diuretic in ICU: a prospective observational echocardiographic evaluation. Crit Care 26:305
Reynolds T, Appleton CP (1991) Doppler flow velocity patterns of the superior vena cava, inferior vena cava, hepatic vein, coronary sinus, and atrial septal defect: a guide for the echocardiographer. J Am Soc Echocardiogr 4:503–512
Ghio S, Recusani F, Sebastiani R, Klersy C, Raineri C, Campana C, Lanzarini L, Gavazzi A, Tavazzi L (2001) Doppler velocimetry in superior vena cava provides useful information on the right circulatory function in patients with congestive heart failure. Echocardiography 18:469–477
Abu-Yousef MM, Kakish M, Mufid M (1996) Pulsatile venous Doppler flow in lower limbs: highly indicative of elevated right atrium pressure. AJR Am J Roentgenol 167:977–980
Sivaciyan V, Ranganathan N (1978) Transcutaneous Doppler jugular venous flow velocity recording. Circulation 57:930–939
Ranganathan N, Sivaciyan V, Pryszlak M, Freeman MR (1989) Changes in jugular venous flow velocity after coronary artery bypass grafting. Am J Cardiol 63:725–729
Ranganathan N, Sivaciyan V (2022) Jugular venous pulse descents patterns—recognition and clinical relevance. CJC Open
Kenny J-ÉS, Barjaktarevic I, Mackenzie DC, Rola P, Haycock K, Eibl AM, Eibl JK (2021) Inferring the Frank–Starling curve from simultaneous venous and arterial Doppler: measurements from a wireless, wearable ultrasound patch. Front Med Technol 3
Kenny J-ÉS, Munding CE, Eibl AM, Eibl JK (2022) Wearable ultrasound and provocative hemodynamics: a view of the future. Crit Care 26:329
Kenny J-ÉS, Barjaktarevic I, Mackenzie DC, Elfarnawany M, Yang Z, Eibl AM, Eibl JK, Kim C-H, Johnson BD (2021) Carotid Doppler ultrasonography correlates with stroke volume in a human model of hypovolaemia and resuscitation: analysis of 48 570 cardiac cycles. Br J Anaesth 127:e60–e63
Kenny J-ÉS (2021) Functional hemodynamic monitoring with a wireless ultrasound patch. J Cardiothorac Vasc Anesth 35:1509–1515
Kenny J-ÉS, Munding CE, Eibl JK, Eibl AM, Long BF, Boyes A, Yin J, Verrecchia P, Parrotta M, Gatzke R (2021) A novel, hands-free ultrasound patch for continuous monitoring of quantitative Doppler in the carotid artery. Sci Rep 11:1–11
Kenny J-ÉS, Barjaktarevic I, Mackenzie DC, Eibl AM, Parrotta M, Long BF, Eibl JK (2020) Diagnostic characteristics of 11 formulae for calculating corrected flow time as measured by a wearable Doppler patch. Intensive Care Med Exp 8:1–11
Kenny JS, Barjaktarevic I, Eibl AM, Parrotta M, Long BF, Elfarnawany M, Eibl JK (2022) Temporal concordance between pulse contour analysis, bioreactance and carotid Doppler during rapid preload changes. PLoS ONE 17:e0265711
Iida N, Seo Y, Sai S, Machino-Ohtsuka T, Yamamoto M, Ishizu T, Kawakami Y, Aonuma K (2016) Clinical implications of intrarenal hemodynamic evaluation by Doppler ultrasonography in heart failure. JACC Heart Failure 4:674–682
Tang WW, Kitai T (2016) Intrarenal venous flow: a window into the congestive kidney failure phenotype of heart failure? JACC Heart Failure 4:683–686
Seo Y, Iida N, Yamamoto M, Machino-Ohtsuka T, Ishizu T, Aonuma K (2017) Estimation of central venous pressure using the ratio of short to long diameter from cross-sectional images of the inferior vena cava. J Am Soc Echocardiogr 30:461–467
Tsutsui RS, Borowski A, Tang WH, Thomas JD, Popović ZB (2014) Precision of echocardiographic estimates of right atrial pressure in patients with acute decompensated heart failure. J Am Soc Echocardiogr 27:1072-1078.e1072
Magder S (2006) Central venous pressure monitoring. Curr Opin Crit Care 12:219–227
Hermansen JL, Nørskov J, Juhl-Olsen P (2022) Effects of changes in position, positive end-expiratory pressure and mean arterial pressure on renal, portal and hepatic Doppler ultrasound perfusion indices: a randomized crossover study in cardiac surgery patients. J Clin Monit Comput 36:1841–1850
Monnet X, Teboul J-L (2008) Passive leg raising. Intensive Care Med 34:659–663
Ma G-G, Xu L-Y, Luo J-C, Hou J-Y, Hao G-W, Su Y, Liu K, Yu S-J, Tu G-W, Luo Z (2021) Change in left ventricular velocity time integral during Trendelenburg maneuver predicts fluid responsiveness in cardiac surgical patients in the operating room. Quant Imaging Med Surg 11:3133
Monnet X, Shi R, Teboul J-L (2022) Prediction of fluid responsiveness. What’s new? Ann Intensive Care 12:46
Terai C, Anada H, Matsushima S, Shimizu S, Okada Y (1995) Effects of mild Trendelenburg on central hemodynamics and internal jugular vein velocity, cross-sectional area, and flow. Am J Emerg Med 13:255–258
Donahue SP, Wood JP, Patel BM, Quinn JV (2009) Correlation of sonographic measurements of the internal jugular vein with central venous pressure. Am J Emerg Med 27:851–855
Lipton B (2000) Estimation of central venous pressure by ultrasound of the internal jugular vein. Am J Emerg Med 18:432–434
Hossein-Nejad H, Mohammadinejad P, Ahmadi F (2016) Internal jugular vein/common carotid artery cross-sectional area ratio and central venous pressure. J Clin Ultrasound 44:312–318
Corl KA, George NR, Romanoff J, Levinson AT, Chheng DB, Merchant RC, Levy MM, Napoli AM (2017) Inferior vena cava collapsibility detects fluid responsiveness among spontaneously breathing critically-ill patients. J Crit Care 41:130–137
Muller L, Bobbia X, Toumi M, Louart G, Molinari N, Ragonnet B, Quintard H, Leone M, Zoric L, Lefrant JY (2012) Respiratory variations of inferior vena cava diameter to predict fluid responsiveness in spontaneously breathing patients with acute circulatory failure: need for a cautious use. Crit Care 16:R188
Magder S, Bafaqeeh F (2007) The clinical role of central venous pressure measurements. J Intensive Care Med 22:44–51
Kenny J-ES, Eibl JK, Mackenzie DC, Barjaktarevic I (2021) Guidance of intravenous fluid by ultrasound will improve with technology. Chest 161:132–133
Godfrey GE, Dubrey SW, Handy JM (2014) A prospective observational study of stroke volume responsiveness to a passive leg raise manoeuvre in healthy non-starved volunteers as assessed by transthoracic echocardiography. Anaesthesia 69:306–313
Kenny J-ÉS, Barjaktarevic I, Mackenzie DC, Elfarnawany M, Math ZYB, Eibl AM, Eibl JK, Kim CH, Johnson BD (2021) Carotid Doppler measurement variability in functional hemodynamic monitoring: an analysis of 17,822 cardiac cycles. Crit care explor 3:e0439
Kenny J-ÉS, Barjaktarevic I, Mackenzie DC, Elfarnawany M, Yang Z, Eibl AM, Eibl JK, Kim C-H, Johnson BD (2022) Carotid artery velocity time integral and corrected flow time measured by a wearable Doppler ultrasound detect stroke volume rise from simulated hemorrhage to transfusion. BMC Res Notes 15:7
Acknowledgements
None.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
JESK—conception, study design, analysis and interpretation, drafting. RP—conception, data acquisition, analysis and interpretation, critical revisions. PR—conception, design, data acquisition, analysis, critical revisions. GM—data acquisition, analysis and interpretation, critical revisions. JKE—conception, analysis and interpretation, critical revisions. KH—conception, data acquisition, analysis and interpretation, critical revisions. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Written and informed consent was obtained for both patients included in this report, and the study was approved by the Peoria Institutional Review Board. The study was performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments.
Consent for publication
Written and informed consent was obtained for both patients for publication of this report and accompanying images.
Competing interests
JESK, GM, JKE work for Flosonics Medical, the start-up building the wearable Doppler ultrasound. RP, KH, PR reports no conflicts; no specific funding supported this work.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kenny, JE.S., Prager, R., Rola, P. et al. The effect of gravity-induced preload change on the venous excess ultrasound (VExUS) score and internal jugular vein Doppler in healthy volunteers. ICMx 11, 19 (2023). https://doi.org/10.1186/s40635-023-00504-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40635-023-00504-8