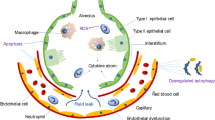
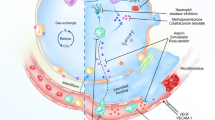
Abstract
This review addresses the plausibility of hydrogen sulfide (H2S) therapy for acute lung injury (ALI) and circulatory shock, by contrasting the promising preclinical results to the present clinical reality. The review discusses how the narrow therapeutic window and width, and potentially toxic effects, the route, dosing, and timing of administration all have to be balanced out very carefully. The development of standardized methods to determine in vitro and in vivo H2S concentrations, and the pharmacokinetics and pharmacodynamics of H2S-releasing compounds is a necessity to facilitate the safety of H2S-based therapies. We suggest the potential of exploiting already clinically approved compounds, which are known or unknown H2S donors, as a surrogate strategy.
Similar content being viewed by others
Background
This review explores the plausibility of hydrogen sulfide (H2S) therapy for acute lung injury (ALI) and circulatory shock. H2S is a toxic gas with a characteristic smell of rotten eggs, and is also produced endogenously by three different enzymes: cystathionine-β-synthase (CBS), cystathionine-γ-lyase (CSE), and 3-mercaptopyruvate-sulfurtransferase (MST) [1]. In 1996 and 1997, physiological roles of H2S in the brain and vascular smooth muscle, respectively [2, 3], were discovered, which led to its classification as the third “endogenous gaso-transmitter” [4], besides nitric oxide and carbon monoxide.
In 2005, in a hallmark study, Blackstone et al. demonstrated that inhaled H2S (80 ppm, ambient temperature 13 °C) can induce a “suspended-animation” like state by reduction of the metabolic rate in spontaneously breathing mice. This was accompanied by a fall in body temperature down to 15 °C [5]. The metabolic rate dropped by 90% after 6 h of H2S exposure. The effect was fully reversible upon transferring the mice into room air and room temperature [5]. These findings led to high hopes and a frenzy of speculation regarding the ability of H2S to induce a hypometabolic state which could be exploited in patient care [6]. However, the fact that this effect was first shown in experimental conditions (low ambient temperature, no maintenance of body temperature, and no anesthesia) that are contrary to the current clinical practice, some drawbacks have to be anticipated in translating this effect to critical care medicine. Interestingly, H2S-induced hypometabolism and hypothermia could be reproduced in mice at room temperature, but could not be confirmed in anesthetized sheep [7]. In anesthetized pigs, Simon et al. did report a sulfide-induced drop in metabolism in a model of aortic occlusion with intravenous sulfide administration [8]. However, in large animals, the effect seems to take longer to manifest and is not as pronounced as in rodents. Thus, the concept of H2S-induced “suspended animation” or hypometabolism should remain in the realm of science fiction (as suggested by Drabek et al. [9]), but it is also true that potentially therapeutic effects of H2S independent of hypometabolism [10,11,12]: anti-inflammatory, antioxidant, organ-specific benefits, regulation of blood pressure, and glucose metabolism [13,14,15,16,17], are encouraging for the clinical development of H2S donors and have not yet been fully explored [18]. After a brief introduction into the role of H2S in the lung, its role in chronic lung diseases and modes of exogenous H2S administration, we will review the current literature of exogenous H2S administration in preclinical models of acute lung injury (ALI, mostly rodents), translationally more relevant models of lung injury and circulatory shock (resuscitated large animal models), and finally conclude with the current status of clinical trials of H2S therapies and an outlook on future clinical development.
The role of H2S in the lung
High levels of H2S gas have been shown to be an environmental hazard, entering the body through the lung and being further distributed via the bloodstream [17]. H2S as a byproduct of various industries and pollutant arising from sewers can cause a “knock-down” effect upon inhalation of > 500 ppm: pulmonary injury, loss of consciousness, cardiopulmonary arrest, and death [19]. Generally, 10–20 ppm of H2S are considered to be safe to inhale acutely [17]. The effects of a chronic low-level exposure to H2S on lung toxicity have not been well characterized, and epidemiological studies are controversial, either reporting no relevant effect [20], or reduced lung function [21]. Bates et al. investigated the effects of naturally occurring H2S in geothermal areas on pulmonary health and found no detrimental effect and surprisingly even suggest a potential benefit on lung function [22].
H2S reportedly plays a role in lung development [23], and a deficiency in the endogenous H2S enzymes impairs alveolarization [24]. In the adult lung, the expression of the endogenous enzymes has been identified in a variety of pulmonary compartments in different species: rodents [25,26,27], bovine [28], and humans [29,30,31,32]. An upregulation of the endogenous H2S enzymes has been reported to play a role in the adaptive response to injury [27, 33]. However, the role of endogenous H2S in the adult lung is not well established.
H2S in chronic lung diseases
Chronic pulmonary diseases have been found to be associated with reduced H2S serum levels in patients [34] and suppressed pulmonary CSE expression [31]. Even though a few preclinical studies report pro-inflammatory effects of H2S in general (e.g., [35, 36]), it seems well established that the predominant H2S effect in the pathophysiology of chronic pulmonary diseases is anti-inflammatory [25, 31, 32, 37, 38]. Interestingly, low expression of the H2S-producing enzymes was shown to compromise the anti-inflammatory effects of glucocorticoid therapy in asthma [31, 39]. Low levels of CSE expression and H2S production in early development have been correlated to a higher susceptibility to allergic asthma in young mice [40]. The protective role of H2S in chronic inflammatory lung diseases has been thoroughly reviewed by Chen and Wang ([41]: animal models [25, 37, 39, 42] and human studies [34, 43]) and reported more recently (animal models: [38, 44] human: [31], human in vitro: [32]). There are numerous studies reporting a potential benefit of exogenous H2S administration in chronic lung diseases [25, 32, 38, 44, 45].
Possible strategies for exogenous administration of H2S
The possible strategies for exogenous administration of H2S have been reviewed recently by Szabo and Papapetropoulos [17] and comprise the following: inhalation of gaseous H2S and intraperitoneal (i.p.) or intravenous (i.v.) administration of various H2S-releasing compounds: H2S-releasing salts (e.g., Na2S, NaHS) and slow H2S-releasing donors (GYY4137, AP39, diallyl-trisulfide (DATS)). Regarding the effects of exogenous H2S on inflammation reveals that short-term free sulfide levels as a consequence of the administration of H2S-releasing salts can have detrimental effects, whereas a slow continuous H2S release from slow-releasing donors attenuated inflammation (demonstrated in vitro by [46] and thoroughly reviewed by [13]). An overview of currently available H2S-releasing compounds is given in Table 1.
Therapeutic potential of H2S during acute lung injury
In the following subsections, 70 articles investigating the effects of exogenous H2S administration in various models of acute lung injury are reviewed. These articles were identified in a literature search on PubMed in August 2019 with the search term “hydrogen sulfide” in combination with either “acute lung injury” or “ventilator-induced lung injury” or “shock” and “lung.” Articles that were not available in English or did not deal with exogenous H2S administration were excluded.
Ventilator-induced lung injury (VILI)
The effects of exogenous H2S in murine models of VILI are mostly reported to be anti-inflammatory. Only one study reports an acceleration of VILI with 60 ppm of H2S gas administration as an inhaled gas [47]. However, in the same study, pre-treatment with an intra-arterial bolus of Na2S (0.55 mg/kg) before starting harmful ventilation could attenuate lung inflammation and oxidative stress [47]. The latter is well in accordance with the protective effects of H2S in VILI reported by Aslami et al. and Wang et al., who observed reduced inflammation and improved lung function in animals with VILI, treated with a continuous infusion of 2 mg/kg/h NaHS or DATS, respectively [48, 49]. In contrast to the harmful effects of gaseous H2S administration (60 ppm) [47], four separate reports from a different group all indicate a beneficial effect of 80 ppm of H2S: anti-inflammatory and anti-apoptotic effects [11], attenuated lung damage [50], antioxidant effects [51], and prevention of edema formation, even with a reduced H2S administration time [52]. These contrasting results might be due to the fact that the latter group used a milder VILI protocol with a tidal volume of 12 ml/kg over a longer time (6 h) [11, 50,51,52] rather than 40 ml/kg for 4 h as [47]. In conclusion, these results suggest an overall beneficial effect of H2S in VILI.
Pancreatitis-induced acute lung injury (ALI)
Up to 1/3 of all pancreatitis patients develop ALI or acute respiratory distress syndrome (ARDS), which accounts for 60% of pancreatitis-related deaths [53]. Inhibition of cystathionine-γ-lyase (CSE) had anti-inflammatory effects in a murine model of pancreatitis-induced lung injury [54]. In a follow-up experiment, Bhatia et al. 2006 reported an induction of lung inflammation and histological damage in response to i.p. injection of 10 mg/kg NaHS in mice [55]. The effects were only present 1 h post-injection and by 3 and 6 h, the inflammatory state had returned to baseline [55], suggesting that the toxic effects were a transitory consequence of NaHS-induced high peak sulfide concentrations, which were quickly cleared. Besides Bhatia et al. 2005 [54], three more studies report a benefit of the inhibition of endogenous H2S production by CSE (either chemically or genetic deletion) on pancreatitis-induced ALI in murine models [56,57,58]. However, as mentioned previously, the effects of H2S on inflammation are controversial: in other studies, both the administration of ACS15 (H2S-releasing diclofenac) and NaHS pre-treatment (10–15 mg/kg) led to an attenuation of inflammation in pancreatitis-induced ALI [59, 60]. The context of H2S administration seems to be crucial: in a healthy animal, 10 mg/kg NaHS induces transient lung inflammation, whereas this kind of pre-treatment is anti-inflammatory in subsequent pancreatitis-induced ALI. Furthermore, the role of CBS in the CSE inhibition experiments is not clear—it could potentially be upregulated in response to CSE inhibition. Neither of the CSE inhibition experiments report pulmonary H2S levels; thus, no causal conclusions about the role of H2S itself in inflammation can be drawn from these studies.
Burn and/or smoke-induced lung injury
Acute lung injury is common in burn injury patients and can also be aggravated by the inhalation of smoke. In a murine model of hot water-induced skin burn, Zhang et al. observed aggravated lung inflammation and histological damage in animals treated with NaHS (10 mg/kg) [61], which could be mediated by transient toxic peak sulfide release, which has to be anticipated with this dose of NaHS. In contrast, in a similar model, Ahmad et al. report attenuated pulmonary cell infiltration and oxidative stress with the administration of AP39 [62]. However, confoundedly, another arm in this study was treated with AOAA, an inhibitor of endogenous H2S enzymes [63], which had the same effects as AP39, prompting their conclusion of a “complex pathogenic role of H2S in burns” [62]. However, the authors neither report H2S levels nor the expression levels of the endogenous enzymes, which makes it difficult to interpret their data. In the lung, the upregulation of the endogenous H2S enzymes can represent an adaptive response to stress [27]. Thus, it is tempting to speculate that their apparently ambivalent results may be attributed to AOAA and AP39 having a similar regulatory effect on the endogenous H2S enzymes, which has not been investigated or reported yet. In fact, Han et al. report attenuated lung injury and antioxidant effects of spontaneous breathing of 80 ppm H2S in a rat model of cotton smoke-induced ALI [64]. In a combined model of smoke- and flame burn-induced lung injury, Esechie et al. were able to demonstrate attenuated inflammation and improved 5 days survival due to subcutaneous Na2S treatment [65]. They were also able to confirm this protective effect of Na2S in a large animal (ovine) model of smoke and burn injury, where a 24-h primed continuous i.v. infusion of Na2S after injury ameliorated pulmonary pathophysiological changes [66]. Overall, H2S seems to mediate protective effects in burn- and/or smoke-induced ALI.
Endotoxin-induced ALI
All studies investigating the effects of exogenous H2S in LPS-induced lung inflammation were performed in rodents and reported beneficial effects, regardless of the mode of LPS (locally or systemically) and H2S (salt, slow-releasing donor, inhalation) administration. Inhalation of 80 ppm H2S after intranasal LPS attenuated lung histological damage and had anti-inflammatory and antioxidative effects [67, 68]. Pre-treatment with GYY4137 also attenuated lung injury and cell infiltration after LPS inhalation [69]. Both GYY4137 and NaHS pre-treatment also attenuated lung injury and inflammation after intratracheal LPS exposure [70, 71]. A therapeutic administration of H2S, either sodium thiosulfate (STS) or GYY4137, after intratracheal LPS ameliorated pulmonary inflammation as well [72, 73]. GYY4137 also attenuated cell infiltration in the lung after i.v. injection with LPS. Pre-treatment with GYY4137 had antioxidant and anti-inflammatory effects in i.p. injection of LPS. NaHS administration 3 h after i.v. LPS attenuated inflammation and oxidative stress and protected the mitochondria in the lung [74].
Polymicrobial sepsis-induced ALI
In contrast to studies investigating endotoxin administration, the role of exogenous H2S in murine models of cecal ligation and puncture (CLP, abdominal sepsis) is controversial: both beneficial and detrimental effects have been reported. In a resuscitated murine model, 100 ppm of inhaled H2S had minor anti-inflammatory effects, though not mediating protective effects in CLP [75]. A variety of studies report aggravation of sepsis-induced lung injury by NaHS [76,77,78,79,80,81,82]. However, in all these models, NaHS was administered as an i.p. bolus and did not comprise any additional resuscitative measures. The route of administration might also be a confounding factor combined with the CLP. Furthermore, the dose of H2S that was used in these studies was much higher than the dose of the previously mentioned LPS experiments (i.e., 10 mg/kg during CLP versus 0.78–3.12 mg/kg i.p. NaHS during LPS). In fact, 1 h i.v. administration of NaHS at a rate of 1 and 3 mg/(kg × h) after CLP attenuated oxidative stress and cell infiltration in the lung [83]. High peak sulfide levels achieved by the bolus administration of a high dose of H2S can exert toxic detrimental effects, whereas achieving a less pronounced elevation of sulfide levels over a longer period of time could exert a benefit [13]. In a model of enterocolitis, the slow-releasing H2S donor GYY4137 attenuated lung inflammation and edema, whereas Na2S (20 mg/kg 3 times daily) had no effect [84].
Oleic acid-induced ALI
ALI is most commonly modeled in mice by an intravenous injection of oleic acid (OA) [85]. Studies investigating exogenous H2S administration in this model consistently report beneficial effects: attenuated edema formation, reduced cell infiltration, and anti-inflammatory and antioxidant effects of NaHS pre-treatment [86,87,88,89].
Oxidative lung injury
In models of hyperoxia- or ozone-induced ALI, NaHS administration exerted anti-inflammatory and antioxidative effects [90,91,92]. However, hyperoxia cannot only induce lung damage, depending on the experimental protocol: hyperoxia, as an experimental therapy in combined fracture healing and blunt chest trauma, exerted lung-protective effects. Interestingly, these protective effects were associated with an amelioration of the stress-induced upregulation of endogenous H2S enzymes and thus restoring the naive state of protein expression [27].
Trauma-induced ALI
Blunt chest trauma induces mechanical and inflammatory injury to the lung [93]. In a resuscitated, murine model of thoracic trauma, a continuous i.v. infusion of Na2S (0.2 mg/(kg × h)) had no effect on lung mechanics and gas exchange, but reduced apoptosis and cytokine production [33]. These effects were even more pronounced in combination with hypothermia [33]. Inhaled H2S (100 ppm) attenuated inflammation and cell infiltration in the lung in a non-resuscitated rat model of thoracic trauma [94]. However, in both these studies, the effects of H2S were rather weak and a clear benefit could not have been determined [33, 94], in contrast to models of other types of injury. Interestingly, an upregulation of pulmonary CSE expression in response to combined acute on chronic lung disease, i.e., thoracic trauma after cigarette smoke exposure, was suggested to be an adaptive response to injury [27, 95], in that a genetic deletion of CSE in the same kind of acute on chronic trauma was associated with aggravated ALI [96].
ALI in various types of ischemia/reperfusion injury (I/R)
In a rat model of lung transplantation, NaHS (0.7 mg/kg i.p.) improved lung function and reduced cell infiltration and oxidative stress [97]. NaHS pre-treatment was beneficial in limb I/R-induced lung injury, due to anti-inflammatory effects and attenuated edema formation [98]. GYY4137 pre-treatment has been tested in infrarenal aortic cross clamping, as well as lung I/R, and beneficial effects have been reported in both types of lung injury: anti-inflammatory and antioxidant activity, respectively [99, 100]. Results in models of hemorrhagic shock are controversial. One study found a beneficial effect of an i.p. bolus of NaHS in a rat model: attenuated edema formation, cell infiltration, and necrosis [101]. Another study of HS in mice determined pulmonary anti-inflammatory effects of AP39; however, the mortality rate in the treated arm of this study was very high due to profound vasodilation [102]. Using a lower dose of AP39 yielded no effects at all [102]. These opposite effects of exogenous H2S administration in these two experiments might be due to the different H2S-releasing compounds used or resuscitative measures. Chai et al. [101] performed the re-transfusion/resuscitation only with fluid administration, whereas Wepler et al. [102] used re-transfusion of shed blood and a full-scale small animal intensive care unit (ICU) setup (see below), which certainly changes the pathophysiology. In general, the role of H2S in hemorrhagic shock is controversial, with either a beneficial [103,104,105,106,107,108], harmful [109, 110], or no impact [111, 112].
Translational medicine—H2S in large animal models of shock
Animal models with the purpose to identify relevant novel therapeutic strategies for patient care should reflect the clinical situation as closely as possible. In the context of ALI and shock research, the clinical practice for patient care in the ICU has to be reflected in experimental models to facilitate the translation from preclinical research to the clinical reality, i.e., temperature management, frequent blood gas analysis, lung-protective mechanical ventilation, hemodynamic monitoring, fluid administration, and catecholamine support titrated to the mean arterial pressure (MAP) [113]. Metabolic and organ-specific differences between small and large animals need to be taken into account [114, 115], as well as the challenge of reproducing the patient’s pathophysiology (e.g., comorbidities and premedication).
In particular for H2S, in a translational scenario, the implementation of intensive care measures (e.g., maintenance of body temperature, anesthesia, fluid resuscitation) might interfere with its effects, thus contributing to the lack of a hypometabolic effect in resuscitated rodent intensive care models [10, 33, 75, 102]. In large animals, the effects of H2S administration, in general, have been less robust, not only due to the intensive care measures, but also due to their large body size and different metabolic and thermoregulatory phenotype [114]. Large resuscitated animal studies reflect (i) no or very limited effects [8, 103, 112, 116,117,118], (ii) organ-specific effects [66], or (iii) beneficial effects restricted to a narrow timing and dosing window [119, 120].
As aforementioned, the induction of suspended animation by H2S inhalation was successful in small animals [5]; however, the translation to larger animals and eventually humans has proven to be challenging. Small animals have a much higher metabolic rate in relation to their body weight than large animals [121]; thus, the induction of a hypometabolic state is much easier to perform in small animals [114]. To induce that same state in a larger animal, a much higher dose of H2S would be needed, harboring the risk of toxicity [114]. However, the challenges of measuring H2S/sulfide in biological samples make it difficult to perform dose-finding studies.
Nonetheless, several studies in large animal models explored the therapeutic potential in various types of ALI. Na2S in an ovine model of burn reduced mortality and improved gas exchange [66]. In porcine models, Na2S was further studied in hemorrhagic shock, where it attenuated lung damage when administered at the time of reperfusion, however largely unrelated to hypothermia [120]. Administration of STS in the acute phase of resuscitation (24 h) after hemorrhagic shock in a porcine comorbid atherosclerotic model showed only a limited effect by improved gas exchange and lung mechanics in comparison to vehicle-treated animals (Table 2, [122]). Nußbaum et al. investigated the effects of GYY4137 during long-term resuscitated septic shock in pigs with atherosclerosis: GYY4137 treatment led to a preferential utilization of carbohydrates; however, they did not observe any major benefit of the treatment, gas exchange was not affected, and they did not further investigate lung tissue [117]. Unfortunately, none of the other large animal studies report lung function or lung histopathology. Still, it seems that exogenous H2S can mediate lung-protective effects in translationally relevant large animal models, when carefully timed and titrated.
Clinical trials of exogenous H2S administration in ALI
To be able to answer the question posted in the title of this review, the clinical development of H2S-releasing compounds has to be taken into consideration as well. As we shift from large animal preclinical studies to clinical trials, a search on clinicaltrials.gov (August 2019) for the term “sulfide” revealed a total of 64 clinical trials (see Fig. 1). Only two trials were found, which focused on a lung pathology (i.e., asthma), falling into the category “observational” in Fig. 1, investigating the potential use of H2S as a biomarker. There are no interventional clinical trials addressing the therapeutic potential of exogenous H2S in lung injury or lung disease. Of the 50 interventional trials identified, only 20 were evaluating H2S donors, 8 evaluated their intervention based on H2S as a biomarker, and 5 suggested H2S as a part of the mechanism of their intervention (see Fig. 1). The category “other” in Fig. 1 includes contrast agents, chemotherapeutics, and dietary supplements with a sulfide moiety. Only 6 of the 20 interventional trials with H2S donors are relevant to intensive care (see Fig. 2), excluding skin diseases, colonoscopy, and arthritis.
Overview of clinical trials found with the search term “sulfide” listed on clinicaltrials.gov
Clinical trials found with the search term “sulfide” on clinicaltrials.gov and relevant to intensive care. Excluded: skin diseases, halitosis, dental conditions, colonoscopy, and similar
IK-1001, a solution generated by bubbling H2S gas into an aqueous solution, was the first compound, designated to administer H2S, under investigation in clinical trials in 2009. The first trial of IK-1001 targeted “renal impairment” (NCT00879645) and was terminated prematurely (actual recruitment of 28 participants) because investigators were unable to determine sulfide levels. The issue of not being able to reliably measure sulfide is of course critical for clinical approval of a compound: how would one ever be able to determine the safety of a compound that cannot be measured? One complexity is the fact that exogenous sulfide is highly volatile and rapidly bound and/or metabolized in vivo [27]. Various sulfide pools are available in biological systems and sulfide engages in many different chemical reactions [123], suggesting that these endogenous pools are highly dynamic. Exogenous administration of H2S might change the balance of this whole system in ways that we do not fully understand yet. The second trial with IK-1001 in coronary artery bypass (NCT00858936) was terminated after recruiting 6 participants with reasons not reported. The third trial in ST-elevation myocardial infarct (STEMI, NCT01007461) was withdrawn by company decision—non-safety related.
As mentioned above, IK-1001 is an aqueous solution of physically dissolved H2S, thus resembling the characteristics of the administration of H2S-releasing salts or inhaled H2S (see also Table 1). Neither administration of H2S via inhalation nor injection of H2S-releasing salts will likely be ever used in clinical practice, due to airway mucosal damage and the potential of toxic peak sulfide concentrations, respectively [27]. In fact, inhalation of 300 ppm H2S, though sub-lethal, is used as a model to study lung injury [124, 125]. Efforts to avoid the airway irritation of gaseous H2S using extracorporeal membrane lung ventilation in a preclinical study were successful, but there was no improvement on the outcome from cardiopulmonary bypass [126].
SG-1002, a mixture of organic sulfide-releasing compounds and salts, has been under investigation in heart failure. A phase I trial revealed the compound to be safe and well tolerated (NCT01989208); a follow-up phase II trial is still in progress with no results posted yet (NCT02278276).
An interesting perspective for H2S-based therapeutics is the reconsideration of compounds that are already clinically approved and have only recently been identified to be able to release H2S: (i) sodium thiosulfate (STS) [17, 127], approved for cyanide detoxification and cisplatin overdosage; (ii) ammonium tetrathiomolybdate (ATTM) [128, 129], approved for Wilson’s disease, a copper metabolism disorder; and (iii) zofenopril [130], an inhibitor of angiotensin converting enzyme approved for hypertension. These compounds all have been tested extensively and are known to have good safety profiles (see also Table 1).
For example, Dyson et al. showed ATTM led to a 50% reduction of infarct size in rat models of myocardial and cerebral I/R as well as improved survival after hemorrhagic shock [129]. The good safety profile of STS [131] in particular might be related to the fact that thiosulfate itself is an endogenous intermediate of oxidative H2S metabolism [127] and is suggested to be “a circulating ‘carrier’ molecule of beneficial effects of H2S” [132], in particular under hypoxic conditions [127]. The clinical trial of IK-1001 in renal impairment even used thiosulfate as an indirect measure of H2S release from their compound (NCT00879645), although ultimately not successful. STS is currently under investigation in a phase 2 clinical trial to preserve cardiac function in STEMI (NCT02899364). With regard to the lung, as mentioned previously, STS was beneficial in murine models of intratracheal LPS and CLP [72]. Our own group’s findings support these results from Sakaguchi et al.: we determined a beneficial effect of STS to the lung, i.e., improved gas exchange and lung mechanics in a translationally relevant large animal model of hemorrhagic shock (Table 2). Thus, STS is a very promising compound for the development of therapeutic H2S administration in ALI in a clinical setting.
Conclusions
Exogenous H2S administration has been demonstrated to be beneficial in various preclinical models of lung injury. However, due to the narrow therapeutic window and width, and potentially toxic effects, the route, dosing, and timing of administration all have to be balanced out very carefully. The development of methods to determine H2S levels and/or the pharmacokinetics and pharmacodynamics of H2S-releasing compounds is absolutely necessary to facilitate the safety of H2S-based therapies. Awaiting the results of currently ongoing clinical trials and the re-evaluation of already approved H2S-releasing compounds for novel indications could likely help to prove that H2S is in fact not a therapeutic dead end [6].
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- ALI:
-
Acute lung injury
- ARDS:
-
Acute respiratory distress syndrome
- ATTM:
-
Ammonium tetrathiomolybdate
- CBS:
-
Cystathionine-β-synthase
- CLP:
-
Cecal ligation and puncture
- CSE:
-
Cystathionine-γ-lyase
- DATS:
-
Diallyl-trisulfide
- H2S:
-
Hydrogen sulfide
- ICU:
-
Intensive care unit
- I/R:
-
Ischemia reperfusion
- i.p.:
-
Intraperitoneal
- i.v.:
-
Intravenous
- LPS:
-
Lipopolysaccharide
- MAP:
-
Mean arterial pressure
- MST:
-
3-mercaptopyruvate-sulfurtransferase
- OA:
-
Oleic acid
- ppm:
-
parts per million
- STS:
-
Sodium thiosulfate
- VILI:
-
Ventilator-induced lung injury
References
Wang R (2012) Physiological implications of hydrogen sulfide: a whiff exploration that blossomed. Physiol Rev 92:791–896. https://doi.org/10.1152/physrev.00017.2011
Abe K, Kimura H (1996) The possible role of hydrogen sulfide as an endogenous neuromodulator. J Neuroscience 16:1066–1071
Hosoki R, Matsuki N, Kimura H (1997) The possible role of hydrogen sulfide as an endogenous smooth muscle relaxant in synergy with nitric oxide. Biochem Biophys Res Commun 237:527–531. https://doi.org/10.1006/bbrc.1997.6878
Wang R (2002) Two’s company, three’s a crowd: can H2S be the third endogenous gaseous transmitter? FASEB J 16:1792–1798. https://doi.org/10.1096/fj.02-0211hyp
Blackstone E, Morrison M, Roth MB (2005) H2S induces a suspended animation-like state in mice. Science 308:518. https://doi.org/10.1126/science.1108581
Leslie M (2008) Medicine. Nothing rotten about hydrogen sulfide’s medical promise. Science 320:1155–1157. https://doi.org/10.1126/science.320.5880.1155
Haouzi P, Notet V, Chenuel B, Chalon B, Sponne I, Ogier V, Bihain B (2008) H2S induced hypometabolism in mice is missing in sedated sheep. Respir Physiol Neurobiol 160:109–115. https://doi.org/10.1016/j.resp.2007.09.001
Simon F, Giudici R, Duy CN, Schelzig H, Oter S, Gröger M, Wachter U, Vogt J, Speit G, Szabó C, Radermacher P, Calzia E (2008) Hemodynamic and metabolic effects of hydrogen sulfide during porcine ischemia/reperfusion injury. Shock 30:359–364. https://doi.org/10.1097/SHK.0b013e3181674185
Drabek T (2012) Hydrogen sulfide-curiouser and curiouser! Crit Care Med 40:2255–2256. https://doi.org/10.1097/CCM.0b013e318251507d
Baumgart K, Wagner F, Gröger M, Weber S, Barth E, Vogt JA, Wachter U, Huber-Lang M, Knöferl MW, Albuszies G, Georgieff M, Asfar P, Szabó C, Calzia E, Radermacher P, Simkova V (2010) Cardiac and metabolic effects of hypothermia and inhaled hydrogen sulfide in anesthetized and ventilated mice. Crit Care Med 38:588–595. https://doi.org/10.1097/ccm.0b013e3181b9ed2e
Faller S, Ryter SW, Choi AM, Loop T, Schmidt R, Hoetzel A (2010) Inhaled hydrogen sulfide protects against ventilator-induced lung injury. Anesthesiology 113:104–115. https://doi.org/10.1097/ALN.0b013e3181de7107
Tokuda K, Kida K, Marutani E, Crimi E, Bougaki M, Khatri A, Kimura H, Ichinose F (2012) Inhaled hydrogen sulfide prevents endotoxin-induced systemic inflammation and improves survival by altering sulfide metabolism in mice. Antioxid Redox Signal 17:11–21. https://doi.org/10.1089/ars.2011.4363
Whiteman M, Winyard PG (2011) Hydrogen sulfide and inflammation: the good, the bad, the ugly and the promising. Expert Rev Clin Pharmacol 4:13–32. https://doi.org/10.1586/ecp.10.134
Kimura Y, Goto Y, Kimura H (2010) Hydrogen sulfide increases glutathione production and suppresses oxidative stress in mitochondria. Antioxid Redox Signal 12:1–13. https://doi.org/10.1089/ars.2008.2282
van Goor H, van den Born JC, Hillebrands JL, Joles JA (2016) Hydrogen sulfide in hypertension. Curr Opin Nephrol Hypertens 25:107–113. https://doi.org/10.1097/MNH.0000000000000206
Untereiner AA, Wang R, Ju Y, Wu L (2016) Decreased gluconeogenesis in the absence of cystathionine gamma-lyase and the underlying mechanisms. Antioxid Redox Signal 24:129–140. https://doi.org/10.1089/ars.2015.6369
Szabo C, Papapetropoulos A (2017) International Union of Basic and Clinical Pharmacology. CII: pharmacological modulation of H2S levels: H2S donors and H2S biosynthesis inhibitors. Pharmacol Rev 69:497–564. https://doi.org/10.1124/pr.117.014050
Leslie M (2016) Whatever happened to. Science 353:1198–1201. https://doi.org/10.1126/science.353.6305.1198
Reiffenstein RJ, Hulbert WC, Roth SH (1992) Toxicology of hydrogen sulfide. Annu Rev Pharmacol Toxicol 32:109–134. https://doi.org/10.1146/annurev.pa.32.040192.000545
Jäppinen P, Vilkka V, Marttila O, Haahtela T (1990) Exposure to hydrogen sulphide and respiratory function. Br J Ind Med 47:824–828. https://doi.org/10.1136/oem.47.12.824
Richardson DB (1995) Respiratory effects of chronic hydrogen sulfide exposure. Am J Ind Med 28(1):99–108
Bates MN, Crane J, Balmes JR, Garrett N (2015) Investigation of hydrogen sulfide exposure and lung function, asthma and chronic obstructive pulmonary disease in a geothermal area of New Zealand. PLoS One 10:e0122062. https://doi.org/10.1371/journal.pone.0122062
Madurga A, Mižíková I, Ruiz-Camp J, Vadász I, Herold S, Mayer K, Fehrenbach H, Seeger W, Morty RE (2014) Systemic hydrogen sulfide administration partially restores normal alveolarization in an experimental animal model of bronchopulmonary dysplasia. Am J Physiol Lung Cell Mol Physiol 306:L684–L697. https://doi.org/10.1152/ajplung.00361.2013
Madurga A, Golec A, Pozarska A, Ishii I, Mižíková I, Nardiello C, Vadász I, Herold S, Mayer K, Reichenberger F, Fehrenbach H, Seeger W, Morty RE (2015) The H2S-generating enzymes cystathionine β-synthase and cystathionine γ-lyase play a role in vascular development during normal lung alveolarization. Am J Physiol Lung Cell Mol Physiol 309:L710–L724. https://doi.org/10.1152/ajplung.00134.2015
Chen YH, Wu R, Geng B, Qi YF, Wang PP, Yao WZ, Tang CS (2009) Endogenous hydrogen sulfide reduces airway inflammation and remodeling in a rat model of asthma. Cytokine 45(2):117–123. https://doi.org/10.1016/j.cyto.2008.11.009
Wang P, Zhang G, Wondimu T, Ross B, Wang R (2011) Hydrogen sulfide and asthma. Exp Physiol 96:847–852. https://doi.org/10.1113/expphysiol.2011.057448
McCook O, Radermacher P, Volani C, Asfar P, Ignatius A, Kemmler J, Möller P, Szabó C, Whiteman M, Wood ME, Wang R, Georgieff M, Wachter U (2014) H2S during circulatory shock: some unresolved questions. Nitric Oxide 41:48–61. https://doi.org/10.1016/j.niox.2014.03.163
Olson KR, Whitfield NL, Bearden SE, St Leger J, Nilson E, Gao Y, Madden JA (2010) Hypoxic pulmonary vasodilation: a paradigm shift with a hydrogen sulfide mechanism. Am J Physiol Regul Integr Comp Physiol 298:R51–R60. https://doi.org/10.1152/ajpregu.00576.2009
Perry MM, Hui CK, Whiteman M, Wood ME, Adcock I, Kirkham P, Michaeloudes C, Chung KF (2011) Hydrogen sulfide inhibits proliferation and release of IL-8 from human airway smooth muscle cells. Am J Respir Cell Mol Biol 45:746–752. https://doi.org/10.1165/rcmb.2010-0304OC
Baskar R, Li L, Moore PK (2007) Hydrogen sulfide-induces DNA damage and changes in apoptotic gene expression in human lung fibroblast cells. FASEB J 21:247–255. https://doi.org/10.1096/fj.06-6255com
Sun Y, Wang K, Li MX, He W, Chang JR, Liao CC, Lin F, Qi YF, Wang R, Chen YH (2015) Metabolic changes of H2S in smokers and patients of COPD which might involve in inflammation, oxidative stress and steroid sensitivity. Sci Rep 5:14971. https://doi.org/10.1038/srep14971
Perry MM, Tildy B, Papi A, Casolari P, Caramori G, Rempel KL, Halayko AJ, Adcock I, Chung KF (2018) The anti-proliferative and anti-inflammatory response of COPD airway smooth muscle cells to hydrogen sulfide. Respir Res 19:85. https://doi.org/10.1186/s12931-018-0788-x
Wagner F, Scheuerle A, Weber S, Stahl B, McCook O, Knöferl MW, Huber-Lang M, Seitz DH, Thomas J, Asfar P, Szabó C, Möller P, Gebhard F, Georgieff M, Calzia E, Radermacher P, Wagner K (2011) Cardiopulmonary, histologic, and inflammatory effects of intravenous Na2S after blunt chest trauma-induced lung contusion in mice. J Trauma 71:1659–1667. https://doi.org/10.1097/TA.0b013e318228842e
Chen YH, Yao WZ, Geng B, Ding YL, Lu M, Zhao MW, Tang CS (2005) Endogenous hydrogen sulfide in patients with COPD. Chest 128:3205–3211. https://doi.org/10.1378/chest.128.5.3205
Zhi L, Ang AD, Zhang H, Moore PK, Bhatia M (2007) Hydrogen sulfide induces the synthesis of proinflammatory cytokines in human monocyte cell line U937 via the ERK-NF-kappaB pathway. J Leukoc Biol 81:1322–1332. https://doi.org/10.1189/jlb.1006599
Bhatia M, Sidhapuriwala JN, Ng SW, Tamizhselvi R, Moochhala SM (2008) Pro-inflammatory effects of hydrogen sulphide on substance P in caerulein-induced acute pancreatitis. J Cell Mol Med 12:580–590. https://doi.org/10.1111/j.1582-4934.2007.00131.x
Chen YH, Wang PP, Wang XM, He YJ, Yao WZ, Qi YF, Tang CS (2011) Involvement of endogenous hydrogen sulfide in cigarette smoke-induced changes in airway responsiveness and inflammation of rat lung. Cytokine 53:334–341. https://doi.org/10.1016/j.cyto.2010.12.006
Zhang G, Wang P, Yang G, Cao Q, Wang R (2013) The inhibitory role of hydrogen sulfide in airway hyperresponsiveness and inflammation in a mouse model of asthma. Am J Pathol 182:1188–1195. https://doi.org/10.1016/j.ajpath.2012.12.008
Li SB, Tong XS, Wang XX, Jin XH, Ye H (2010) Regulative mechanism of budesonide on endogenous hydrogen sulfide, cystathionine-gamma-lyase and cystathionine-beta-synthase system in asthmatic rats. Zhongguo Dang Dai Er Ke Za Zhi 12:654–657
Wang P, Wu L, Ju Y, Fu M, Shuang T, Qian Z, Wang R (2017) Age-dependent allergic asthma development and cystathionine gamma-lyase deficiency. Antioxid Redox Signal 27:931–944. https://doi.org/10.1089/ars.2016.6875
Chen Y, Wang R (2013) The message in the air: hydrogen sulfide metabolism in chronic respiratory diseases. Respir Physiol Neurobiol 184:130–138. https://doi.org/10.1016/j.resp.2012.03.009
Han W, Dong Z, Dimitropoulou C, Su Y (2011) Hydrogen sulfide ameliorates tobacco smoke-induced oxidative stress and emphysema in mice. Antioxid Redox Signal 15:2121–2134. https://doi.org/10.1089/ars.2010.3821
Chen YH, Yao WZ, Ding YL, Geng B, Lu M, Tang CS (2008) Effect of theophylline on endogenous hydrogen sulfide production in patients with COPD. Pulm Pharmacol Ther. 21:40–46. https://doi.org/10.1016/j.pupt.2006.11.002
Ding HB, Liu KX, Huang JF, Wu DW, Chen JY, Chen QS (2018) Protective effect of exogenous hydrogen sulfide on pulmonary artery endothelial cells by suppressing endoplasmic reticulum stress in a rat model of chronic obstructive pulmonary disease. Biomed Pharmacother 105:734–741. https://doi.org/10.1016/j.biopha.2018.05.131
Wang T, Wang L, Zaidi SR, Sammani S, Siegler J, Moreno-Vinasco L, Mathew B, Natarajan V, Garcia JG (2012) Hydrogen sulfide attenuates particulate matter-induced human lung endothelial barrier disruption via combined reactive oxygen species scavenging and Akt activation. Am J Respir Cell Mol Biol 47:491–496. https://doi.org/10.1165/rcmb.2011-0248OC
Whiteman M, Li L, Rose P, Tan CH, Parkinson DB, Moore PK (2010) The effect of hydrogen sulfide donors on lipopolysaccharide-induced formation of inflammatory mediators in macrophages. Antioxid Redox Signal 12:1147–1154. https://doi.org/10.1089/ars.2009.2899
Francis RC, Vaporidi K, Bloch KD, Ichinose F, Zapol WM (2011) Protective and detrimental effects of sodium sulfide and hydrogen sulfide in murine ventilator-induced lung injury. Anesthesiology 115:1012–1021. https://doi.org/10.1097/ALN.0b013e31823306cf
Aslami H, Heinen A, Roelofs JJ, Zuurbier CJ, Schultz MJ, Juffermans NP (2010) Suspended animation inducer hydrogen sulfide is protective in an in vivo model of ventilator-induced lung injury. Intensive Care Med 36:1946–1952. https://doi.org/10.1007/s00134-010-2022-2
Wang L, Yu H, Zhang Y, Dong C, Liu B (2017) Intravenous controlled-release hydrogen sulfide protects against ventilator-induced lung injury. Exp Lung Res 43:370–377. https://doi.org/10.1080/01902148.2017.1381780
Spassov S, Pfeifer D, Strosing K, Ryter S, Hummel M, Faller S, Hoetzel A (2014) Genetic targets of hydrogen sulfide in ventilator-induced lung injury--a microarray study. PLoS One 15;9:e102401. https://doi.org/10.1371/journal.pone.0102401
Spassov SG, Donus R, Ihle PM, Engelstaedter H, Hoetzel A, Faller S (2017) Hydrogen sulfide prevents formation of reactive oxygen species through PI3K/Akt signaling and limits ventilator-induced lung injury. Oxid Med Cell Longev 2017:3715037. https://doi.org/10.1155/2017/3715037
Faller S, Seiler R, Donus R, Engelstaedter H, Hoetzel A, Spassov SG (2017) Pre- and posttreatment with hydrogen sulfide prevents ventilator-induced lung injury by limiting inflammation and oxidation. PLoS One 12:e0176649. https://doi.org/10.1371/journal.pone.0176649
Shields CJ, Winter DC, Redmond HP (2002) Lung injury in acute pancreatitis: mechanisms, prevention, and therapy. Curr Opin Crit Care 8:158–163
Bhatia M, Wong FL, Fu D, Lau HY, Moochhala SM, Moore PK (2005) Role of hydrogen sulfide in acute pancreatitis and associated lung injury. FASEB J 19:623–625. https://doi.org/10.1096/fj.04-3023fje
Bhatia M, Zhi L, Zhang H, Ng SW, Moore PK (2006) Role of substance P in hydrogen sulfide-induced pulmonary inflammation in mice. Am J Physiol Lung Cell Mol Physiol 291:L896–L904. https://doi.org/10.1152/ajplung.00053.2006
Tamizhselvi R, Moore PK, Bhatia M (2008) Inhibition of hydrogen sulfide synthesis attenuates chemokine production and protects mice against acute pancreatitis and associated lung injury. Pancreas 36:e24–e31. https://doi.org/10.1097/MPA.0b013e31816857bb
Ang AD, Rivers-Auty J, Hegde A, Ishii I, Bhatia M. The effect of CSE gene deletion in caerulein-induced acute pancreatitis in the mouse. Am J Physiol Gastrointest Liver Physiol 305:G712-21. doi: https://doi.org/10.1152/ajpgi.00044.2013.
Qu Z, Jiang Y, Wu BQ, Duan YF, Sun ZD, Luo GH (2014) Cystathionine-gamma-lyase inhibitor attenuates acute lung injury induced by acute pancreatitis in rats. Arch Med Sci 10:825–829. https://doi.org/10.5114/aoms.2014.44873
Bhatia M, Sidhapuriwala JN, Sparatore A, Moore PK (2008) Treatment with H2S-releasing diclofenac protects mice against acute pancreatitis-associated lung injury. Shock 29:84–88. https://doi.org/10.1097/shk.0b013e31806ec26
Sidhapuriwala JN, Ng SW, Bhatia M (2009) Effects of hydrogen sulfide on inflammation in caerulein-induced acute pancreatitis. J Inflamm (Lond) 6:35. https://doi.org/10.1186/1476-9255-6-35
Zhang J, Sio SW, Moochhala S, Bhatia M (2010) Role of hydrogen sulfide in severe burn injury-induced inflammation in mice. Mol Med 16:417–424. https://doi.org/10.2119/molmed.2010.00027
Ahmad A, Szabo C2 (2016) Both the H2S biosynthesis inhibitor aminooxyacetic acid and the mitochondrially targeted H2S donor AP39 exert protective effects in a mouse model of burn injury. Pharmacol Res 113:348-355. doi: https://doi.org/10.1016/j.phrs.2016.09.013.
Asimakopoulou A, Panopoulos P, Chasapis CT, Coletta C, Zhou Z, Cirino G, Giannis A, Szabo C, Spyroulias GA, Papapetropoulos A (2013) Selectivity of commonly used pharmacological inhibitors for cystathionine β synthase (CBS) and cystathionine γ lyase (CSE). Br J Pharmacol 169:922–932. https://doi.org/10.1111/bph.12171
Han ZH, Jiang YI, Duan YY, Wang XY, Huang Y, Fang TZ (2015) Protective effects of hydrogen sulfide inhalation on oxidative stress in rats with cotton smoke inhalation-induced lung injury. Exp Ther Med 10:164–168. https://doi.org/10.3892/etm.2015.2482
Esechie A, Kiss L, Olah G, Horváth EM, Hawkins H, Szabo C, Traber DL (2008) Protective effect of hydrogen sulfide in a murine model of acute lung injury induced by combined burn and smoke inhalation. Clin Sci (Lond) 115:91–97. https://doi.org/10.1042/CS20080021
Esechie A, Enkhbaatar P, Traber DL, Jonkam C, Lange M, Hamahata A, Djukom C, Whorton EB, Hawkins HK, Traber LD, Szabo C (2009) Beneficial effect of a hydrogen sulphide donor (sodium sulphide) in an ovine model of burn- and smoke-induced acute lung injury. Br J Pharmacol 158:1442–1453. https://doi.org/10.1111/j.1476-5381.2009.00411.x
Faller S, Zimmermann KK, Strosing KM, Engelstaedter H, Buerkle H, Schmidt R, Spassov SG, Hoetzel A (2012) Inhaled hydrogen sulfide protects against lipopolysaccharide-induced acute lung injury in mice. Med Gas Res 2:26. https://doi.org/10.1186/2045-9912-2-26
Zimmermann KK, Spassov SG, Strosing KM, Ihle PM, Engelstaedter H, Hoetzel A, Faller S (2018) Hydrogen sulfide exerts anti-oxidative and anti-inflammatory effects in acute lung injury. Inflammation 41:249–259. https://doi.org/10.1007/s10753-017-0684-4
Faller S, Hausler F, Goeft A, von Itter MA, Gyllenram V, Hoetzel A, Spassov SG (2018) Hydrogen sulfide limits neutrophil transmigration, inflammation, and oxidative burst in lipopolysaccharide-induced acute lung injury. Sci Rep 8:14676. https://doi.org/10.1038/s41598-018-33101-x
Xu X, Li H, Gong Y, Zheng H, Zhao D (2018) Hydrogen sulfide ameliorated lipopolysaccharide-induced acute lung injury by inhibiting autophagy through PI3K/Akt/mTOR pathway in mice. Biochem Biophys Res Commun 507:514–518. https://doi.org/10.1016/j.bbrc.2018.11.081
Tian F, Ling Y, Chen Y, Wang Z (2017) Effects of CCK-8 and cystathionine γ-lyase/hydrogen sulfide system on acute lung injury in rats. Inflammation 40:174–183. https://doi.org/10.1007/s10753-016-0466-4
Sakaguchi M, Marutani E, Shin HS, Chen W, Hanaoka K, Xian M, Ichinose F (2014) Sodium thiosulfate attenuates acute lung injury in mice. Anesthesiology 121:1248–1257. https://doi.org/10.1097/ALN.0000000000000456
Jiang L, Jiang Q, Yang S, Huang S, Han X, Duan J, Pan S, Zhao M, Guo S (2019) GYY4137 attenuates LPS-induced acute lung injury via heme oxygenase-1 modulation. Pulm Pharmacol Ther 54:77–86. https://doi.org/10.1016/j.pupt.2018.12.004
Du Q, Wang C, Zhang N, Li G, Zhang M, Li L, Zhang Q, Zhang J (2014) In vivo study of the effects of exogenous hydrogen sulfide on lung mitochondria in acute lung injury in rats. BMC Anesthesiol 14:117. https://doi.org/10.1186/1471-2253-14-117
Wagner F, Wagner K, Weber S, Stahl B, Knöferl MW, Huber-Lang M, Seitz DH, Asfar P, Calzia E, Senftleben U, Gebhard F, Georgieff M, Radermacher P, Hysa V (2011) Inflammatory effects of hypothermia and inhaled H2S during resuscitated, hyperdynamic murine septic shock. Shock 35:396–402. https://doi.org/10.1097/SHK.0b013e3181ffff0e
Zhang H, Zhi L, Moore PK, Bhatia M (2006) Role of hydrogen sulfide in cecal ligation and puncture-induced sepsis in the mouse. Am J Physiol Lung Cell Mol Physiol 290:L1193–L1201
Zhang H, Zhi L, Moochhala SM, Moore PK, Bhatia M (2007) Endogenous hydrogen sulfide regulates leukocyte trafficking in cecal ligation and puncture-induced sepsis. J Leukoc Biol 82:894–905
Zhang H, Zhi L, Moochhala S, Moore PK, Bhatia M (2007) Hydrogen sulfide acts as an inflammatory mediator in cecal ligation and puncture-induced sepsis in mice by upregulating the production of cytokines and chemokines via NF-kappaB. Am J Physiol Lung Cell Mol Physiol 292:L960–L971
Zhang H, Moochhala SM, Bhatia M (2007) Endogenous hydrogen sulfide regulates inflammatory response by activating the ERK pathway in polymicrobial sepsis. J Immunol 181:4320–4331
Zhang H, Hegde A, Ng SW, Adhikari S, Moochhala SM, Bhatia M (2007) Hydrogen sulfide up-regulates substance P in polymicrobial sepsis-associated lung injury. J Immunol 179:4153–4160
Ang SF, Moochhala SM, Bhatia (2010) Hydrogen sulfide promotes transient receptor potential vanilloid 1-mediated neurogenic inflammation in polymicrobial sepsis. Crit Care Med 38:619-628. doi: https://doi.org/10.1097/CCM.0b013e3181c0df00.
Ang SF, Sio SW, Moochhala SM, MacAry PA, Bhatia M (2011) Hydrogen sulfide upregulates cyclooxygenase-2 and prostaglandin E metabolite in sepsis-evoked acute lung injury via transient receptor potential vanilloid type 1 channel activation. J Immunol 187:4778–4787. https://doi.org/10.4049/jimmunol.1101559
Ahmad A, Druzhyna N, Szabo C (2016) Delayed treatment with sodium hydrosulfide improves regional blood flow and alleviates cecal ligation and puncture (CLP)-induced septic shock. Shock 46:183–193. https://doi.org/10.1097/SHK.0000000000000589
Drucker NA, Jensen AR, Ferkowicz M, Markel TA (2018) Hydrogen sulfide provides intestinal protection during a murine model of experimental necrotizing enterocolitis. J Pediatr Surg 53:1692–1698. https://doi.org/10.1016/j.jpedsurg.2017.12.003
Schuster DP (1994) ARDS: clinical lessons from the oleic acid model of acute lung injury. Am J Respir Crit Care Med 149:245–260. https://doi.org/10.1164/ajrccm.149.1.8111590
Li T, Zhao B, Wang C, Wang H, Liu Z, Li W, Jin H, Tang C, Du J (2008) Regulatory effects of hydrogen sulfide on IL-6, IL-8 and IL-10 levels in the plasma and pulmonary tissue of rats with acute lung injury. Exp Biol Med (Maywood) 233:1081–1087. https://doi.org/10.3181/0712-RM-354
Wang C, Wang HY, Liu ZW, Fu Y, Zhao B (2011) Effect of endogenous hydrogen sulfide on oxidative stress in oleic acid-induced acute lung injury in rats. Chin Med J (Engl) 124:3476–3480
Liu WL, Liu ZW, Li TS, Wang C, Zhao B (2013) Hydrogen sulfide donor regulates alveolar epithelial cell apoptosis in rats with acute lung injury. Chin Med J (Engl) 126:494–499
Liu ZW, Wang HY, Guan L, Zhao B (2015) Regulatory effects of hydrogen sulfide on alveolar epithelial cell endoplasmic reticulum stress in rats with acute lung injury. World J Emerg Med 6:67–73. https://doi.org/10.5847/wjem.j.1920-8642.2015.01.012
Faller S, Spassov SG, Zimmermann KK, Ryter SW, Buerkle H, Loop T, Schmidt R, Strosing KM, Hoetzel A (2013) Hydrogen sulfide prevents hyperoxia-induced lung injury by downregulating reactive oxygen species formation and angiopoietin-2 release. Curr Pharm Des 19:2715–2721. https://doi.org/10.2174/1381612811319150006
Li HD, Zhang ZR, Zhang QX, Qin ZC, He DM, Chen JS (2013) Treatment with exogenous hydrogen sulfide attenuates hyperoxia-induced acute lung injury in mice. Eur J Appl Physiol 113:1555–1563. https://doi.org/10.1007/s00421-012-2584-5
Zhang P, Li F, Wiegman CH, Zhang M, Hong Y, Gong J, Chang Y, Zhang JJ, Adcock I, Chung KF, Zhou X (2015) Inhibitory effect of hydrogen sulfide on ozone-induced airway inflammation, oxidative stress, and bronchial hyperresponsiveness. Am J Respir Cell Mol Biol 52:129–137. https://doi.org/10.1165/rcmb.2013-0415OC
Flierl MA, Perl M, Rittirsch D, Bartl C, Schreiber H, Fleig V, Schlaf G, Liener U, Brueckner UB, Gebhard F, Huber-Lang MS (2008) The role of C5a in the innate immune response after experimental blunt chest trauma. Shock 29:25–31
Seitz DH, Fröba JS, Niesler U, Palmer A, Veltkamp HA, Braumüller ST, Wagner F, Wagner K, Bäder S, Wachter U, Calzia E, Radermacher P, Huber-Lang MS, Zhou S, Gebhard F, Knöferl MW (2012) Inhaled hydrogen sulfide induces suspended animation, but does not alter the inflammatory response after blunt chest trauma. Shock 37:197–204. https://doi.org/10.1097/SHK.0b013e31823f19a0
Hartmann C, Gröger M, Noirhomme JP, Scheuerle A, Möller P, Wachter U, Huber-Lang M, Nussbaum B, Jung B, Merz T, McCook O, Kress S, Stahl B, Calzia E, Georgieff M, Radermacher P, Wepler M (2019) In-depth characterization of the effects of cigarette smoke exposure on the acute trauma response and hemorrhage in mice. Shock 51:68–77. https://doi.org/10.1097/SHK.0000000000001115
Hartmann C, Hafner S, Scheuerle A, Möller P, Huber-Lang M, Jung B, Nubaum B, McCook O, Gröger M, Wagner F, Weber S, Stahl B, Calzia E, Georgieff M, Szabó C, Wang R, Radermacher P, Wagner K (2017) The role of cystathionine-γ-lyase in blunt chest trauma in cigarette smoke exposed mice. Shock 47:491–499. https://doi.org/10.1097/SHK.0000000000000746
Wu J, Wei J, You X, Chen X, Zhu H, Zhu X, Liu Y, Xu M (2013) Inhibition of hydrogen sulfide generation contributes to lung injury after experimental orthotopic lung transplantation. J Surg Res 182:e25–e33. https://doi.org/10.1016/j.jss.2012.09.028
Qi QY, Chen W, Li XL, Wang YW, Xie XH (2014) H2S protecting against lung injury following limb ischemia-reperfusion by alleviating inflammation and water transport abnormality in rats. Biomed Environ Sci 27:410–418. https://doi.org/10.3967/bes2014.070
Tang B, Ma L, Yao X, Tan G, Han P, Yu T, Liu B, Sun X (2015) Hydrogen sulfide ameliorates acute lung injury induced by infrarenal aortic cross-clamping by inhibiting inflammation and angiopoietin 2 release. J Vasc Surg 65:501-508.e1. https://doi.org/10.1016/j.jvs.2015.10.010
Jiang T, Liu Y, Meng Q, Lv X, Yue Z, Ding W, Liu T, Cui X (2019) Hydrogen sulfide attenuates lung ischemia-reperfusion injury through SIRT3-dependent regulation of mitochondrial function in type 2 diabetic rats. Surgery 165:1014–1026. https://doi.org/10.1016/j.surg.2018.12.018 Epub 2019 Feb 26
Chai W, Wang Y, Lin JY, Sun XD, Yao LN, Yang YH, Zhao H, Jiang W, Gao CJ, Ding Q (2012) Exogenous hydrogen sulfide protects against traumatic hemorrhagic shock via attenuation of oxidative stress. J Surg Res 176:210–219. https://doi.org/10.1016/j.jss.2011.07.016
Wepler M, Merz T, Wachter U, Vogt J, Calzia E, Scheuerle A, Möller P, Gröger M, Kress S, Fink M, Lukaschewski B, Rumm G, Stahl B, Georgieff M, Huber-Lang M, Torregrossa R, Whiteman M, McCook O, Radermacher P, Hartmann C (2019) The mitochondria-targeted H2S-donor AP39 in a murine model of combined hemorrhagic shock and blunt chest trauma. Shock 52:230–239. https://doi.org/10.1097/SHK.0000000000001210
Satterly SA, Salgar S, Hoffer Z, Hempel J, DeHart MJ, Wingerd M, Raywin H, Stallings JD, Martin M (2015) Hydrogen sulfide improves resuscitation via non-hibernatory mechanisms in a porcine shock model. J Surg Res 199:197–210. https://doi.org/10.1016/j.jss.2015.04.001
Gao C, Xu DQ, Gao CJ, Ding Q, Yao LN, Li ZC (2012) Chai W (2012) An exogenous hydrogen sulphide donor, NaHS, inhibits the nuclear factor κB inhibitor kinase/nuclear factor κb inhibitor/nuclear factor-κB signaling pathway and exerts cardioprotective effects in a rat hemorrhagic shock model. Biol Pharm Bull. 35(7):1029–1034. https://doi.org/10.1248/bpb.b110679
Ganster F, Burban M, de la Bourdonnaye M, Fizanne L, Douay O, Loufrani L, Mercat A, Calès P, Radermacher P, Henrion D, Asfar P, Meziani F (2010) Effects of hydrogen sulfide on hemodynamics, inflammatory response and oxidative stress during resuscitated hemorrhagic shock in rats. Crit Care 14:R165. https://doi.org/10.1186/cc9257
Morrison ML, Blackwood JE, Lockett SL, Iwata A, Winn RK, Roth MB (2008) Surviving blood loss using hydrogen sulfide. J Trauma 65:183–188. https://doi.org/10.1097/TA.0b013e3181507579
Knöller E, Stenzel T, Broeskamp F, Hornung R, Scheuerle A, McCook O, Wachter U, Vogt JA, Matallo J, Wepler M, Gässler H, Gröger M, Matejovic M, Calzia E, Lampl L, Georgieff M, Möller P, Asfar P, Radermacher P, Hafner S (2016) Effects of hyperoxia and mild therapeutic hypothermia during resuscitation from porcine hemorrhagic shock. Crit Care Med 44:e264–e277. https://doi.org/10.1097/CCM.0000000000001412
Nussbaum BL, Stenzel T, Merz T, Scheuerle A, McCook O, Wachter U, Vogt JA, Matallo J, Gässler H, Gröger M, Matejovic M, Calzia E, Lampl L, Georgieff M, Möller P, Asfar P, Radermacher P, Hafner S (2017) Hyperoxia or therapeutic hypothermia during resuscitation from non-lethal hemorrhagic shock in swine. Shock 48:564–570. https://doi.org/10.1097/SHK.0000000000000884
Mok YY, Atan MS, Yoke Ping C, Zhong Jing W, Bhatia M, Moochhala S, Moore PK (2004) Role of hydrogen sulphide in haemorrhagic shock in the rat: protective effect of inhibitors of hydrogen sulphide biosynthesis. Br J Pharmacol 143:881–889. https://doi.org/10.1038/sj.bjp.0706014
Mok YY, Moore PK (2008) Hydrogen sulphide is pro-inflammatory in haemorrhagic shock. Inflamm Res 57:512–518. https://doi.org/10.1007/s00011-008-7231-6
Gröger M, Wepler M, Wachter U, Merz T, McCook O, Kress S, Lukaschewski B, Hafner S, Huber-Lang M, Calzia E, Georgieff M, Nagahara N, Szabó C, Radermacher P, Hartmann C (2019) The effects of genetic 3-mercaptopyruvate sulfurtransferase deficiency in murine traumatic-hemorrhagic shock. Shock 51:472–478. https://doi.org/10.1097/SHK.0000000000001165
Drabek T, Kochanek PM, Stezoski J, Wu X, Bayir H, Morhard RC, Stezoski SW, Tisherman SA (2011) Intravenous hydrogen sulfide does not induce hypothermia or improve survival from hemorrhagic shock in pigs. Shock 35:67–73. https://doi.org/10.1097/SHK.0b013e3181e86f49
Guillon A, Preau S, Aboab J, Azabou E, Jung B, Silva S, Textoris J, Uhel F, Vodovar D, Zafrani L, de Prost N, Radermacher P (2019) Preclinical septic shock research: why we need an animal ICU. Ann Intensive Care 9:66. https://doi.org/10.1186/s13613-019-0543-6
Aslami H, Schultz MJ, Juffermans NP (2009) Potential applications of hydrogen sulfide-induced suspended animation. Curr Med Chem 16:1295–1303
Asfar P, Calzia E, Radermacher P (2014) Is pharmacological, H2S-induced ‘suspended animation’ feasible in the ICU? Crit Care 18:215. https://doi.org/10.1186/cc13782
Derwall M, Westerkamp M, Löwer C, Deike-Glindemann J, Schnorrenberger NK, Coburn M, Nolte KW, Gaisa N, Weis J, Siepmann K, Häusler M, Rossaint R, Fries M (2010) Hydrogen sulfide does not increase resuscitability in a porcine model of prolonged cardiac arrest. Shock 34:190–195. https://doi.org/10.1097/SHK.0b013e3181d0ee3d
Nußbaum BL, Vogt J, Wachter U, McCook O, Wepler M, Matallo J, Calzia E, Gröger M, Georgieff M, Wood ME, Whiteman M, Radermacher P, Hafner S (2017) Metabolic, cardiac, and renal effects of the slow hydrogen sulfide-releasing molecule GYY4137 during resuscitated septic shock in swine with pre-existing coronary artery disease. Shock 48:175–184. https://doi.org/10.1097/SHK.0000000000000834
Bredthauer A, Lehle K, Scheuerle A, Schelzig H, McCook O, Radermacher P, Szabo C, Wepler M, Simon F (2018) Intravenous hydrogen sulfide does not induce neuroprotection after aortic balloon occlusion-induced spinal cord ischemia/reperfusion injury in a human-like porcine model of ubiquitous arteriosclerosis. Intensive Care Med Exp 6(1):44. https://doi.org/10.1186/s40635-018-0209-y
Simon F, Scheuerle A, Gröger M, Stahl B, Wachter U, Vogt J, Speit G, Hauser B, Möller P, Calzia E, Szabó C, Schelzig H, Georgieff M, Radermacher P, Wagner F (2011) Effects of intravenous sulfide during porcine aortic occlusion-induced kidney ischemia/reperfusion injury. Shock 35:156–163. https://doi.org/10.1097/SHK.0b013e3181f0dc91
Bracht H, Scheuerle A, Gröger M, Hauser B, Matallo J, McCook O, Seifritz A, Wachter U, Vogt JA, Asfar P, Matejovic M, Möller P, Calzia E, Szabó C, Stahl W, Hoppe K, Stahl B, Lampl L, Georgieff M, Wagner F, Radermacher P, Simon F (2012) Effects of intravenous sulfide during resuscitated porcine hemorrhagic shock. Crit Care Med 40:2157–2167. https://doi.org/10.1097/CCM.0b013e31824e6b30
Dirkes MC, Milstein DM, Heger M, van Gulik TM (2015) Absence of hydrogen sulfide-induced hypometabolism in pigs: a mechanistic explanation in relation to small nonhibernating mammals. Eur Surg Res 54:178–191. https://doi.org/10.1159/000369795 Epub 2015 Feb 12
Datzmann T, Hoffmann A, McCook O, Merz T, Wachter U, Preuss J, Vettorazzi S, Calzia E, Gröger M, Kohn F, Schmid A, Denoix N, Radermacher P, Wepler M (2019) Effects of sodium thiosulfate (Na2S2O3) during resuscitation from hemorrhagic shock in swine with preexisting atherosclerosis. Pharmacol Res 14:104536. https://doi.org/10.1016/j.phrs.2019.104536
Nagy P, Pálinkás Z, Nagy A, Budai B, Tóth I, Vasas A (2014) Chemical aspects of hydrogen sulfide measurements in physiological samples. Biochim Biophys Acta 1840:876–891. https://doi.org/10.1016/j.bbagen.2013.05.037
Wu N, Du X, Wang D, Hao F (2011) Myocardial and lung injuries induced by hydrogen sulfide and the effectiveness of oxygen therapy in rats. Clin Toxicol (Phila) 49:161–166. https://doi.org/10.3109/15563650.2011.565419
Wang J, Zhang H, Su C, Chen J, Zhu B, Zhang H, Xiao H, Zhang J (2014) Dexamethasone ameliorates H2S-induced acute lung injury by alleviating matrix metalloproteinase-2 and -9 expression. PLoS One 9:e94701. https://doi.org/10.1371/journal.pone.0094701
Derwall M, Francis RC, Kida K, Bougaki M, Crimi E, Adrie C, Zapol WM, Ichinose F (2011) Administration of hydrogen sulfide via extracorporeal membrane lung ventilation in sheep with partial cardiopulmonary bypass perfusion: a proof of concept study on metabolic and vasomotor effects. Crit Care 15:R51. https://doi.org/10.1186/cc10016
Olson KR, Deleon ER, Gao Y, Hurley K, Sadauskas V, Batz C, Stoy GF (2013) Thiosulfate: a readily accessible source of hydrogen sulfide in oxygen sensing. Am J Physiol Regul Integr Comp Physiol 305:R592–R603. https://doi.org/10.1152/ajpregu.00421.2012
Xu S, Yang CT, Meng FH, Pacheco A, Chen L, Xian M (2016) Ammonium tetrathiomolybdate as a water-soluble and slow-release hydrogen sulfide donor. Bioorg Med Chem Lett 26:1585–1588. https://doi.org/10.1016/j.bmcl.2016.02.005
Dyson A, Dal-Pizzol F, Sabbatini G, Lach AB, Galfo F, Dos Santos Cardoso J, Pescador Mendonça B, Hargreaves I5, Bollen Pinto B, Bromage DI, Martin JF, Moore KP, Feelisch M, Singer M (2017) Ammonium tetrathiomolybdate following ischemia/reperfusion injury: chemistry, pharmacology, and impact of a new class of sulfide donor in preclinical injury models. PLoS Med 14:e1002310. doi: https://doi.org/10.1371/journal.pmed.1002310.
Donnarumma E, Ali MJ, Rushing AM, Scarborough AL, Bradley JM, Organ CL, Islam KN, Polhemus DJ, Evangelista S, Cirino G, Jenkins JS, Patel RA, Lefer DJ, Goodchild TT (2016) Zofenopril protects against myocardial ischemia-reperfusion injury by increasing nitric oxide and hydrogen sulfide bioavailability. J Am Heart Assoc 5:e003531. https://doi.org/10.1161/JAHA.116.003531
Neuwelt EA, Gilmer-Knight K, Lacy C, Nicholson HS, Kraemer DF, Doolittle ND, Hornig GW, Muldoon LL (2006) Toxicity profile of delayed high dose sodium thiosulfate in children treated with carboplatin in conjunction with blood-brain-barrier disruption. Pediatr Blood Cancer 47:174–182
Marutani E, Yamada M, Ida T, Tokuda K, Ikeda K, Kai S, Shirozu K, Hayashida K, Kosugi S, Hanaoka K, Kaneki M, Akaike T, Ichinose F (2015) Thiosulfate mediates cytoprotective effects of hydrogen sulfide against neuronal ischemia. J Am Heart Assoc 4:e002125. https://doi.org/10.1161/JAHA.115.002125
Acknowledgements
Not applicable
About this supplement
This article has been published as part of Intensive Care Medicine Experimental Volume 8 Supplement 1, 2020: Proceedings from the Fourth International Symposium on Acute Pulmonary Injury and Translation Research (INSPIRES IV). The full contents of the supplement are available at https://icm-experimental.springeropen.com/articles/supplements/volume-8-supplement-1.
Funding
PR received funding from the DFG CRC 1149, the DFG GRK 2203, and the German Ministry of Defense. The work has been funded by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) - Projektnummer 251293561 - SFB 1149. Publication costs are covered from the budget of the Institute for Anesthesiological Pathophysiology and Process Engineering (DFG CRC 1149 and DFG GRK 2203).
Author information
Authors and Affiliations
Contributions
TM drafted the manuscript. OM and PR critically reviewed and edited the manuscript. TM, ND, MW, HG, DACM, CH, TD, and OM were involved in the acquisition and interpretation of data. All authors read and approved the final version.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Merz, T., Denoix, N., Wepler, M. et al. H2S in acute lung injury: a therapeutic dead end(?). ICMx 8 (Suppl 1), 33 (2020). https://doi.org/10.1186/s40635-020-00324-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40635-020-00324-0